Abstract
Background
The majority of Plasmodium falciparum infections, constituting the reservoir in all ages, are asymptomatic in high-transmission settings in Africa. The role of this reservoir in the evolution and spread of drug resistance was explored.
Methods
Population genetic analyses of the key drug resistance–mediating polymorphisms were analyzed in a cross-sectional survey of asymptomatic P. falciparum infections across all ages in Bongo District, Ghana.
Results
Seven years after the policy change to artemisinin-based combination therapies in 2005, the pfcrt K76 and pfmdr1 N86 wild-type alleles have nearly reached fixation and have expanded via soft selective sweeps on multiple genetic backgrounds. By constructing the pfcrt-pfmdr1-pfdhfr-pfdhps multilocus haplotypes, we found that the alleles at these loci were in linkage equilibrium and that multidrug-resistant parasites have not expanded in this reservoir. For pfk13, 32 nonsynonymous mutations were identified; however, none were associated with artemisinin-based combination therapy resistance.
Conclusions
The prevalence and selection of alleles/haplotypes by antimalarials were similar to that observed among clinical cases in Ghana, indicating that they do not represent 2 subpopulations with respect to these markers. Thus, the P. falciparum reservoir in all ages can contribute to the maintenance and spread of antimalarial resistance.
Keywords: malaria, Plasmodium falciparum, Ghana, drug resistance, population genetics, asymptomatic reservoir, artemisinin-based combination therapies, antimalarials
The majority of Plasmodium falciparum infections, constituting the reservoir in all ages, are asymptomatic in high-transmission settings in Africa. The role of this reservoir in the evolution and spread of drug resistance was investigated in northern Ghana.
Plasmodium falciparum resistance to antimalarials including chloroquine (CQ), sulfadoxine-pyrimethamine (SP), and artemisinin (ART)-based combination therapies (ACTs) threatens control efforts, particularly in sub-Saharan Africa where >90% of malaria-related deaths occur annually [1]. In the early 2000s widespread resistance to CQ, both globally and locally, prompted Ghana to change its first-line treatment policy to ACTs for uncomplicated malaria. Accordingly, in 2005 the Ministry of Health in Ghana replaced CQ with artesunate-amodiaquine (ASAQ) as the first-line treatment, with the addition of artemether-lumefantrine (AL) in 2008 [2]. With the widespread use of ACTs, ART resistance, characterized by a slow parasite clearance phenotype, has been associated with the presence of mutations in the “propeller” region of the P. falciparum kelch gene encoded on chromosome 13 (pfk13) [3–5]. Although ART resistance has spread across Southeast Asia (SEA) [4, 6–8], it has yet to become established in sub-Saharan Africa, despite the identification of pfk13 mutations at low frequency on African genetic backgrounds [6–10].
Monitoring for shifts in antimalarial drug efficacy using genetic markers, including pfcrt and pfmdr1 (for CQ, ASAQ, and AL), pfdhps and pfdhfr (for SP), and pfk13 (for ART), is essential to properly guide treatment. P. falciparum genetic diversity facilitates adaptation to environmental changes, including antimalarial drug pressure [11, 12]. In regions with low transmission, such as SEA, P. falciparum populations have been characterized by single-clone infections, low genetic variation, reduced rates of recombination, and strong linkage disequilibrium (LD) (ie, nonrandom associations among loci) [11]. Given that aggressive strategies have been implemented to eliminate P. falciparum from SEA and that inbreeding predominates, it is not surprising that multidrug resistance (MDR) to antimalarials with different modes of action have arisen and spread [13–16]. Although studies in Ghana have provided longitudinal data on the trends for polymorphisms in the genes mediating resistance, they have not addressed (1) whether MDR haplotypes are circulating and/or expanding in the P. falciparum population and/or (2) whether LD exists between/among the alleles at these drug resistance loci.
Current antimalarial resistance monitoring in Ghana has focused on screening and genotyping children with clinical P. falciparum infections [17–20]. However, the majority of P. falciparum infections in Ghana, like most of sub-Saharan Africa, are asymptomatic and, despite persisting throughout the wet and dry seasons across all ages, are generally not included in routine surveillance for antimalarial resistance [21, 22].
Hence, we aimed to examine the population genetics of antimalarial drug resistance markers in this neglected reservoir in this high-transmission African setting, where malaria will be most difficult to eliminate. The prevalence, diversity, and mechanisms of selection for the key drug resistance–mediating polymorphisms was examined in an age-stratified cross-sectional survey of asymptomatic P. falciparum infections in all ages in Bongo District, Ghana, 7-years after the introduction of ACTs. This study is one of the first to investigate antimalarial drug resistance markers in the asymptomatic P. falciparum reservoir across all ages in sub-Saharan Africa. It is also notable as it explores the relationship among antimalarial drug resistance markers, usually analyzed independently.
METHODS
Study Design
This age stratified cross-sectional survey was conducted at the end of the dry season over a 2-week period before the start of the heavy rains (June 2012) among 698 participants (aged 1–85 years) in Bongo District, Ghana. Details on the study population, data collection procedures, and epidemiology have been published elsewhere [21, 22]. This study was approved by the ethics committees at the Navrongo Health Research Centre (Ghana), Noguchi Memorial Institute for Medical Research (Ghana), University of Ghana (Ghana), and The University of Melbourne (Australia).
pfcrt, pfmdr1, pfdhfr, pfdhps, and pfk13 Genotyping
For isolates with confirmed P. falciparum infections by microscopy, modified nested polymerase chain reaction protocols with adapted primers (Supplementary Table 1) were performed to amplify and detect the key single-nucleotide polymorphisms (SNPs) using Sanger sequencing (Supplementary Methods) for pfcrt (chromosome 7; codons 72–76), pfmdr1 (chromosome 5; codons 86 and 184), pfdhfr (chromosome 4; codons 51, 59, 108, and 164), and pfdhps (chromosome 8; codons 436, 437, 540, 581, and 613) [17]. For each gene the key SNPs were classified as either wild type or mutant. Multiplicity of infection (MOI) was evaluated using previously published msp2 genotyping data [21]. Multilocus haplotypes were constructed for the various loci investigated, including (1) between/among codons within each gene (termed alleles or haplotypes) and (2) between/among the genes (termed multigene haplotypes), for only those isolates with confirmed single-clone infections (MOI = 1).
Isolates with complete genotyping data were further analyzed for pfk13. To identify pfk13 (chromosome 13; codons 427–650) SNPs, specific genomic regions of the propeller domain of pfk13 were amplified using forward primers that included a multiplex identifier (Supplementary Table 1 and Supplementary Figure 1) and sequenced using an Illumina MiSeq platform (Supplementary Methods). For each isolate, the pfk13 SNPs were classified as either synonymous or nonsynonymous amino acid changes. The unique pfk13 sequences identified in Bongo were then aligned with 406 published pfk13 sequences from Mali, Uganda, Kenya, and SEA to construct a neighbor-joining phylogenetic tree (Supplementary Methods). All sequence data reported in this article have been submitted to GenBank. Accession numbers: MK687571-MK688372. Project number: PRJNA630847.
Microsatellite Loci Typing
To explore selection on pfcrt and pfmdr1, the microsatellite loci flanking these genes (designated selected) were investigated using modified polymerase chain reaction protocols with adapted primers (Supplementary Table 2). For this analysis only isolates with an MOI ≤2 (based on msp2 genotyping) were included for pfcrt (n = 66) and pfmdr1 (n = 69). Eleven flanking microsatellites were typed, with 4 loci flanking pfcrt (−4.9, −4.6, 1.6, and 4.2 kb) and 7 flanking pfmdr1 (−1.6, −1.4, −1.2, −0.9, −0.2, 0.3, and 0.6 kb). Since these loci are proximal to genes under selection and considered non-neutral, previously published data from 12 putatively neutral microsatellites (designated unselected) were included for comparison [21]. The flanking microsatellites were used to construct the multilocus microsatellite haplotypes to define the parasite genetic lineages circulating.
Population Genetic Analyses
All data were recorded and formatted in Excel and imported into R software, version 3.5.0 [23], for the genetic analyses (Supplementary Methods). For the drug resistance genes, genetic diversity was analyzed using the number of alleles per locus (A), the number of unique haplotypes (h), the expected heterozygosity (He), and the evenness score. In addition, diversity was evaluated using the Shannon-Weiner index (H) and Simpson’s index (D). The standardized index of association () (note: 10 000 permutations) by Agapow and Burt [24] was used to measure LD between/among the loci investigated. For the microsatellite loci, genetic diversity was analyzed using A and He. To investigate selection on pfcrt and pfmdr1, genetic differentiation (GST, a generalization of the fixation index (FST) that accommodates for multiple alleles and loci) was calculated for the 11 flanking and the 12 putatively neutral microsatellites. To estimate genetic relatedness we calculated the pairwise allele sharing (PAS) statistic between all pairs of isolates for the microsatellites flanking pfcrt, pfmdr1, and the neutral loci [21]. To construct genetic relatedness networks, a threshold of PAS ≥ 0.70 was selected to visualize the relationships among the different alleles/haplotypes.
Statistical Analyses
The statistical analyses were performed using R software, version 3.5.0 [23]. The discrete variables are presented using the calculated/observed prevalence, with Fisher exact or χ 2 used for univariate analyses of discrete variables to compare proportions. The mean He between the wild-type and mutant alleles/haplotypes and the mean GST between the flanking and neutral microsatellite loci were compared using the Mann-Whitney test. For the multigene and multilocus microsatellite haplotypes selected based on MOI (ie, MOI of 1 or ≤2, respectively), this selection did not significantly bias the isolates included, except where indicated (Supplementary Results). A result was deemed statistically significant if the P value was <.05.
RESULTS
Study Population
The demographics and parasitological characteristics of the study population have been previously described by Ruybal-Pesántez et al [21]. Briefly, during their survey at the end of the dry season, 267 participants (38.3%) were positive for an asymptomatic P. falciparum infection, with only 10.5% reporting antimalarial use in the 2-weeks before being surveyed. MOI was successfully genotyped for 251 of the 267 P. falciparum positive isolates (94.0%) and ranged from 1 to 6 (median = 2; interquartile range = 1-3), with 152 isolates (60.6%) having an MOI ≥2 [21].
Genotyping
For a detailed breakdown of the isolates used and their corresponding genotyping and statistical results, see the Supplementary Results (Supplementary Figure 2 and Supplementary Tables 3–5).
Prevalence of Resistance-Mediating Polymorphisms in pfcrt and pfmdr1
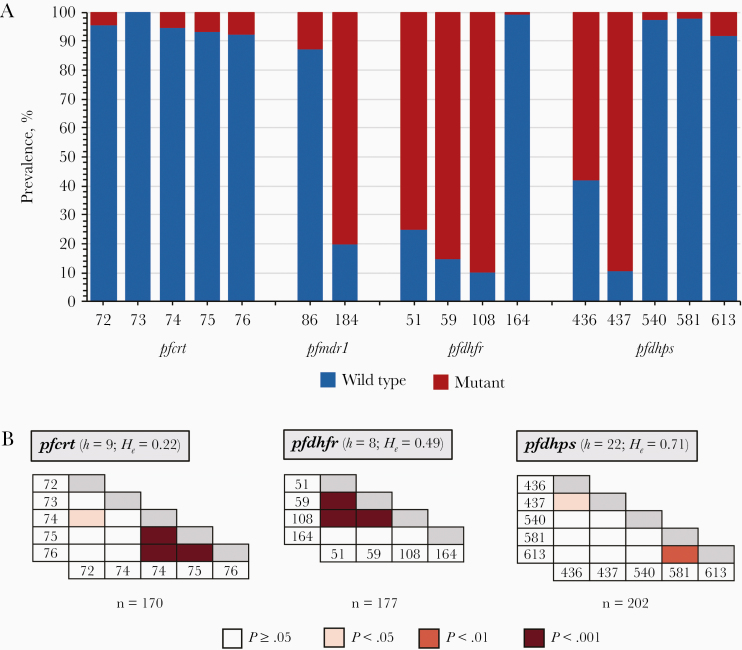
The pfcrt K76 and pfmdr1 N86 wild-type alleles, associated with CQ sensitivity, were identified in 92.4% and 87.4% of isolates, respectively (Figure 1 and Supplementary Table 6). Nine unique pfcrt haplotypes were identified in this asymptomatic reservoir, with the CQ-sensitive CVMNK haplotype the most prevalent (88.2%) (Supplementary Table 7). The African/Asian mutant CVIET haplotype, associated with CQ treatment failure, was observed in only 3.5% of isolates. For pfmdr1, the wild-type NY (17.7%) and the single mutant NF (69.7%) haplotypes were the most prevalent.
Figure 1.
A, Prevalence of wild-type and mutant sequences at the indicated alleles for pfcrt (n = 170), pfmdr1 (n = 198), pfdhfr (n = 177), and pfdhps (n = 202) in the asymptomatic Plasmodium falciparum reservoir (see Supplementary Table 7 for additional details on the unique haplotypes identified). (Note: pfmdr1 codon 1246 was not polymorphic. All isolates genotyped carried the D1246 wild-type allele.) B, Pairwise linkage disequilibrium (LD) between all loci (codons) for pfcrt, pfdhfr, and pfdhps. Gray shading along the horizontal is used to denote within codon comparisons, for which the calculation of LD is not possible. For each gene, the number of unique haplotypes (h) and heterozygosity (He) are provided, as well as the number of isolates included. Pfmdr1 is not included, since there was no significant pairwise LD between codons 86 and 184. (For pfcrt and pfmdr1 previous studies with clinical isolates have observed LD between codons pfcrt 76 and pfmdr1 86 due to directional selection by chloroquine [25, 26]. No significant LD was detected between the codon pair ( = 0.0121; P = .62)).
Prevalence of Resistance-Mediating Polymorphisms in pfdhfr and pfdhps
The pfdhfr mutant 51I, 59R, and 108N alleles, necessary for pyrimethamine resistance, were characterized in 75.1%, 85.3%, and 89.8% of isolates, respectively (Figure 1 and Supplementary Table 6). For pfdhps used to monitor for sulfadoxine resistance, 89.1% of isolates carried the mutant 437G allele, whereas only 1.5% of isolates carried the 581G mutation, which is associated with high-level resistance and uncommon in Africa. For pfdhfr, 8 unique haplotypes were observed with the majority of isolates (91.0%) carrying mutant haplotypes (Supplementary Table 7). The most prevalent were the double (NRNI) and triple (IRNI) mutants, both involved in modulating intermediate resistance to pyrimethamine, identified in 15.3% and 68.9% isolates, respectively. For pfdhps there were 22 unique haplotypes with 96.5% of isolates carrying mutant haplotypes. The 2 predominant haplotypes observed included the single (SGKAA) and double (AGKAA) mutants at 33.7% and 41.6%, respectively.
Prevalence of Resistance-Mediating Polymorphisms in pfk13
Of the 109 isolates with pfk13 sequence data, 418 unique sequences or haplotypes were identified (Supplementary Results) with 92.3% having nonsynonymous SNPs. A total of 53 pfk13 SNPs were identified, with 60.4% being nonsynonymous (Supplementary Table 8). Of these nonsynonymous pfk13 SNPs, only the 578S, identified in 12 isolates (11.0%), has been characterized in vitro, although it has not been associated with ART resistance [9]. In Bongo the most prevalent mutation was 648E (nonsynonymous), identified in 83 isolates (76.1%), with the remainder observed in <5% of isolates. In the phylogenetic analysis the Bongo pfk13 sequences formed a separate cluster with other African isolates and were distinct from the SEA isolates included (Figure 2).
Figure 2.
Inferred phylogeny of the pfk13 sequences from Bongo District, Ghana. An unrooted neighbor-joining phylogenetic tree was constructed using sequences from West Africa (Bongo District, Ghana [n = 418] and Mali [n = 206]), East Africa (Uganda [n = 66] and Kenya [n = 70]) (GenBank accession nos. KT901412-KT901454 and KT955978-KT956003), and Southeast Asia (n = 60). Asterisks at major nodes indicate bootstrap values ≥65%.
Genetic Diversity
For the codons, the mean He was lowest for pfcrt (He = 0.09) compared with pfmdr1, pfdhps, and pfdhfr, whose mean He values were more than two times greater than pfcrt, ranging from 0.20 to 0.27 (Supplementary Table 9). For the haplotypes, multiple unique alleles/haplotypes were identified for all drug resistance genes investigated (Supplementary Table 7), with He lowest for pfcrt (He = 0.22) and highest for pfdhps (He = 0.71) (Table 1). When these genes were compared, pfcrt had the lowest diversity index (H = 0.58; D = 0.22), pfmdr1 and pfdhfr were intermediate, and pfdhps was the most diverse (H = 1.70; D = 0.71) (Table 1 and Supplementary Table 10). For the constructed multigene haplotypes (Supplementary Tables 11 and 12), He ranged from 0.39 to 0.57, indicative of medium to high levels of heterozygosity (Table 1). Overall, both H and D were high, demonstrating that at the end of the dry season the multigene haplotypes circulating in the P. falciparum reservoir were genetically diverse (Supplementary Table 10).
Table 1.
Patterns of Genetic Diversity and Standardized Index of Association Testing for Linkage Disequilibrium Between/Among the Alleles/Haplotypes for the Drug Resistance Genes Investigated
| Drug Resistance Gene(s) | Isolates, No. | Unique Haplotypes, No. | H e | (LD) | P Value |
|---|---|---|---|---|---|
| Alleles/haplotypesa | |||||
| pfcrt | 170 | 9 | 0.22 | 0.491 | <.001 |
| pfmdr1 | 198 | 4 | 0.48 | −0.003 | NS |
| pfdhfr | 177 | 8 | 0.49 | 0.341 | <.001 |
| pfdhps | 202 | 22 | 0.71 | 0.038 | .009 |
| Multigene haplotypesb | |||||
| pfcrt-pfmdr1 | 56 | 10 | 0.39 | 0.087 | NS |
| pfdhfr-pfdhps | 62 | 21 | 0.57 | 0.071 | NS |
| pfcrt-pfmdr1-pfdhfr-pfdhps | 44 | 31 | 0.50 | −0.014 | NS |
Abbreviations: He, expected heterozygosity; LD, linkage disequilibrium; NS, not significant (P ≥ .05); , standardized index of association.
aAlleles/haplotypes were constructed using the key polymorphic loci genotyped for each drug resistance gene.
bThe multigene haplotypes were constructed by combining the gene-specific alleles/haplotypes for isolates with single-clone infections (MOI = 1). The multigene haplotypes used for the LD analyses were constructed based on the genes used for determining the response to various antimalarial drugs, specifically pfcrt-pfmdr1 for chloroquine, artesunate-amodiaquine, and artemether-lumefantrine, pfdhfr-pfdhps for sulfadoxine-pyrimethamine, and pfcrt-pfmdr1-pfdhfr-pfdhps for chloroquine, artesunate-amodiaquine, artemether-lumefantrine, and sulfadoxine-pyrimethamine.
Multilocus LD
For the haplotypes, significant multilocus LD was observed for pfcrt, pfdhfr, and pfdhps (P < .01; Table 1), with the pairwise LD indicating linkage between specific codon pairs for each gene as expected (P < .05; Figure 1). These results demonstrate that specific haplotypes are prevalent in this reservoir (Supplementary Table 7) and have likely expanded in the population, since they confer fitness advantages under the current antimalarial drug pressures. For pfcrt-pfmdr1 (n = 56), only 1 isolate (1.8%) was identified with a CQ-resistant multigene haplotype (CVIET-YY). More importantly, 75% of isolates carried CQ-sensitive haplotypes (CVMNK-NY or CVMNK-NF) (Supplementary Table 11). In contrast, for pfdhfr-pfdhps (n = 62), 59.7% of isolates were found to have the quadruple haplotype (IRN-G) associated with intermediate resistance to SP (RII-SP) on 5 unique multigene haplotypes (Supplementary Table 11). For pfcrt-pfmdr1-pfdhfr-pfdhps (n = 44) only 3 isolates (6.9%), each with a unique multigene haplotype, were identified with MDR for CQ and RII-SP. For the multigene haplotypes examined, no evidence of significant multilocus LD was observed, despite the detection of P. falciparum isolates with MDR haplotypes (Table 1).
Evidence of Selection at pfcrt and pfmdr1
For pfcrt there were a limited number of isolates with the mutant CVIET haplotype (n = 2), thus comparisons with the wild-type CVMNK haplotype (n = 48) were not possible. To undertake the analyses, isolates with the pfcrt mutant 76T allele (n = 6) were compared with those isolates with the wild-type K76 allele (n = 49), as described by Laufer et al [27]. The mean He values at the 4 microsatellites flanking the pfcrt 76T mutant allele (He = 0.17) were significantly lower (<50%; P < .03) than for the pfcrt K76 wild-type allele (He = 0.76), supporting positive directional selection (Figure 3). The pfcrt selection patterns were also further supported by the locus-by-locus GST analyses, with the mean GST for the microsatellites flanking pfcrt being significantly greater compared with the neutral loci (Supplementary Figure 3; P = .002), confirming selection. For pfmdr1, although there was a reduction in He around the double-mutant YF (He = 0.71; n = 3) compared with both the single-mutant NF (He = 0.84; n = 35), and the wild-type NY (He = 0.92l n = 8) haplotypes (Figure 3), suggesting positive directional selection, there were no significant differences in mean He between YF and NF (P = .70) or NY (P = .70). When the selection patterns were investigated, the mean GST at the microsatellites flanking pfmdr1 did not differ significantly from the neutral microsatellites (Supplementary Figure 3; P = .65), indicating no evidence of strong selection.
Figure 3.
Patterns of expected heterozygosity (He) in the microsatellite loci flanking (selected) pfcrt (A) and pfmdr1 (B) alleles/haplotypes. On the x-axis, microsatellite locus distance (kb) upstream and downstream of the drug resistance genes are indicated with negative and positive values, respectively. Dashed line crossing the y-axis represents the mean He of the 12 neutral microsatellite loci (unselected); error bars indicated standard deviation. (For additional details on the number of alleles and the He for each locus, see Supplementary Table 13.)
Genetic Lineages of pfcrt and pfmdr1
For pfcrt, 46 of the 49 isolates (93.9%) with the wild-type K76 allele had unique multilocus microsatellite haplotypes, whereas the 6 isolates with the mutant 76T allele belonged to 3 closely related haplotypes (Supplementary Figure 4). To explore these relationships, genetic relatedness networks based on PAS were constructed (Figure 4). Isolates with the mutant 76T allele clustered in the network and were found to be nearly identical to those previously identified in Ghana (Supplementary Figure 5) [17]. For pfmdr1, all wild-type NY (n = 8) and single-mutant NF (n = 35) haplotypes had unique multilocus microsatellite haplotypes (Supplementary Figure 4). Thus, the 184F mutation has emerged independently on multiple genetic backgrounds (soft selective sweeps). For the double-mutant YF (n = 3), all isolates had unique multilocus microsatellite haplotypes (Supplementary Figure 4); however, given the limited sample size and lack of historical data, it is difficult to make any inferences about their genetic lineages due to previous CQ drug pressure. As expected, this high pfmdr1 microsatellite haplotype diversity resulted in limited genetic relatedness between isolates, since most isolates (>70%), regardless of haplotype, were not connected at the PAS ≥ 0.70 threshold (Figure 4).
Figure 4.
The genetic relatedness networks constructed for pfcrt (A) and pfmdr1 (B). The multilocus microsatellite haplotypes (−4.9, −4.6, 1.6, and 4.2-kb loci flanking pfcrt and −1.6, −1.4, −1.2, −0.9, −0.2, 0.3, and 0.6 kb loci flanking pfmdr1) were constructed for each gene to generate the networks. Each node represents an isolate, with the color of each node signifying the pfcrt 76 allele (K/T) or pfmdr1 haplotype (NY/NF/YF). The edges in the networks denote the pairwise relatedness between isolates at the selected pairwise allele sharing (PAS) ≥0.70 threshold (ie, identical at ≥3 loci for pfcrt and ≥5 loci for pfmdr1). This threshold was selected to visualize the genetic similarity between isolates that likely share a recent transmission history. For further details on the pfcrt networks with the pfcrt haplotypes defined and comparisons to the Ghanaian isolates collected 3-years after the introduction of artemisinin-based combination therapies, see Supplementary Figure 5. The PAS networks were constructed using the ggraph and tidygraph R software packages.
DISCUSSION
By surveying this reservoir, we established that >90% of P. falciparum isolates carried the pfcrt wild-type or CQ-sensitive allele (K76) that has rebounded via soft selective sweeps on multiple genetic backgrounds (local and/or imported) following the replacement of CQ with ACTs in 2005. This recovery of the CQ-sensitive allele in Bongo is a result of both the removal of CQ, such that parasites with the pfcrt 76T mutation no longer carry a survival advantage, and the recent introduction of AL, which specifically selects for the K76 allele [19, 28–31]. By constructing genetic relatedness networks, we observed that isolates with the CQ-resistant allele (76T) had nearly identical multilocus microsatellite haplotypes and were closely related to CQ-resistant genetic lineages collected previously in Ghana (2007–2008) that expanded via a hard selective sweep [17]. It is important to note that while the prevalence of the 76T allele has rapidly declined in northern Ghana since 2005 it has not been eliminated from this reservoir, likely owing to continued usage of ASAQ, which has similar selection effects as CQ on pfcrt [32, 33].
For pfmdr1, which modulates drug resistance in conjunction with pfcrt [34–36], we showed that pfmdr1 haplotypes (ie, NY and NF) have spread via soft selective sweeps that have been maintained in the population through balancing selection. Previous studies have shown that although pfmdr1 N86 is required for reduced susceptibility to AL, it is not sufficient, and that other pfmdr1 mutations, including 184F, play a role in the gradual acquisition of AL tolerance [37–39]. Our results support the hypothesis that independent lineages of pfmdr1 184F allele emerged in Ghana before and/or after the removal of CQ and introduction of ACTs. Using these data, we also observed that there was limited genetic relatedness among the isolates with no evidence of strong selection, contrasting with what Alam et al described in 2007–2008 [17].
The most logical explanation for this shift is the introduction of AL starting in 2008 to treat clinical infections as a substitute for ASAQ, due to adverse drug reactions [2]. Since, AL and ASAQ have different partner drugs (ie, lumefantrine and amodiaquine) that exert opposing selective pressures on pfcrt and pfmdr1 simultaneously, we examined the multigene haplotypes to address this interplay. In this reservoir, 75% of isolates carried either the CVMNK-NY or CVMNK-NF haplotype, which have both been shown to provide a fitness advantage to the parasite under AL pressure [28, 37, 40, 41]. The prevalence of these haplotypes suggests that the use of AL is common as an alternative treatment for ASAQ [2, 42]. Despite the identification of these pfcrt-pfmdr1 multigene haplotypes, the alleles were in linkage equilibrium on diverse genetic backgrounds.
Although Ghana changed its policy to restrict the use of SP for intermittent preventive treatment in pregnancy exclusively in 2005, the mutations mediating resistance have not declined; 91.0% and 96.5% of isolates in this study carried mutations in pfdhfr and pfdhps, respectively [17, 18, 43]. By constructing the pfdhfr-pfdhps multigene haplotypes, 59.7% of isolates were identified as carrying the quadruple haplotype (IRN-G) associated with RII-SP, although the alleles at these loci were in linkage equilibrium. Over the next several years, it is anticipated that SP usage will increase in Bongo through the deployment of seasonal malaria chemoprevention to all children <5 years old, regardless of infection status. With this escalation in drug exposure, ongoing surveillance for the prevalence of resistance mutations will be necessary to predict if and when SP may no longer be efficacious for intermittent preventive treatment in pregnancy and/or seasonal malaria chemoprevention.
Recent cross-sectional data from uncomplicated cases across Ghana have shown that after a decade of ACT use there has been a steady increase in pfk13 mutations [44]. In the current study, 32 nonsynonymous mutations were identified in the asymptomatic reservoir in Bongo. Although none of the validated mutations associated with ART resistance were detected, monitoring for these polymorphisms is important, so that if resistance does appear, we will be better prepared to validate these markers conferring resistance. Consistent with these data, recent therapeutic studies in Ghana have shown that ACTs are still >90% effective for the treatment of uncomplicated malaria [45, 46]. Through phylogenetic analyses, we established that the pfk13 mutations identified in Bongo were distinct from those in SEA and more similar to those seen elsewhere in Africa [8, 47, 48]. The low frequency of these pfk13 mutations in Ghana reflects standing genetic variation, suggesting that pfk13 is not currently undergoing strong directional selection by ART.
The conventional approach for monitoring drug resistance is by looking at the prevalence of drug resistance markers individually. Although informative, this does not address whether MDR haplotypes are circulating or expanding in this region. By constructing the pfcrt-pfmdr1-pfdhfr-pfdhps multigene haplotypes, the current study was able to identify 3 isolates (6.9%) with predicted clinical resistance to both CQ and RII-SP. The detection of these MDR haplotypes, although useful for monitoring purposes, are not an immediate cause for concern, since we observed random associations between/among the multigene haplotypes investigated. Unlike SEA, in high-transmission settings such as Bongo, epidemiological barriers exist that impede the rapid spread of MDR, including host immunity, high MOI, high genetic diversity, and/or increased rates of outcrossing [21, 49, 50].
The current study aimed to gain population genetic insights into the prevalence and mechanisms of selection of drug resistance–mediating polymorphisms in the P. falciparum reservoir across all ages in Bongo, Ghana. Of note, we found the prevalence and selection of these alleles to be similar to that found among clinical cases prior to treatment in Ghana [17–20], suggesting that they do not represent 2 subpopulations and have been sampled from the same underlying population. This implies that exposure of the reservoir in all ages to antimalarials may be more widespread than just the treatment of febrile episodes in children. Our data showed that this reservoir is composed of diverse haplotypes that have been selected by the use of several antimalarials (ie, ASAQ, AL, and SP) with different modes of action. Thus, these alleles/haplotypes are being maintained because they offer a survival advantage to parasites as drug pressures change and/or frequent outcrossing during sexual recombination facilitates spread in the reservoir upon exposure to drugs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the participants, the communities, and the Ghana Health Service in Bongo District, Ghana for their willingness to participate in this study. We also thank the field teams for their technical assistance in the field and the laboratory personnel at the Navrongo Health Research Centre for undertaking the sample collection and parasitological assessment/expertise. We are grateful to Charles Robin and Alyssa Barry for their useful comments on the initial drafts of the manuscript and Mercedes Pascual for valuable input related to this work.
Author contributions. Conceived and designed the study: K. A. K. and K. P. D. Contributed to study design: A. G., A. R. O., and K. E. T. Coordinated fieldwork: A. R. O. Performed the laboratory experiments: C. A. N. and C. O. O. Analyzed the data: C. A. N., A. G., M. F. D., S. R-P., K. P. D, and K. E. T. Wrote the manuscript: C. A. N. and K. E. T. Critically revised the manuscript: A. G., M. F. D., S. R-P., A. R. O., K. A. K., and K. P. D. Funding acquisition: K. A. K. and K. P. D.
Financial support. This work was supported by the Fogarty International Center at the National Institutes of Health, Ecology and Evolution of Infectious Diseases (R01-TW009670 awarded to K. A. K. and K. P. D., salary support to K. E. T.); Australian Government Research Training Program Scholarship (to C. A. N.); The University of Melbourne International Engagement Award (to S. R-P.); The University of Melbourne salary support (to M. F. D. and K. E. T.). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Potential conflict of interest statement. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part. Seventh Multilateral Initiative on Malaria Pan African Malaria Conference, Dakar, Senegal, April 2018; American Society for Tropical Medicine and Hygiene Annual Conference, National Harbor, MD, November 2019.
References
- 1. World Health Organization. World malaria report 2019. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 2. Ministry of Health. Guidelines for case management of malaria in Ghana. 3rd ed. Ghana: Ministry of Health, 2014. [Google Scholar]
- 3. Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium . Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 2008; 359:2619–20. [DOI] [PubMed] [Google Scholar]
- 4. Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009; 361:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014; 505:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ashley EA, Dhorda M, Fairhurst RM, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2014; 371:411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phyo AP, Nkhoma S, Stepniewska K, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 2012; 379:1960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MalariaGEN. Genomic epidemiology of artemisinin resistant malaria. Elife 2016; 5:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ménard D, Khim N, Beghain J, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 2016; 374:2453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamau E, Campino S, Amenga-Etego L, et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis 2014; 211:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson TJ, Haubold B, Williams JT, et al. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol 2000; 17:1467–82. [DOI] [PubMed] [Google Scholar]
- 12. Rosenthal PJ. The interplay between drug resistance and fitness in malaria parasites. Mol Microbiol 2013; 89:1025–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mackinnon MJ, Hastings IM. The evolution of multiple drug resistance in malaria parasites. Trans R Soc Trop Med Hyg 1998; 92:188–95. [DOI] [PubMed] [Google Scholar]
- 14. Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis 2002; 2:209–18. [DOI] [PubMed] [Google Scholar]
- 15. Amato R, Pearson RD, Almagro-Garcia J, et al. Origins of the current outbreak of multidrug-resistant malaria in Southeast Asia: a retrospective genetic study. Lancet Infect Dis 2018; 18:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miotto O, Almagro-Garcia J, Manske M, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet 2013; 45:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alam MT, de Souza DK, Vinayak S, et al. Selective sweeps and genetic lineages of Plasmodium falciparum drug -resistant alleles in Ghana. J Infect Dis 2011; 203:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duah NO, Quashie NB, Abuaku BK, Sebeny PJ, Kronmann KC, Koram KA. Surveillance of molecular markers of Plasmodium falciparum resistance to sulphadoxine-pyrimethamine 5 years after the change of malaria treatment policy in Ghana. Am J Trop Med Hyg 2012; 87:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duah NO, Matrevi SA, de Souza DK, et al. Increased pfmdr1 gene copy number and the decline in pfcrt and pfmdr1 resistance alleles in Ghanaian Plasmodium falciparum isolates after the change of anti-malarial drug treatment policy. Malar J 2013; 12:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mensah BA, Aydemir O, Myers-Hansen JL, Opoku M. Antimalarial drug resistance profiling of Plasmodium falciparum infections in Ghana using molecular inversion probes and next generation sequencing. Antimicrob Agents Chemother 2020; 64:e01423–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruybal-Pesántez S, Tiedje KE, Rorick MM, et al. Lack of geospatial population structure yet significant linkage disequilibrium in the reservoir of Plasmodium falciparum in Bongo District, Ghana. Am J Trop Med Hyg 2017; 97:1180–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tiedje KE, Oduro AR, Agongo G, et al. Seasonal variation in the epidemiology of asymptomatic Plasmodium falciparum infections across two catchment areas in Bongo District, Ghana. Am J Trop Med Hyg 2017; 97:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018. http://www.r-project.org/. Accessed 4 May 2020. [Google Scholar]
- 24. Agapow PM, Burt A. Indices of multilocus linkage disequilibrium. Mol Ecol Notes 2001; 1:101–102. [Google Scholar]
- 25. Sutherland CJ, Alloueche A, Curtis J, et al. Gambian children successfully treated with chloroquine can harbor and transmit Plasmodium falciparum gametocytes carrying resistance genes. Am J Trop Med Hyg 2002; 67:578–85. [DOI] [PubMed] [Google Scholar]
- 26. Tumwebaze P, Tukwasibwe S, Taylor A, et al. Changing antimalarial drug resistance patterns identified by surveillance at three sites in Uganda. J Infect Dis 2017; 215:631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laufer MK, Takala-Harrison S, Dzinjalamala FK, Stine OC, Taylor TE, Plowe CV. Return of chloroquine-susceptible falciparum malaria in Malawi was a reexpansion of diverse susceptible parasites. J Infect Dis 2010; 202:801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Venkatesan M, Gadalla NB, Stepniewska K, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am J Trop Med Hyg 2014; 91:833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sisowath C, Petersen I, Veiga MI, et al. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis 2009; 199:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laufer MK, Thesing PC, Eddington ND, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med 2006; 355:1959–66. [DOI] [PubMed] [Google Scholar]
- 31. Ord R, Alexander N, Dunyo S, et al. Seasonal carriage of pfcrt and pfmdr1 alleles in Gambian Plasmodium falciparum imply reduced fitness of chloroquine-resistant parasites. J Infect Dis 2007; 196:1613–1619. [DOI] [PubMed] [Google Scholar]
- 32. Ecker A, Lehane AM, Clain J, Fidock DA. PfCRT and its role in antimalarial drug resistance. Trends Parasitol 2012; 28:504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tinto H, Guekoun L, Zongo I, Guiguemdé RT, D’Alessandro U, Ouédraogo JB. Chloroquine-resistance molecular markers (Pfcrt T76 and Pfmdr-1 Y86) and amodiaquine resistance in Burkina Faso. Trop Med Int Health 2008; 13:238–40. [DOI] [PubMed] [Google Scholar]
- 34. Fidock DA, Nomura T, Talley AK, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell 2000; 6:861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babiker HA, Pringle SJ, Abdel‐Muhsin A, Mackinnon M, Hunt P, Walliker D. High‐level chloroquine resistance in Sudanese isolates of Plasmodium falciparum is associated with mutations in the chloroquine resistance transporter gene pfcrt and the multidrug resistance gene pfmdr1. J Infect Dis 2001; 183:1535–1538. [DOI] [PubMed] [Google Scholar]
- 36. Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 2000; 403:906–9. [DOI] [PubMed] [Google Scholar]
- 37. Humphreys GS, Merinopoulos I, Ahmed J, et al. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob Agents Chemother 2007; 51:991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sisowath C, Ferreira PE, Bustamante LY, et al. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Trop Med Int Health 2007; 12:736–42. [DOI] [PubMed] [Google Scholar]
- 39. Malmberg M, Ferreira PE, Tarning J, et al. Plasmodium falciparum drug resistance phenotype as assessed by patient antimalarial drug levels and its association with pfmdr1 polymorphisms. J Infect Dis 2013; 207:842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Somé AF, Séré YY, Dokomajilar C, et al. Selection of known Plasmodium falciparum resistance-mediating polymorphisms by artemether-lumefantrine and amodiaquine-sulfadoxine-pyrimethamine but not dihydroartemisinin-piperaquine in Burkina Faso. Antimicrob Agents Chemother 2010; 54:1949–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Veiga MI, Dhingra SK, Henrich PP, et al. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat Commun 2016; 7:11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abuaku BK, Duah N, Quaye L, et al. Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine combinations in the treatment of uncomplicated malaria in two ecological zones in Ghana. Malar J. BioMed Central 2016; 15:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mockenhaupt FP, Bedu‐Addo G, Eggelte TA, et al. Rapid increase in the prevalence of sulfadoxine‐pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis 2008; 198:1545–9. [DOI] [PubMed] [Google Scholar]
- 44. Matrevi SA, Opoku-Agyeman P, Quashie NB, et al. Plasmodium falciparum kelch propeller polymorphisms in clinical isolates from Ghana: 2007–2016. Antimicrob Agents Chemother 2019; 63:e00802–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abuaku BK, Mensah BA, Ofori MF, et al. Efficacy of artesunate/amodiaquine in the treatment of uncomplicated Malaria among children in Ghana. Am J Trop Med Hyg 2017; 97:690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abuaku B, Duah-Quashie NO, Quaye L, et al. Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine combinations for uncomplicated malaria in 10 sentinel sites across Ghana: 2015–2017. Malar J 2019; 18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Z, Wang Y, Cabrera M, et al. Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrob Agents Chemother 2015; 59:6952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mita T, Culleton R, Takahashi N, et al. Little polymorphism at the K13 propeller locus in worldwide Plasmodium falciparum populations prior to the introduction of artemisinin combination therapies. Antimicrob Agents Chemother 2016; 60:3340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duah NO, Matrevi SA, Quashie NB, Abuaku B, Koram KA. Genetic diversity of Plasmodium falciparum isolates from uncomplicated malaria cases in Ghana over a decade. Parasit Vectors 2016; 9:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ataide R, Ashley EA, Powell R, et al. Host immunity to Plasmodium falciparum and the assessment of emerging artemisinin resistance in a multinational cohort. Proc Natl Acad Sci 2017; 114:3515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.