Abstract
Background and Aim
Circulating levels of interleukin (IL)-6, a well-known inflammatory cytokine, are often elevated in coronavirus disease-2019 (COVID-19). Elevated IL-6 levels are also observed in patients with metabolic dysfunction-associated fatty liver disease (MAFLD). Our study aimed to describe the association between circulating IL-6 levels and MAFLD at hospital admission with risk of severe COVID-19.
Methods
A total of 167 patients with laboratory-confirmed COVID-19 from three Chinese hospitals were enrolled. Circulating levels of IL-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ were measured at admission. All patients were screened for fatty liver by computed tomography. Forty-six patients were diagnosed as MAFLD.
Results
Patients with MAFLD (n = 46) had higher serum IL-6 levels (median 7.1 [interquartile range, 4.3–20.0] vs. 4.8 [2.6–11.6] pg/mL, p = 0.030) compared to their counterparts without MAFLD (n = 121). After adjustment for age and sex, patients with MAFLD had a ~2.6-fold higher risk of having severe COVID-19 than those without MAFLD. After adjustment for age, sex and metabolic co-morbidities, increased serum IL-6 levels remained associated with higher risk of severe COVID-19, especially among infected patients with MAFLD (adjusted-odds ratio 1.14, 95% CI 1.05–1.23; p = 0.002). There was a significant interaction effect between serum IL-6 levels and MAFLD for risk of severe COVID-19 (p for interaction = 0.008).
Conclusions
Patients with MAFLD and elevated serum IL-6 levels at admission are at higher risk for severe illness from COVID-19.
Keywords: COVID-19, SARS-CoV-2, MAFLD, cytokine, IL-6, NAFLD
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to attract worldwide attention. The occurrence of a virus-induced cytokine ‘storm’ is associated with greater disease severity and unfavorable in-hospital outcomes (1, 2). Elevated levels of serum interleukin-6 (IL-6) are a hallmark inflammatory signature commonly seen in patients with severe COVID-19 and the use of IL-6-receptor blocking antibodies has recently been approved in China for treatment of COVID-19 patients with serious pulmonary damage and elevated serum IL-6 levels (3).
Metabolic dysfunction-associated fatty liver disease (MAFLD), the newly proposed name for non-alcoholic fatty liver disease (NAFLD) (4), has reached epidemic proportions, affecting up to a quarter of the world’s adult population (5). MAFLD is known to be associated with various hepatic and extra-hepatic complications, such as cirrhosis, liver cancer, cardiovascular disease, type 2 diabetes and chronic kidney disease (6). We recently showed that younger patients with MAFLD have higher odds of severe COVID-19 (7).
Patients with MAFLD were characterized by impaired hepatic immunity (8, 9). Hepatic macrophages are associated with fatty liver disease through their effects on chronic inflammation, including cytokine and adipokine secretion (9). Previous studies reported that the status of inflammation associated with MAFLD might contribute to the cytokine ‘storm’, which further exacerbates the infection in patients with COVID-19 (10–12). The angiotensin-converting enzyme (ACE) 2 is the receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). An exploratory analysis found that ACE 2 mRNA expression in visceral fat (VF) positively correlated with BMI, and the engagement of SARS-CoV-2 on ACE 2 in the VF would impair the enzymatic activity of ACE 2 and enhance the production of inflammatory cytokines and their release into the systemic circulation (13). Therefore, we postulated the presence of MAFLD might exacerbate the virus-induced cytokine ‘storm’ associated with COVID-19, possibly through the hepatic release of multiple pro-inflammatory cytokines.
In this study, we investigated the association between peripheral blood IL-6 levels and MAFLD at admission and risk of having more severe illness in hospitalized patients with COVID-19.
Materials and Methods
Patients and Study Design
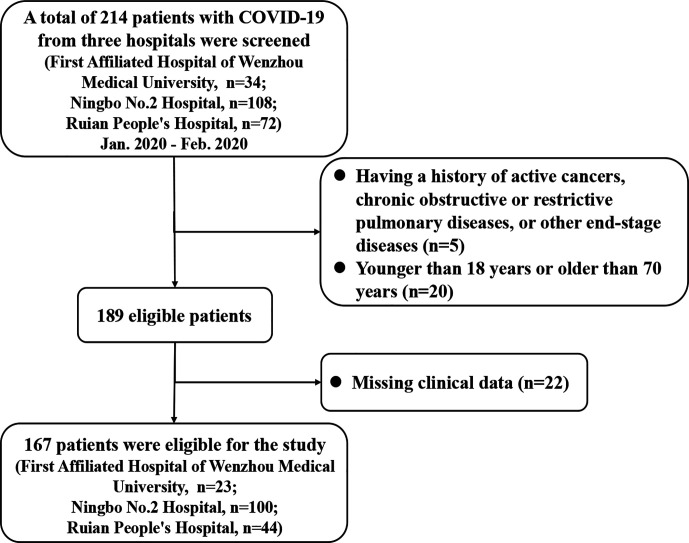
We enrolled 214 patients with laboratory-confirmed COVID-19 from three Chinese hospitals (the First Affiliated Hospital of Wenzhou Medical University, the Ningbo No.2 Hospital, and the Ruian People’s Hospital) between 17th January and 11th February 2020. As detailed in Figure 1, 47 subjects were excluded for the following reasons (1): younger than 18 years or older than 70 years; (2) having a history of active cancers, chronic obstructive or restrictive pulmonary diseases, or other end-stage diseases; (3) incomplete clinical/biochemical data. As a result of these exclusions, a sample of 167 patients was included in the final analysis. Some of these patients have been reported in a previous study (14). All patients had received standard treatments according to the Chinese COVID-19 management guidance.
Figure 1.
The flow chart for the study.
The local ethics committees of the hospitals approved the study protocol (2020-002, 5 February 2020). All procedures performed involving the participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration. The requirement for written informed consent was waived due to the retrospective and anonymous nature of the study design.
Laboratory and Clinical Data
Demographic and laboratory data, including age, sex, serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl-transpeptidase (GGT) and various cytokines were collected on the first day of hospital admission. In particular, circulating levels of IL-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ were measured using a flow cytometer (FACSCanto™ plus, America) and cytometric bead array techniques. The neutrophil-to-lymphocyte ratio (NLR), i.e., an established marker of systemic inflammation, was also calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes. FIB-4 score was calculated from the published formula (15).
Body mass index (BMI) was calculated using the formula weight (kilograms) divided by height (meters) squared. Overweight and obesity were defined, respectively, as BMI between 23 and 24.9 kg/m2 and BMI ≥ 25 kg/m2 in this Asian population. Type 2 diabetes mellitus (T2DM) was diagnosed as either self-reported history of disease, fasting glucose levels ≥7.0 mmol/L, hemoglobin A1c ≥6.5% (≥48 mmol/mol) or use of any anti-hyperglycemic drugs. Hypertension and dyslipidemia were diagnosed according to the consensus criteria (16).
In the whole cohort of patients, COVID-19 was diagnosed as a positive result by high-throughput sequencing or real-time reverse transcriptase-polymerase chain reaction assay of oropharyngeal swab specimens. Severity of COVID-19 was assessed during hospitalization and classified as two subtypes (severe and non-severe) based on the Chinese guidelines for management of COVID-19 (see Supplementary Table 1) (17).
Liver Imaging
All patients were screened for fatty liver by computed tomography (CT). Liver and spleen measurements were performed using a CT post-processing workstation, and only one image (level of portal vein into the liver) of each patient was selected to complete attenuation measurements of liver and spleen. Three regions of interest in the liver were selected to avoid blood vessels, bile ducts and calcification. Two regions of interest in the spleen were selected at the same level. The respective means of the three attenuation values of the liver and two attenuation values of the spleen were calculated. Fatty liver was diagnosed by widely accepted CT characteristics, namely attenuation value of liver parenchyma (CTLP) <48 HU, or attenuation ratio of liver and spleen (LS ratio) <1.0, or attenuation difference of liver and spleen (LS diff.) <5 HU, respectively.
MAFLD Definition
MAFLD was diagnosed as the presence of hepatic steatosis on CT scan and one of the following criteria: 1) overweight or obesity as defined by a BMI ≥23.0 kg/m2; 2) established T2DM; or 3) presence of at least two metabolic risk abnormalities (18). These metabolic risk abnormalities were defined as follows: 1) waist circumference ≥90/80 cm in men and women; 2) blood pressure ≥130/85 mmHg or drug treatment; 3) plasma triglycerides ≥1.70 mmol/L or drug treatment; 4) plasma HDL-cholesterol <1.03 mmol/L for men and <1.29 mmol/L for women or drug treatment; and 5) prediabetes (i.e., fasting glucose levels 5.6 to 6.9 mmol/L, or HbA1c 5.7% to 6.4%) (18).
Statistical Analysis
Continuous variables are expressed as means ± SD or medians (inter-quartile ranges [IQR]) and compared using either the unpaired Student’s t-test or the Mann-Whitney test as appropriate. Categorical variables are expressed as numbers (percentages) and compared using the chi-squared test or the Fisher’s exact test as appropriate. The odds ratios (OR) and 95% confidence intervals (CIs) for the risk of having severe COVID-19 (as the outcome) associated with serum IL-6 levels and MAFLD at hospital admission were estimated using binary logistic regression analyses with adjustment for age, sex and metabolic co-morbidities (overweight/obesity, T2DM and hypertension). Interaction tests were used to evaluate associations between exposure variables and the outcome. Statistical analyses were two-sided and significance was set at p < 0.05. All statistical tests were performed using SPSS version 23.0 (SPSS Inc., Chicago, USA).
Results
A cohort of 167 (42.5% men; mean age 49 years) hospitalized patients with laboratory-confirmed COVID-19 was included in the study. The median time from symptom onset to hospital admission was 9.0 (IQR 4.0–13.0) days. Forty-six (27.5%) of these patients had imaging-defined MAFLD and 32 (19.2%) patients were classified as having severe COVID-19. Among MAFLD patients, patients with severe COVID-19 had significantly higher FIB-4 score than those with non-severe COVID-19 (1.64 IQR [1.23–2.57] vs. 1.03 IQR [0.71–1.61], p = 0.047). The data about the cytokine levels is presented in the Supplementary Table 2.
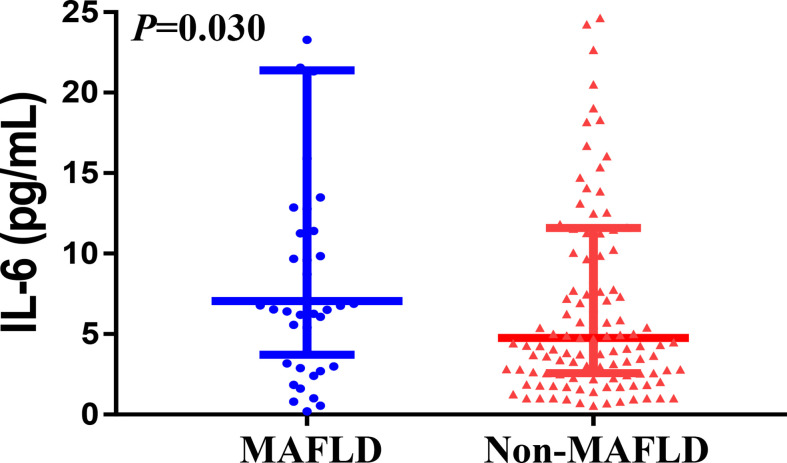
As shown in Table 1, patients with imaging-defined MAFLD had significantly higher levels at admission of serum IL-6 (median 7.1 [IQR, 4.3–20.0] vs. 4.8 [2.6–11.6] pg/mL, p = 0.030; Figure 2), liver enzymes and a higher proportion of overweight/obesity and dyslipidemia compared to those without MAFLD. After adjustment for age and sex, patients with MAFLD had an approximate 2.6-fold higher risk of severe COVID-19 than those without MAFLD (odds ratio 2.61, 95% CI 1.10–6.23, p = 0.030)).
Table 1.
Baseline characteristics of COVID-19 patients, stratified by MAFLD status at hospital admission.
| Without MAFLD (n = 121) | With MAFLD (n = 46) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 49.9 ± 13.2 | 47.7 ± 13.9 | 0.343 |
| Male sex, n (%) | 52 (43.0%) | 19 (41.3%) | 0.845 |
| Coexisting disorders | |||
| Type 2 diabetes, n (%) | 15 (12.4%) | 10 (21.7%) | 0.131 |
| Hypertension, n (%) | 22 (18.2%) | 13 (28.3%) | 0.153 |
| BMI ≥23 kg/m2, n (%) | 75 (62.5%) | 40 (87.0%) | 0.002 |
| Dyslipidemia, n (%) | 85 (70.2%) | 43 (93.5%) | 0.002 |
| Laboratory parameters | |||
| WBC, x109/L | 4.8 (3.9–6.5) | 5.0 (4.3–6.7) | 0.458 |
| Lymphocyte count, x109/L | 1.1 (0.8–1.4) | 1.2 (0.9–1.6) | 0.208 |
| NLR | 2.8 (1.8–4.9) | 2.5 (1.7–3.6) | 0.282 |
| C-reactive protein, mg/L | 11.5 (2.3–29.8) | 17.2 (4.2–41.0) | 0.122 |
| Procalcitonin, ng/mL | 0.03 (0.01–0.05) | 0.03 (0.01–0.06) | 0.474 |
| D-dimer, mg/L | 0.28 (0.14–0.66) | 0.36 (0.20–0.59) | 0.702 |
| ALT, U/L | 21.0 (15.0–31.0) | 26.0 (20.0–39.8) | 0.024 |
| AST, U/L | 22.0 (17.0–29.0) | 27.0 (20.2–34.8) | 0.028 |
| GGT, U/L | 24.0 (16.0–39.0) | 31.0 (21.2–50.0) | 0.037 |
| TBIL, μmol/L | 10.0 (6.6–14.0) | 10.2 (7.5–14.0) | 0.395 |
| Cytokines | |||
| IL-2, pg/mL | 0.9 (0.6–1.4) | 0.8 (0.5–1.4) | 0.670 |
| IL-4, pg/mL | 1.3 (1.0–2.1) | 1.0 (1.0–2.0) | 0.518 |
| IL-6, pg/mL | 4.8 (2.6–11.6) | 7.1 (4.3–20.0) | 0.030 |
| IL-10, pg/mL | 2.6 (1.0–4.5) | 3.6 (1.0–5.5) | 0.251 |
| TNF-α, pg/mL | 1.1 (0.9–1.5) | 1.0 (0.9–1.5) | 0.306 |
| IFN-γ, pg/mL | 1.0 (1.0–1.6) | 1.0 (0.9–1.9) | 0.790 |
| Time from symptom onset to hospital admission, days | 9.0 (4.5–13.0) | 7.0 (4.0–11.3) | 0.267 |
| Severe COVID-19, n (%) | 19 (15.7%) | 13 (28.3%) | 0.065 |
Data are expressed as means ± SD, medians (IQRs) and number (percentages).
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; GGT, gamma glutamyl-transpeptidase; IFN-γ, interferon gamma; IL, interleukin; MAFLD, metabolic dysfunction-associated fatty liver disease; NLR, Neutrophil-to-lymphocyte ratio; TBIL, total bilirubin; TNF-α, tumor necrosis factor-α; WBC, white blood cell.
Bold values represent statistical significant P values.
Figure 2.
The association between IL-6 levels and MAFLD status among COVID-19 patients.
As shown in Table 2, compared to those with non-severe COVID-19, patients with severe COVID-19 had significantly higher circulating levels of neutrophil-to-lymphocyte ratio, C-reactive protein, procalcitonin, D-dimer, IL-6 and IL-10 both in MAFLD and in non-MAFLD patients. Among non-MAFLD patients, those with severe COVID-19 were older, had significantly higher levels of WBC, liver enzymes (ALT, AST, GGT, and TBIL), and also had a higher proportion of T2DM, hypertension, and overweight/obesity than those with non-severe COVID-19. However, the significant differences in the aforementioned parameters were not observed among MAFLD patients, after stratification by severe COVID-19.
Table 2.
Baseline characteristics of COVID-19 patients, stratified by both COVID-19 severity and MAFLD status.
| With MAFLD (n = 46) | Without MAFLD (n = 121) | |||||
|---|---|---|---|---|---|---|
| Non-severe COVID-19 (n = 33) | SevereCOVID-19 (n = 13) | P value | Non-severe COVID-19 (n = 102) | SevereCOVID-19 (n = 19) | P value | |
| Demographics | ||||||
| Age, years | 46.8 ± 14.1 | 50.2 ± 13.8 | 0.453 | 48.3 ± 13.3 | 58.2 ± 8.6 | 0.003 |
| Male sex, n (%) | 12 (36.4%) | 7 (53.8%) | 0.278 | 39 (38.2%) | 13 (68.4%) | 0.015 |
| Coexisting disorders | ||||||
| Type 2 diabetes, n (%) | 9 (27.3%) | 4 (30.8%) | 0.813 | 10 (9.8%) | 10 (52.6%) | <0.001 |
| Hypertension, n (%) | 8 (24.2%) | 5 (38.5%) | 0.335 | 15 (14.7%) | 7 (36.8%) | 0.022 |
| BMI ≥23 kg/m2, n (%) | 29 (87.9%) | 11 (84.6%) | 0.767 | 60 (58.8%) | 15 (83.3%) | 0.048 |
| Dyslipidemia, n (%) | 31 (93.9%) | 12 (92.3%) | 0.840 | 74 (72.5%) | 11 (57.9%) | 0.200 |
| Laboratory parameters | ||||||
| WBC, x109/L | 5.0 (3.9–6.4) | 5.4 (4.8–7.2) | 0.183 | 4.7 (3.7–5.9) | 7.4 (5.4–10.2) | <0.001 |
| Lymphocyte count, x109/L | 1.3 (0.9–1.7) | 1.1 (0.9–1.2) | 0.094 | 1.2 (0.9–1.6) | 0.7 (0.5–0.8) | <0.001 |
| NLR | 2.5 (1.6–3.7) | 10.2 (7.1–16.8) | <0.001 | 2.1 (1.6–3.1) | 3.7 (3.2–4.4) | 0.012 |
| C-reactive protein, mg/L | 9.7 (3.1–24.5) | 47.6 (18.9–74.0) | 0.001 | 9.2 (2.0–24.9) | 32.0 (14.8–67.8) | <0.001 |
| Procalcitonin, ng/mL | 0.01 (0.01–0.04) | 0.06 (0.04–0.08) | 0.013 | 0.01 (0.01–0.04) | 0.05 (0.04–0.07) | <0.001 |
| D-dimer, mg/L | 0.23 (0.12–0.34) | 0.59 (0.47–1.21) | 0.002 | 0.22 (0.13–0.30) | 0.68 (0.55–0.83) | <0.001 |
| ALT, U/L | 30.0 (20.0–49.0) | 22.0 (20.0–24.0) | 0.241 | 20.0 (15.0–27.8) | 26.0 (21.5–41.5) | 0.009 |
| AST, U/L | 27.0 (19.0–35.0) | 28.0 (25.0–34.0) | 0.329 | 22.0 (17.0–28.0) | 32.0 (21.5–40.5) | 0.003 |
| GGT, U/L | 30.0 (20.0–51.0) | 31.0 (24.0–47.0) | 0.413 | 22.0 (15.2–32.8) | 43.0 (27.5–84.5) | <0.001 |
| TBIL, μmol/L | 10.0 (7.1–13.6) | 13.2 (9.3–14.0) | 0.121 | 9.4 (6.4–13.6) | 12.0 (9.2–18.5) | 0.033 |
| Cytokines | ||||||
| IL-2, pg/mL | 0.9 (0.5–1.6) | 0.6 (0.5–1.1) | 0.508 | 0.9 (0.5–1.5) | 0.9 (0.7–1.0) | 0.568 |
| IL-4, pg/mL | 1.0 (1.0–2.0) | 1.0 (0.7–1.7) | 0.267 | 1.4 (1.0–2.1) | 1.2 (0.6–1.7) | 0.058 |
| IL-6, pg/mL | 6.4 (2.9–9.8) | 26.3 (12.9–45.4) | <0.001 | 4.6 (2.3–10.2) | 4.9 (3.3–31.9) | 0.043 |
| IL-10, pg/mL | 2.2 (1.0–4.2) | 6.5 (4.3–12.5) | <0.001 | 2.3 (1.0–4.0) | 4.8 (3.2–7.0) | <0.001 |
| TNF-α, pg/mL | 1.0 (1.0–1.5) | 0.7 (0.2–1.4) | 0.120 | 1.2 (1.0–1.6) | 0.6 (0.2–1.5) | 0.007 |
| IFN-γ, pg/mL | 1.0 (1.0–1.6) | 1.4 (0.6–2.3) | 0.834 | 1.0 (1.0–1.5) | 1.3 (0.8–1.8) | 0.439 |
| Time from symptom onset to hospital admission, days | 8.0 (4.0–12.0) | 7.0 (5.5–9.5) | 0.922 | 9.0 (4.0–13.0) | 8.0 (5.0–10.0) | 0.778 |
Data are expressed as means ± SD, medians (IQR) and number (percentages).
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; GGT, gamma glutamyl-transpeptidase; IFN-γ, interferon gamma; IL, interleukin; MAFLD, metabolic associated fatty liver disease; NLR, Neutrophil-to-lymphocyte ratio; TBIL, total bilirubin; TNF-α, tumor necrosis factor-α; WBC, white blood cell.
Bold values represent statistical significant P values.
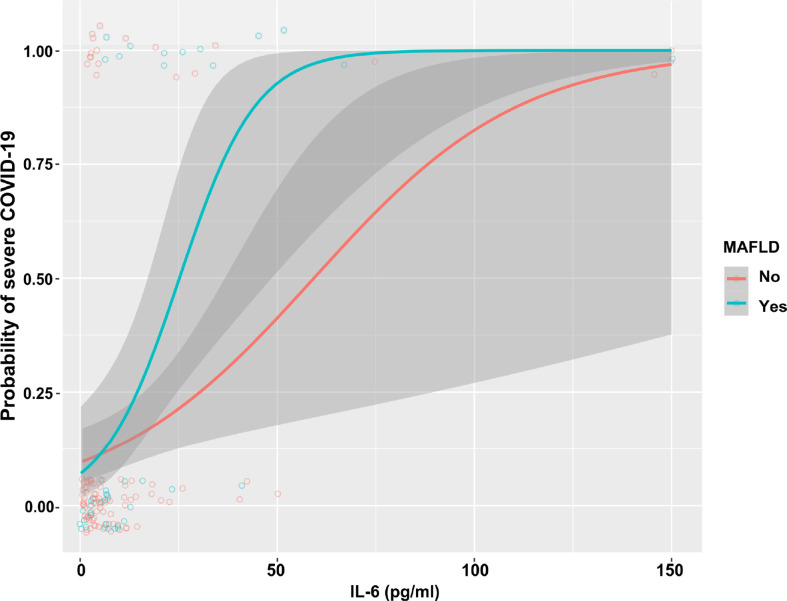
As shown in Table 3, in the whole cohort of COVID-19 patients, there was a significant association between higher serum IL-6 levels and risk of having severe COVID-19 (unadjusted model: OR 1.06 [95% CI 1.03–1.09], p<0.001). Notably, this association remained significant even after adjustment for age, sex, overweight/obesity, T2DM, and hypertension (adjusted model 2: OR 1.04 [95% CI 1.02–1.09], p = 0.002). We also performed subgroup analyses and interaction tests to evaluate the existence of significant differences in serum IL-6 levels between patients with and without MAFLD at hospital admission, and to investigate interactions between exposure variables and the outcome. In the unadjusted model, elevated serum IL-6 levels were significantly associated with a higher risk of severe COVID-19 in both groups of patients. However, after adjustment for age and sex (adjusted model 1) and other metabolic co-morbidities (adjusted model 2), the significant association between elevated serum IL-6 levels and higher risk of severe COVID-19 persisted only in patients with MAFLD (adjusted model 2: OR 1.14 [95% CI 1.05–1.23], p = 0.002). The interaction test between IL-6 and MAFLD status on risk of severe COVID-19 was found to be statistically significant (p-value for interaction = 0.008). As shown in Figure 3, there was a clear dose-effect relationship between increasing values of IL-6 and the proportion of patients with severe COVID-19 in both MAFLD and non-MAFLD patients. Notably, elevated IL-6 levels had higher odds of severe COVID-19 among MAFLD patients than non-MAFLD patients.
Table 3.
Association between serum IL-6 levels (as exposure) and severity of COVID-19 (as the outcome) in infected patients with and without MAFLD at hospital admission.
| Total (n = 167) | Without MAFLD (n = 121) | With MAFLD (n = 46) | P for interaction | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Unadjusted Model | 1.06 (1.03–1.09) | <0.001 | 1.04 (1.00–1.07) | 0.028 | 1.11 (1.04–1.19) | 0.003 | 0.055 |
| Adjusted Model 1 | 1.05 (1.02–1.09) | <0.001 | 1.03 (0.99–1.06) | 0.086 | 1.12 (1.04–1.21) | 0.004 | 0.022 |
| Adjusted Model 2 | 1.04 (1.02–1.09) | 0.002 | 1.02 (0.99–1.05) | 0.091 | 1.14 (1.05–1.23) | 0.002 | 0.008 |
Data are expressed as odds ratio (OR) and 95% confidence intervals as tested by univariable (unadjusted) and multivariable (adjusted) logistic regression analysis.
Adjusted Model 1: adjusted for age and sex
Adjusted Model 2: adjusted for age, sex, overweight/obesity, type 2 diabetes, and hypertension.
Bold values represent statistical significant P values.
Figure 3.
The association between increasing IL-6 levels and COVID-19 severity, stratified by MAFLD status.
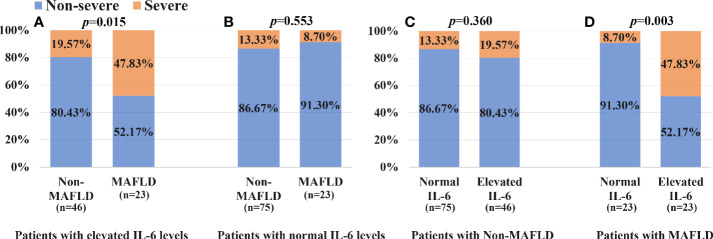
As shown in Figure 4A, patients with MAFLD and elevated IL-6 levels had a significantly higher proportion of severe COVID-19 than those with elevated IL-6 levels but without MAFLD (47.83% vs. 19.57%, p = 0.015). Among patients with normal IL-6 levels (Figure 4B), there was no significant difference in the percentage of severe COVID-19 between MAFLD and non-MAFLD patients (8.70% vs. 13.33%, p = 0.553). As shown in Figure 4C, among non-MAFLD patients, patients with elevated IL-6 levels appeared to have a higher proportion of severe COVID-19 than those with normal IL-6 levels but the effect was not statistically significant (19.57% vs. 13.33%, p = 0.360). There was a significantly lower proportion of patients with severe COVID-19 in MAFLD patients with normal IL-6 levels, than in those with elevated IL-6 levels (8.70% vs. 47.83%, p = 0.003; Figure 4D).
Figure 4.
Association between IL-6 levels, MAFLD status and COVID-19 severity: (A) patients with elevated IL-6 levels; (B) patients with normal IL-6 levels; (C) patients with Non-MAFLD; (D) patients with MAFLD.
Discussion
Our study shows for the first time that patients with laboratory-confirmed COVID-19 and imaging-defined MAFLD at hospital admission have significantly higher circulating IL-6 levels than their counterparts without MAFLD; increased IL-6 levels are also associated with higher odds of severe COVID-19; elevated IL-6 levels had higher odds of severe COVID-19 among MAFLD patients than non-MAFLD patients; and there is a significant interaction between elevated IL-6 levels and MAFLD with severe COVID-19. Notably, these significant associations persisted after adjusting for age, sex and coexisting metabolic co-morbidities. These results remind health care professionals caring for COVID-19 patients should be cognizant of the increased likelihood of severe COVID-19 in patients with elevated IL-6 levels, especially those with MAFLD.
It is known that serum IL-6 levels are elevated in metabolic syndrome, cardiovascular diseases and chronic inflammatory airways diseases (19). Wang et al. reported that fatty liver was independently associated with elevated IL-6 levels (20). However, the association between MAFLD and elevated IL-6 levels is currently uncertain. Our study showed that patients with MAFLD had higher levels of IL-6 than those without MAFLD.
Previous studies have shown that the inflammatory response plays an important role in COVID-19 severity (21, 22). The clinical deterioration observed in some infected patients has been related to the occurrence of a virus-induced cytokine ‘storm’ (23). Liu et al. have reported that the serum levels of IL-6 and C-reactive protein may effectively assess disease severity and predict adverse in-hospital outcomes in patients with COVID-19 (24). Besides, tocilizumab, i.e., a recombinant humanized monoclonal antibody IL-6 receptor inhibitor, has recently emerged as an alternative treatment for COVID-19 patients at risk of cytokine ‘storm’ (25). Accordingly, the results of the present study demonstrated that patients with severe COVID-19 had higher levels of several inflammatory biomarkers (e.g., NLR, CRP, procalcitonin, PCT, IL-6, and IL-10 levels).
Among COVID-19 patients, risk of severe infection and mortality are increased by co-morbidities such as obesity, T2DM, hypertension, cardiovascular disease, and cancer (26). Our previous studies showed that the presence of MAFLD in COVID-19 patients is associated with increased odds of severe COVID-19 (27, 28). A recent study by Petersen et al. also found that greater visceral adipose tissue accumulation was associated with a higher risk of ICU admission in COVID-19 patients (29). Since the diagnosis of MAFLD is based on the evidence of hepatic steatosis in addition to one or more metabolic disorders (namely overweight/obesity, presence of T2DM, or evidence of metabolic dysregulation), it is reasonable to hypothesize that the aggregation of these metabolic risk abnormalities in MAFLD may further aggravate the severity of COVID-19 (30, 31).
We do not know the exact underlying mechanisms by which MAFLD influences the association between elevated IL-6 levels and greater COVID-19 severity, but it is possible that the presence of MAFLD exacerbates the virus-induced cytokine ‘storm’, possibly through the hepatic release of multiple pro-inflammatory cytokines (including IL-6). Moreover, it is also possible to hypothesize that COVID-19 increases the risk of gut-derived endotoxemia, which may lead to macrophage activation and increased secretion of IL-6, thus further contributing to the virus-induced cytokine ‘storm’, especially in infected patients with coexisting abnormal liver function (9, 32). Thus, our findings further highlight the need for clinicians to pay close attention to COVID-19 patients with elevated serum IL-6 levels at hospital admission, especially those with coexisting MAFLD. Moreover, similar to liver, it is commonly to see COVID-19 affects the skeletal muscle such as loss of muscle mass, strength, and physical function (33). Skeletal muscle is also an essential source of IL-6 (34). Therefore, the COVID-19-muscle-IL-6 triangle is also worth investigating.
Our study has some limitations, including the relatively small sample size, the Asian ancestry of the cohort, and the lack of serial monitoring of circulating IL-6 levels during the hospital stay. Therefore, the results of our study will require further validation in larger cohorts of Asian and non-Asian patients with COVID-19. We excluded patients under the age of 18 years and over the age of 70 years, whether our research conclusions are applicable to these patients still needs to be verified. Moreover, sex hormone might affect the severity of MAFLD through the MyD88-dependent IL-6 signaling pathway (35), and recent studies highlighted the role of bio-immunological sex disparities underlying differences in the susceptibility to develop SARS-CoV-2 infection and its sequelae between females and males (36–38). Considering the MAFLD and COVID-19 are both sexually dimorphic and, therefore, additional and more extensive studies are needed to process data separately by sex (39). Finally, relying on CT imaging to diagnose fatty liver might misclassify mild MAFLD cases, and patients were recruited from three different Chinese hospitals, which might have introduced inter-individual variability in the assessment of MAFLD.
In conclusion, the results of this multicenter study show for the first time that there is an independent association and an interaction between serum IL-6 levels and MAFLD in hospitalized patients with severe COVID-19.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committees of the First Affiliated Hospital of Wenzhou Medical University, Ningbo No.2 Hospital, and Ruian People's Hospital. The ethics committees of the First Affiliated Hospital of Wenzhou Medical University, Ningbo No.2 Hospital, and Ruian People's Hospital waived the requirement of written informed consent for participation.
Author Contributions
Study concept and design: FG, KZ, and M-HZ. Acquisition of data: H-DY, Q-FS, K-HP, T-YW, and Y-PC. Analysis and interpretation of data: FG and KZ. Drafting of the manuscript: FG and KZ. Critical revision of the manuscript for important intellectual content: GT, CB, and JG. Study supervision: M-HZ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82070588), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2018ZD039), Ruian Science and Technology Bureau (2020023), High Level Creative Talents from Department of Public Health in Zhejiang Province and Project of New Century 551 Talent Nurturing in Wenzhou. GT is supported in part by grants from the University School of Medicine of Verona, Verona, Italy. CB is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.604100/full#supplementary-material
Abbreviations
CI, confidence intervals; COVID-19, coronavirus disease-2019; IL, interleukin; OR, odds ratio; MAFLD, metabolic dysfunction-associated fatty liver disease; TBIL, total bilirubin.
References
- 1. Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest (2020) 130(5):2202–5. 10.1172/jci137647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest (2020) 130(5):2620–9. 10.1172/jci137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ascierto PA, Fox B, Urba W, Anderson AC, Atkins MB, Borden EC, et al. Insights from immuno-oncology: the Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J Immunother Cancer (2020) 8(1):e000878. 10.1136/jitc-2020-000878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J (2020) 133(19):2271–3. 10.1097/cm9.0000000000000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eslam M, Sanyal AJ, George J. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology (2020) 158(7):1999–2014.e1. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 6. Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic Complications of Nonalcoholic Fatty Liver Disease: When the Liver Is Not an Innocent Bystander. Semin Liver Dis (2015) 35(3):236–49. 10.1055/s-0035-1562944 [DOI] [PubMed] [Google Scholar]
- 7. Zhou Y, Zheng K, Wang X, Yan H, Sun Q, Pan K, et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol (2020) 73(3):719–21. 10.1016/j.jhep.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paquissi FC. Immune Imbalances in Non-Alcoholic Fatty Liver Disease: From General Biomarkers and Neutrophils to Interleukin-17 Axis Activation and New Therapeutic Targets. Front Immunol (2016) 7:490. 10.3389/fimmu.2016.00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol (2019) 16(3):145–59. 10.1038/s41575-018-0082-x [DOI] [PubMed] [Google Scholar]
- 10. Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, et al. Detrimental effects of metabolic dysfunction-associated fatty liver disease and increased neutrophil-to-lymphocyte ratio on severity of COVID-19. Diabetes Metab (2020) 46(6):505–7. 10.1016/j.diabet.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: A meta-analysis. Digest Liver Dis (2020) 53(2):153–7. 10.1016/j.dld.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dongiovanni P, Meroni M, Longo M, Fracanzani AL. MAFLD in COVID-19 patients: an insidious enemy. Expert Rev Gastroenterol Hepatol (2020) 14(10):867–72. 10.1080/17474124.2020.1801417 [DOI] [PubMed] [Google Scholar]
- 13. Favre G, Legueult K, Pradier C, Raffaelli C, Ichai C, Iannelli A, et al. visceral fat is associated to the severity of COVID-19. Metab: Clin Exp (2020) 115:154440. 10.1016/j.metabol.2020.154440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metab: Clin Exp (2020) 108:154244. 10.1016/j.metabol.2020.154244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol (Baltimore Md) (2006) 43(6):1317–25. 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 16. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic Med J Br Diabetic Assoc (2006) 23(5):469–80. 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 17. National Health Commission & State Administration of Traditional Chinese Medicine . Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). (2020). [EB/OL]. 2020.03.03).
- 18. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol (2020) 73(1):202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 19. Wannamethee SG, Whincup PH, Rumley A, Lowe GD. Inter-relationships of interleukin-6, cardiovascular risk factors and the metabolic syndrome among older men. J Thromb Haemostasis JTH (2007) 5(8):1637–43. 10.1111/j.1538-7836.2007.02643.x [DOI] [PubMed] [Google Scholar]
- 20. Wang PW, Hsieh CJ, Psang LC, Cheng YF, Liou CW, Weng SW, et al. Fatty liver and chronic inflammation in Chinese adults. Diabetes Res Clin Pract (2008) 81(2):202–8. 10.1016/j.diabres.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 21. Dietz M, Chironi G, Claessens YE, Farhad RL, Rouquette I, Serrano B, et al. COVID-19 pneumonia: relationship between inflammation assessed by whole-body FDG PET/CT and short-term clinical outcome. Eur J Nucl Med Mol Imaging (2021) 48(1):260–8. 10.1007/s00259-020-04968-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inciardi RM, Solomon SD, Ridker PM, Metra M. Coronavirus 2019 Disease (COVID-19), Systemic Inflammation, and Cardiovascular Disease. J Am Heart Assoc (2020) 9(16):e017756. 10.1161/jaha.120.017756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect (2020) 80(6):607–13. 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol (2020) 127:104370. 10.1016/j.jcv.2020.104370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol (2020) 92(7):814–8. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res (2020) 116(10):1666–87. 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol (2020) 36(1):204–7. 10.1111/jgh.15112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int (2020) 40(9):2160–3. 10.1111/liv.14575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petersen A, Bressem K, Albrecht J, Thieß HM, Vahldiek J, Hamm B, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metab: Clin Exp (2020) 110:154317. 10.1016/j.metabol.2020.154317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care (2020) 43(7):e72–e4. 10.2337/dc20-0682 [DOI] [PubMed] [Google Scholar]
- 31. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract (2020) 162:108142. 10.1016/j.diabres.2020.108142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev (2020) 19(6):102537. 10.1016/j.autrev.2020.102537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gonzalez A, Orozco-Aguilar J, Achiardi O, Simon F, Cabello-Verrugio C. SARS-CoV-2/Renin-Angiotensin System: Deciphering the Clues for a Couple with Potentially Harmful Effects on Skeletal Muscle. Int J Mol Sci (2020) 21(21):7904. 10.3390/ijms21217904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rogeri PS, Gasparini SO, Martins GL, Costa LKF, Araujo CC, Lugaresi R, et al. Crosstalk Between Skeletal Muscle and Immune System: Which Roles Do IL-6 and Glutamine Play? Front Physiol (2020) 11:582258. 10.3389/fphys.2020.582258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xin G, Qin S, Wang S, Wang X, Zhang Y, Wang J. Sex hormone affects the severity of non-alcoholic steatohepatitis through the MyD88-dependent IL-6 signaling pathway. Exp Biol Med (Maywood NJ) (2015) 240(10):1279–86. 10.1177/1535370215570189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salvati L, Biagioni B, Vivarelli E, Parronchi P. A gendered magnifying glass on COVID-19. Clin Mol Allergy CMA (2020) 18:14. 10.1186/s12948-020-00129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology (2020) 161(9):bqaa127. 10.1210/endocr/bqaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lévy Y, Lacabaratz C, Ellefsen-Lavoie K, Stöhr W, Lelièvre JD, Bart PA, et al. Optimal priming of poxvirus vector (NYVAC)-based HIV vaccine regimens for T cell responses requires three DNA injections. Results of the randomized multicentre EV03/ANRS VAC20 Phase I/II Trial. PLoS Pathog (2020) 16(6):e1008522. 10.1371/journal.ppat.1008522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet (Lond Engl) (2020) 396(10250):565–82. 10.1016/s0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.