Abstract
While mother-to-child transmission is believed to play in important role in early childhood infection with Kaposi sarcoma-associated herpesvirus (KSHV), the maternal immune response remains largely uncharacterized. This study aimed to characterize the longitudinal humoral response to KSHV in a cohort of HIV-infected Zambian mothers without KS and identify potential factors that may influence transmission. In total, 86/124 (69.4%) mothers were found to be KSHV seropositive. Longitudinal KSHV titers were fairly stable over time, although seroreversion was still common. Of the total 124 mothers, 81 had at least 1 child KSHV seroconvert during the 2 years analyzed, while the remaining 43 mothers had KSHV-seronegative children. Mothers of KSHV-negative children had higher geometric mean titers than mothers of KSHV-positive children; however, there was no difference in the presence of neutralizing antibodies. This suggests that a strong anti-KSHV immune response, and potentially nonneutralizing antibodies, may reduce transmission.
Keywords: KSHV, seroprevalence, transmission, MTCT, HIV, mother, child, antibodies
Mothers of children who remained KSHV negative demonstrated higher antibody titers than mothers of KSHV-positive children; however, there was no difference in the prevalence of neutralizing antibodies. Low titers and seroreversion were also common.
The causative agent of Kaposi sarcoma (KS) primary effusion lymphoma, and some forms of multicentric Castleman disease, Kaposi sarcoma-associated herpesvirus (KSHV), was first identified 25 years ago [1, 2]. In contrast to other herpesviruses, KSHV seroprevalence varies globally. Certain high-risk groups, such as men who have sex with men, southern Mediterranean males, Amazonian tribes, and Uyghurs, are known to have higher prevalence of KS and KSHV. Outside of these populations, KSHV seroprevalence in the general population is very low (<10%) in Europe, Asia, and the Americas, but is high in sub-Saharan African countries where it is frequently over 50% [3, 4]. In these endemic regions, primary KSHV infection is acquired early in childhood, and KS is an important cause of morbidity and mortality, particularly in those living with human immunodeficiency virus (HIV) [4–7].
Previous studies conducted by our laboratory and others identified several risk factors for primary KSHV infection in young children. HIV infection was found to be a major risk factor; however, the implementation of antiretroviral therapy (ART) in the region has largely mitigated this effect [6–9]. ART-treated HIV-infected children acquire KSHV infection at the same rate and mount similar serological responses as their HIV-uninfected peers [9, 10]. Regardless of ART status, higher CD4+ T-cell counts associate with decreased risk of KSHV infection among children younger than 4 years [9].
Acquisition of KSHV, particularly in young children, has been tied to several factors outside of HIV status. Other infectious agents like malaria and the helminth Schistosoma mansoni have been associated with an increased risk of KSHV seropositivity [8, 11, 12]. Higher numbers of KSHV-seropositive individuals in the household, as well as a KSHV-seropositive mother or primary caregiver, were associated with increased risk of infection in children [8, 13, 14]. However, previous studies have not investigated any maternal characteristics, outside of KSHV serostatus, that may provide protection against transmission or early childhood infection with KSHV. Despite an appreciation of the significance of horizontal mother-to-child transmission [5, 15], and that breastfeeding decreased odds of KSHV seroconversion in infants [13], the impact of the maternal anti-KSHV immune response, specifically around the time of a child’s seroconversion, remains largely uncharacterized. Here we longitudinally evaluated humoral anti-KSHV responses in a cohort of HIV-positive mothers whose children acquired KSHV infection compared to those whose children remained seronegative.
METHODS
Study Design and Cohort
A subset of mothers from a previously established Zambian maternal-child cohort focused on quantifying KSHV infection and risk factors in infants [9, 10, 16] was used for this study. Mother-child pairs were followed for up to 5 years postenrollment, with a sampling interval of 3 months. Informed consent was obtained and the study was approved by the Institutional Review Board of the University of Nebraska and the University of Zambia Biomedical Research Ethics Committee. All mothers in the initial study were HIV-infected without KS. KSHV serostatus of the mothers was unknown prior to the current study. For inclusion in the current study, mothers needed at least 2 years of follow-up after study enrollment. To clearly differentiate between mothers of KSHV-positive and -negative children, the children of these mothers needed at least 2 years of seronegative results, determined by monoclonal antibody-enhanced immunofluorescence assay (mIFA), or at least 2 years of follow-up post-KSHV seroconversion. From this group, 7 additional mothers were excluded as their 2 children were determined to have differing KSHV serostatus. However, mothers with multiple children with the same KSHV serostatus were included, leaving a final number of 124 mothers with 130 children. Six mothers had 2 children each, while the remaining 118 mothers had only a single child enrolled. Of the 6 sets of siblings, 2 pairs were KSHV negative and 4 were KSHV positive.
KSHV Seroprevalence and Antibody Titer
KSHV binding antibodies were detected using an mIFA previously standardized in our laboratory [13]. Briefly, plasma samples were diluted 1:40 in phosphate-buffered saline and incubated with permeabilized and fixed tetradecanoyl phorbal ester acetate-stimulated BC3 cells to induce lytic gene expression, allowing for detection of both latent and lytic KSHV proteins. Mouse monoclonal anti-human IgG (CRL-1786; American Type Culture Collection) was used as a secondary antibody, with Cy-2 conjugated donkey anti-mouse IgG (Jackson ImmunoResearch) serving as the tertiary antibody. Samples were considered positive for KSHV binding antibodies if the sample was deemed positive on 2 independent mIFAs. Samples positive at the 1:40 dilution were then titrated by 2-fold dilutions to 1:5120 and tested using the mIFA. The inverse of the most dilute positive sample is reported as the antibody titer. Mothers are deemed KSHV seropositive if at least 1 sample was ruled positive at the 1:40 dilution over the 2 years studied. The overall seroprevalence is reported as the number of seropositive mothers out of the total number of mothers. Seroprevalence at each timepoint was calculated as the number of seropositive samples out of the total number of samples at that time.
KSHV Neutralizing Antibody Assay
KSHV-seropositive samples, as determined by mIFA, were tested for the presence of KSHV neutralizing antibodies (nAb) using our previously standardized assay [17]. Briefly, a 1:50 dilution of heat-inactivated plasma (56°C 1 hour) was incubated for 1 hour with rKSHV.219 virus at 37°C. The virus-plasma mixture was then used to infect 293T cells via spinoculation (400g, 20 minutes). After a 72-hour incubation, the level of infection was determined as the percentage of green fluorescent protein-expressing cells quantified by flow cytometry (Accuri C6 Plus; BD Biosciences). Samples were analyzed in triplicate and were defined as nAb positive if the average inhibition of infection for 3 independent experiments was greater than 50% when compared to the inhibition of infection mediated by KSHV-seronegative control plasma.
Statistical Analyses
Student t test was used for all analyses, unless stated otherwise. Differences between geometric mean titers were calculated by first transforming each titer (log2) and then determining the arithmetic mean of the transformed values. A t test was used to assess statistical differences between the groups and the means were reported in nonlogarithmic form. Child HIV-1 status and maternal titer group were analyzed for differences in distribution with a Χ2 test, while sex of the children, maternal ART status, maternal KSHV serostatus, and nAb prevalence were assessed via Fisher exact test. All statistical analysis was conducted in GraphPad Prism 8.3.0.
RESULTS
Cohort Characteristics
Table 1 describes the 85 KSHV-seropositive and 45 KSHV-seronegative children of the mothers followed for this study. There were no significant differences in sex or HIV status between KSHV-positive and KSHV-negative children. However, there was a significant difference in age, with KSHV-negative children being an average of 2 months older at enrollment. Importantly, all but 5 of the HIV-positive children were taking ART upon enrollment and they were all on ART by the third follow-up time point.
Table 1.
Demographics of Children
| Characteristic | KSHV Positive (n = 85) | KSHV Negative (n = 45) |
|---|---|---|
| Age at enrollment, moa | 6.6 | 8.5 |
| Sex, No. (%) | ||
| Male | 45 (52.9) | 20 (44.4) |
| Female | 40 (47.1) | 25 (55.6) |
| HIV status, No. (%) | ||
| Positive | 41 (48.2) | 18 (40.0) |
| Negative | 27 (31.8) | 20 (44.4) |
| Unknown | 17 (20.0) | 7 (15.6) |
Abbreviations: HIV, human immunodeficiency virus; KSHV, Kaposi sarcoma-associated herpesvirus.
a P = .0072.
Demographic information for all 124 mothers is shown in Table 2. All mothers were HIV positive, although the duration of HIV infection was unknown. There were no differences in age, CD4+ T-cell count, ART status, or duration of ART between mothers of KSHV-positive and -negative children. Additionally, no significant difference was found in CD4+ T-cell count between women who reported ever taking ART compared to those who reported never taking ART (Supplementary Figure 1).
Table 2.
Demographics of Mothers
| Characteristic | Whole Cohort (n = 124) | Mothers of KSHV + Children (n = 81) | Mothers of KHSV− Children (n = 43) |
|---|---|---|---|
| Mother’s age, y (range) | 30.0 (18–45) | 29.7 | 30.6 |
| KSHV seropositive, No. (%) | 86 (69.4) | 54 (66.7) | 32 (74.4) |
| CD4 T-cell count (range) | 475.07 (43.75–978.33) | 492.78 (43.75–795.75) | 441.70 (122.13–978.33) |
| ART status at enrollment, No. (%) | |||
| Ever | 68 (54.8) | 41 (50.6) | 27 (62.8) |
| Never | 56 (45.2) | 40 (49.4) | 16 (37.2) |
| Years on ART at enrollment (range) | 1.92 (1 mo–14 y) | 1.92 (2 mo–6 y) | 1.69 (1 mo–14 y) |
Abbreviations: ART, antiretroviral therapy; KSHV, Kaposi sarcoma-associated herpesvirus.
Maternal Immune Response
In total, 86 of the 124 mothers (69.4%) were KSHV seropositive for at least 1 time point over the 2 years examined. KSHV antibody titer was not found to correlate with CD4+ T-cell count (Supplementary Figure 2). There was a small but significant difference in KSHV antibody titer by ART status, with mothers who reported ever taking ART at enrollment showing slightly higher titers (Supplementary Figure 3).
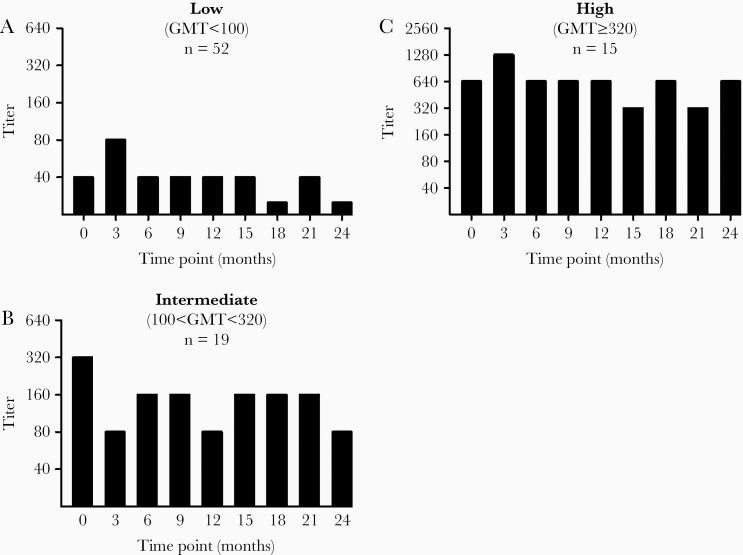
Overall, the maternal humoral response against KSHV was much more consistent than the variation observed in children by Olp et al following primary seroconversion [10]. Mothers demonstrated stable antibody titers that could be stratified into 3 groups. Mothers had a geometric mean titer (GMT) less than or equal to 100 were classified as low titer, and over half of the seropositive women were in this category (52 of 86). Mothers with a GMT between 100 and 320 were classified as intermediate, and those with titers greater than or equal to 320 were classified as high titer. Representative longitudinal KSHV titer graphs for each group are shown in Figure 1.
Figure 1.
Representative longitudinal titers for each of the 3 titer strata: low (A), intermediate (B), and high (C). Titer was determined by monoclonal antibody-enhanced immunofluorescence assay. Bars with a height lower than 40 indicate samples negative at the 1:40 dilution. The numbers of mothers belonging to each stratum are listed in each graph. Abbreviation: GMT, geometric mean titer.
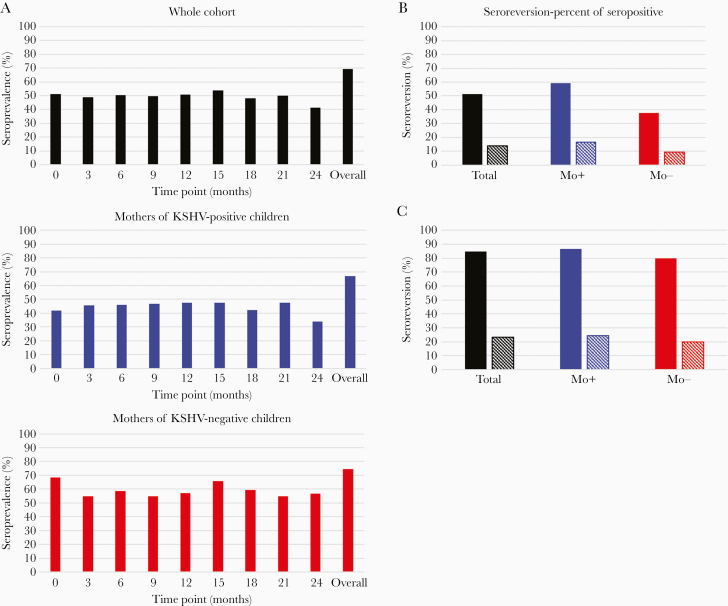
Despite the stability of the KSHV antibody response, seroreversion, or undetectable KSHV antibodies at a time point after KSHV antibodies were previously detected, was common (Figure 2). As seen in Figure 2A, seroreversion altered the seroprevalence estimates at each time point. For the cohort as a whole, maternal KSHV seroprevalence at any single time point was 15.7%–28.3% lower than the overall seroprevalence (Figure 2A, top graph). Over half of the mothers designated as KSHV seropositive underwent seroreversion at least once during follow-up, and 12 mothers (14.0%) tested positive by mIFA only once over the 2-year study duration (Figure 2B, black bars). Mothers of both KSHV-positive and -negative children showed temporal variations in seroprevalence; however, the magnitude of the difference between the overall seroprevalence and the seroprevalence at any given time point was more pronounced among mothers of KSHV-positive children (Figure 2A, middle and bottom graphs). Mothers of KSHV-positive children were more likely to have undergone seroreversion or to have tested seropositive only at a single time point (Figure 2B). Seroreversion occurred exclusively among individuals in the low-titer strata, where approximately 85% seroreverted and 23% were positive at only 1 time point (Figure 2C). Notably, the prevalences of both seroreversion and mothers positive at single time point were similar between mothers of KSHV-positive and -negative children when restricted to the low-titer strata (Figure 2C).
Figure 2.
Change in seroprevalence over time (A) and the frequency of seroreversion among seropositive mothers (B) and low-titer mothers (C). A, Overall seroprevalence was determined as the number of seropositive mothers over the total number of mothers, while seroprevalence at each timepoint was defined as the number of seropositive samples out of the total number of samples available at that time. B and C, Solid bars indicate the percentage of mothers who seroreverted at least once during follow-up, while striped bars indicate mothers who tested positive for KSHV antibodies at only a single time point. Abbreviations: Mo+, mothers of KSHV positive children; Mo−, mothers of KSHV negative children; KSHV, Kaposi sarcoma-associated herpesvirus.
As outlined in Table 3, the KSHV seroprevalence was slightly elevated among mothers of KSHV-negative children; however, this difference was not statistically significant (P = .42). Mothers of KSHV-negative children did show a significantly higher anti-KSHV GMT (22.1 vs 8.87, P < .0001). The difference in GMT was not due to the higher seroprevalence among mothers of KSHV-negative children as it remained significant when only seropositive women were included in the analysis (68.5 vs 26.7, P < .0001). While a larger proportion of mothers of KSHV-negative children fell into the high-titer strata, this difference failed to reach significance (P = .078). Together, these data suggest mothers of KSHV-negative children were more likely to mount a stronger anti-KSHV antibody response than mothers of KSHV-positive children.
Table 3.
Difference in Serology Between Mothers of Seropositive and Seronegative Children
| Characteristic | Mothers of KSHV + Children (n = 81) | Mothers of KSHV− Children (n = 43) |
|---|---|---|
| KSHV seroprevalence, No. (%) | 54 (66.7) | 32 (74.4) |
| GMTa | ||
| All | 8.87 | 22.1 |
| Seropositive | 26.7 | 68.5 |
| Titer group as % of KSHV+, No. (%) | ||
| Low | 37 (68.5) | 15 (46.9) |
| Intermediate | 11 (20.4) | 8 (25.0) |
| High | 6 (11.1) | 9 (28.1) |
| Neutralizing antibodies | 3 (3.7) | 2 (4.7) |
Abbreviations: GMT, geometric mean titer; KSHV, Kaposi sarcoma-associated herpesvirus.
a P < .0001.
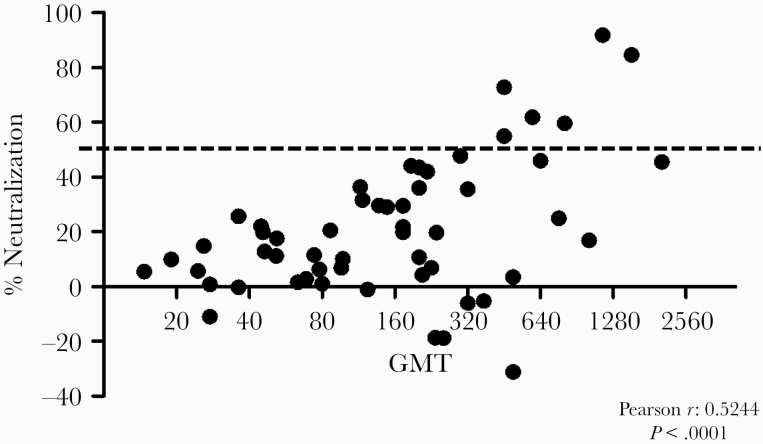
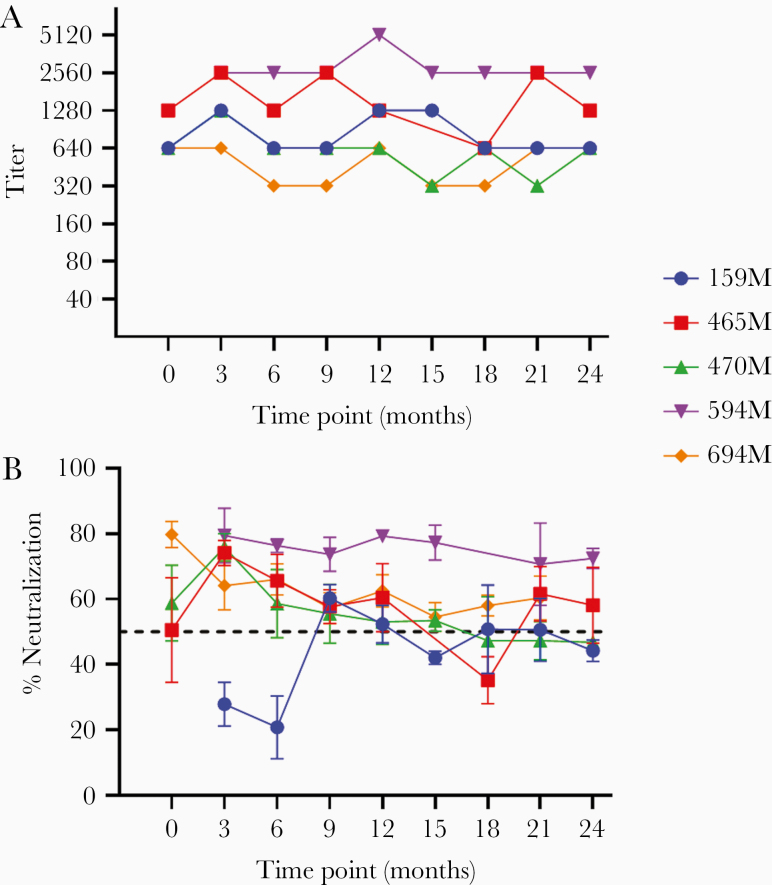
Interestingly, despite the higher seroprevalence and overall antibody response, there was no difference in the prevalence of nAb between the 2 groups. Initial screening revealed a significant positive correlation between neutralizing activity and GMT (Pearson r = 0.52, P < .0001; Figure 3). Due to this correlation, only high-titer mothers (GMT ≥ 320) were tested for nAb at all time points by 3 independent experiments. In total, 5 mothers (3 mothers of KSHV-positive and 2 mothers of KSHV-negative children) showed nAb at least once. One additional mother, whose child was KSHV negative, approached but did not traverse the 50% threshold required to be deemed neutralizing after the completion of 3 replicates. Figure 4 shows KSHV binding antibody titer (Figure 4A) and neutralizing activity (Figure 4B) longitudinally for the 5 mothers with nAb. Binding titer for all 5 mothers showed little variation over time. Despite this consistency and the correlation between neutralizing activity and GMT, neutralization was not stable longitudinally. Only 2 of the 5 mothers (594M and 649M) showed neutralization greater than 50% at all time points, and among all mothers neutralizing activity tended to decay over time. Mothers 159M, 465M, and 470M did show spikes in neutralizing activity, although the response began to fade at the next time point. Mothers 470M, 594M, and 649M had children who seroconverted at time points 15, 12, and 3, respectively. No change in neutralizing activity prior to the child’s seroconversion was seen in any case. This demonstrates that while there was clearly longitudinal consistency in overall KSHV antibody titers as determined by mIFA, neutralization, when present, was variable.
Figure 3.
Positive correlation (Pearson r = 0.52, P < .0001) between neutralizing activity (% neutralization) and geometric mean titer (GMT) is shown. The dashed line indicates the required 50% threshold to be deemed positive for neutralizing antibodies. The data are from an initial screen where each sample was tested in triplicate.
Figure 4.
Binding titer (A) and neutralization profiles (B) at each time point for the 5 mothers with neutralizing antibodies. Mothers are listed by identification number. Titer was determined as the lowest positive dilution by monoclonal antibody-enhanced immunofluorescence assay and therefore does not have error. Neutralization is shown as percent reduction in infection compared to Kaposi sarcoma-associated herpesvirus–seronegative control plasma. Samples were tested in triplicate and the mean and standard deviation from 3 independent experiments are shown. The dashed line indicates the 50% threshold differentiating neutralizing and nonneutralizing responses.
DISCUSSION
We observed an overall KSHV seroprevalence of 69.4% (86/124) among HIV-infected Zambian mothers, consistent with other reports from the region. A cross-sectional analysis observed a KSHV seroprevalence over 50% for HIV-1–positive women in Zambia, while estimates in Ugandan mothers range from 61% to 69% [8, 11, 18]. In Ethiopia, HIV-infected pregnant women were found to have a KSHV seroprevalence of 69% [19]. Longitudinal antibody titers among the 86-seropositive mothers analyzed in this study showed much greater consistency than titers reported by Olp et al in KSHV-seropositive children [10]. Almost half of the children were shown to have variance in titers, either increasing, decreasing, or inconsistent. In contrast, maternal titers seldom showed a variance greater than 2 log2. While the stability of the maternal humoral response could be due to a more mature, fully developed immune system in adults, it is more likely KSHV titers stabilize with a longer duration of infection and cycles of viral production through sporadic lytic induction. Although it is not clear when the mothers were infected with KSHV, it is logical to assume that the mothers were also infected in childhood and ultimately stabilized the humoral immune response against KSHV after exhibiting similar variance to that previously observed in children over the first 2 years following seroconversion.
Previous studies examining risk factors for childhood acquisition of KSHV found children are at an increased risk of acquiring KSHV if the primary caregiver is seropositive, especially if the primary caregiver is the mother [13, 14]. Given the increased risk of KSHV infection in children of KSHV-seropositive women, a higher KSHV seroprevalence was expected in mothers of KSHV-positive children. However, we observed no difference in KSHV seroprevalence between mothers of KSHV-positive and -negative children, and seroprevalence was actually slightly higher among mothers of KSHV-negative children. This discrepancy is likely due to differences in study design, as the current study was implemented to compare maternal immune responses in relation to infection in their children, not to detect risk factors in children. It is also important to note that all mothers in this study were HIV-infected and, as such, are not representative of the maternal population as a whole.
While the seroprevalence was comparable between mothers of KSHV-positive and -negative children, there was a significant difference in KSHV antibody titer between the 2 groups. Mothers of KSHV-negative children had an average titer approximately 2.5 times higher than mothers of KSHV-positive children. This difference was not an effect of the slight variation in seroprevalence as the average titer remained significantly higher among mothers of KSHV-negative children whether seronegative women were included or excluded. Correspondingly, more mothers of KSHV-negative children fell into the high-titer strata than mothers of KSHV-positive children (28.1% vs 11.1%, respectively).
Similar to the 6.5% nAb prevalence seen in asymptomatic KSHV-seropositive individuals reported by Kumar et al [17], only 5 of the 86 (5.8%) seropositive mothers had nAb responses. A significant correlation (P < .0001) between Ab titer and neutralizing activity was detected, and all 5 individuals with nAb had a high KSHV GMT. However, despite the higher KSHV antibody titer found among mothers of KSHV-negative children and the correlation between nAb and titer, there was no difference in the prevalence of nAb between the mothers of KSHV-positive and -negative children. Additionally, the 3 mothers of KSHV-positive children with nAb did not show any change in activity prior to the seroconversion of their children. This could suggest the involvement of nonneutralizing antibodies in controlling KSHV infection or in reducing transmission, or that the specific antigen targeted by the neutralizing response is more important than the presence or absence of nAb in general. The rarity of nAb among asymptomatic populations has led some to posit that high KSHV titers, and the presence of KSHV nAb, may serve as a risk indicator of KS development [20]. For this reason, we intend to follow-up those subjects with nAb to monitor for signs of KS.
Roughly 60% (52/86) of all seropositive mothers showed a low KSHV titer, and seroreversion was almost ubiquitous in this group (84.6%, 44/52). Furthermore, 12 women had detectable KSHV antibodies at only a single time point over the 2 years examined. These findings demonstrate the need for longitudinal follow-up in order to accurately determine KSHV seroprevalence. Additionally, KSHV seroprevalence at any given time point underestimated the overall seroprevalence by approximately 20%. This suggests that in addition to incorrectly classifying an individual’s KSHV serostatus, cross-sectional studies likely underestimate KSHV seroprevalence at the population level. Other studies utilizing immunofluorescence and enzyme-linked immunosorbent assay (ELISA) methods to detect KSHV infection have also reported seroreversion [21–24]. Two studies observed a higher frequency of seroreversion using IFA compared to ELISA; however, in both studies IFAs only assessed latent proteins, while the ELISA was against a lytic protein, either K8.1 or ORF65.2 [21, 23]. In contrast, our mIFA detects responses against both latent and lytic proteins, therefore it is difficult to evaluate the sensitivity of our assay compared to the others without direct comparisons. Unfortunately, we were unable to assess samples using an additional method at this time. It is again worth noting that all mothers in this study were HIV-infected, and low KSHV titers and seroreversion may be a more common event among HIV-infected persons. The finding that mothers who reported ART experience showed a slight, but significant, increase in KSHV antibody titer compared to mothers who reported never taking ART reinforces the idea that control of HIV infection may lead to stronger anti-KSHV responses. However, there was no correlation between CD4+ T-cell count and KSHV antibody titer, suggesting that the impact of ART may be due to reduction of systemic inflammation or a more balanced Th1/Th2 adaptive response rather than an increase in CD4+ T cells.
The commonality of seroreversion as well as changes in neutralizing activity over time suggest the lack of a dominant antibody response to KSHV. Indeed, when Labo et al analyzed seroreactivity of individuals with KSHV-associated malignancies, most of whom were HIV infected, they observed a diverse range of antibody responses against the KSHV proteome [25]. Individualized, suboptimal responses against KSHV could be due to a changing repertoire, low antigenicity of KSHV, a lack of essential B-cell maintenance signals, or a lack of circulating viral particles for antigenic stimulation. While the antibody response was able to be quantified, more research into how the quality of KSHV antibodies changes or stabilizes longitudinally is needed. The data show the overall strength of the KSHV response is fairly stable over time; however, the changes in neutralization despite consistent titers suggest antibody responses may show much greater qualitative changes.
One limitation to this study was the inability to assess KSHV or HIV viral load or oral shedding of KSHV as such samples were not available. Unfortunately, this prevents the determination of any relationships between these variables and the immune responses in the mothers, as well as how oral KSHV shedding in the mothers may relate to the KSHV status of the child in this cohort. A 2018 study by Newton et al did not find a higher risk of KSHV in Ugandan children whose mothers shed detectable KSHV, although the children in that study were already 6 years old. However, they did find a positive correlation between K8.1 antibody titer and the frequency of KSHV shedding [26]. These data imply that mothers of KSHV-negative children would be expected to have higher levels of KSHV shedding in the oral cavity, as they had higher average antibody titers. In contrast, other groups have reported a decrease in the frequency of oral KSHV shedding among individuals with higher titers, in studies conducted among HIV-positive men in the United States [27–29]. More studies examining the frequency of KSHV shedding in relation to antibody levels and other immune responses, especially among mothers and primary caregivers, are needed to clarify these differences and expand our understanding of viral transmission and early childhood infections.
It should also be noted that while mother-to-child transmission is believed to play an important role in childhood acquisition of KSHV, approximately one-third of the mothers of KSHV-positive children were seronegative over the 2 years surrounding the child’s seroconversion. Thus, nonmaternal sources are also a prominent factor for early KSHV infection [5, 30]. Interestingly, a few of these seronegative mothers did test positive for KSHV antibodies at later time points not included in this study (data not shown). In these cases, it is possible the mothers’ antibody levels were undetectable during the 2 years examined despite preexisting KSHV infection, or the mothers may have acquired KSHV after, or even from, their children.
In conclusion, we observed stable longitudinal KSHV antibody titers among a cohort of HIV-positive mothers in Zambia, although low titers and seroreversion were still common. Mothers of KSHV-negative children showed significantly higher antibody titers, but no difference in the prevalence of neutralizing antibodies. This suggests a strong immune response, and potentially nonneutralizing antibodies, may play a role in controlling KSHV infection and preventing transmission.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all of the individuals involved in the study for their participation, as well as the staff at Lusaka Teaching Hospital in Lusaka Zambia, and Landon Olp and Veenu Minhas for their support and database organization.
Financial support. This work was supported by the National Cancer Institute (grant numbers U54 CA221204 and NCI 75903); Fogarty International (grant number D43TW010354); National Institute of Allergy and Infectious Diseases Ruth L. Kirschstein National Research Service Award (grant number T32 AI125207 to C. W.); and National Institute of General Medical Sciences (grant number P20 GM103427). L. K. P. is a Ruth L. Kirchstein fellow.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Conference on EBV and KSHV 2018, Madison, WI, 28 July–1 August 2018.
References
- 1. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994; 266:1865–9. [DOI] [PubMed] [Google Scholar]
- 2. Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer 2010; 10:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minhas V, Wood C. Epidemiology and transmission of Kaposi’s sarcoma-associated herpesvirus. Viruses 2014; 6:4178–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uldrick TS, Whitby D. Update on KSHV epidemiology, Kaposi sarcoma pathogenesis, and treatment of Kaposi sarcoma. Cancer Lett 2011; 305:150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butler LM, Dorsey G, Hladik W, et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis 2009; 200:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minhas V, Crabtree KL, Chao A, et al. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am J Epidemiol 2008; 168:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minhas V, Brayfield BP, Crabtree KL, Kankasa C, Mitchell CD, Wood C. Primary gamma-herpesviral infection in Zambian children. BMC Infect Dis 2010; 10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wakeham K, Webb EL, Sebina I, et al. Risk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in Uganda. J Acquir Immune Defic Syndr 2013; 63:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olp LN, Minhas V, Gondwe C, et al. Effects of antiretroviral therapy on Kaposi’s sarcoma-associated herpesvirus (KSHV) transmission among HIV-infected Zambian children. J Natl Cancer Inst 2015; 107:djv189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olp LN, Minhas V, Gondwe C, et al. Longitudinal analysis of the humoral response to Kaposi’s sarcoma-associated herpesvirus after primary infection in children. J Med Virol 2016; 88:1973–81. [DOI] [PubMed] [Google Scholar]
- 11. Nalwoga A, Cose S, Wakeham K, et al. Association between malaria exposure and Kaposi’s sarcoma-associated herpes virus seropositivity in Uganda. Trop Med Int Health 2015; 20:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nalwoga A, Webb EL, Chihota B, et al. Kaposi’s sarcoma-associated herpesvirus seropositivity is associated with parasite infections in Ugandan fishing communities on Lake Victoria islands. PLoS Negl Trop Dis 2019; 13:e0007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crabtree KL, Wojcicki JM, Minhas V, et al. Risk factors for early childhood infection of human herpesvirus-8 in Zambian children: the role of early childhood feeding practices. Cancer Epidemiol Biomarkers Prev 2014; 23:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr 2007; 44:351–5. [DOI] [PubMed] [Google Scholar]
- 15. Etta EM, Alayande DP, Mavhandu-Ramarumo LG, Gachara G, Bessong PO. HHV-8 seroprevalence and genotype distribution in Africa, 1998–2017: a systematic review. Viruses 2018; 10:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minhas V, Crabtree KL, Chao A, et al. The Zambia children’s KS-HHV8 study: rationale, study design, and study methods. Am J Epidemiol 2011; 173:1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar P, Kuwa NY, Minhas V, et al. Higher levels of neutralizing antibodies against KSHV in KS patients compared to asymptomatic individuals from Zambia. PLoS One 2013; 8:e71254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He J, Bhat G, Kankasa C, et al. Seroprevalence of human herpesvirus 8 among Zambian women of childbearing age without Kaposi’s sarcoma (KS) and mother-child pairs with KS. J Infect Dis 1998; 178:1787–90. [DOI] [PubMed] [Google Scholar]
- 19. Lemma E, Constantine NT, Kassa D, et al. Human herpesvirus 8 infection in HIV-1-infected and uninfected pregnant women in Ethiopia. Ethiop Med J 2009; 47:205–11. [PubMed] [Google Scholar]
- 20. Wakeham K, Johnston WT, Nalwoga A, et al. Trends in Kaposi’s sarcoma-associated herpesvirus antibodies prior to the development of HIV-associated Kaposi’s sarcoma: a nested case-control study. Int J Cancer 2015; 136:2822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biggar RJ, Engels EA, Whitby D, Kedes DH, Goedert JJ. Antibody reactivity to latent and lytic antigens to human herpesvirus-8 in longitudinally followed homosexual men. J Infect Dis 2003; 187:12–8. [DOI] [PubMed] [Google Scholar]
- 22. McDonald AC, Jenkins FJ, Bunker CH, Wilson JW, Patrick AL, Weissfeld JL. Human herpesvirus 8 seroconversion in a population-based cohort of men in Tobago. J Med Virol 2015; 87:642–7. [DOI] [PubMed] [Google Scholar]
- 23. Quinlivan EB, Wang RX, Stewart PW, et al. ; Swiss HIV Cohort Study . Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV cohort 4.7 years before KS. J Med Virol 2001; 64:157–66. [DOI] [PubMed] [Google Scholar]
- 24. Zavitsanou A, Sypsa V, Petrodaskalaki M, et al. Human herpesvirus 8 infection in hemodialysis patients. Am J Kidney Dis 2006; 47:167–70. [DOI] [PubMed] [Google Scholar]
- 25. Labo N, Miley W, Marshall V, et al. Heterogeneity and breadth of host antibody response to KSHV infection demonstrated by systematic analysis of the KSHV proteome. PLoS Pathog 2014; 10:e1004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newton R, Labo N, Wakeham K, et al. Determinants of gammaherpesvirus shedding in saliva among Ugandan children and their mothers. J Infect Dis 2018; 218:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laney AS, Cannon MJ, Jaffe HW, et al. Human herpesvirus 8 presence and viral load are associated with the progression of AIDS-associated Kaposi’s sarcoma. AIDS 2007; 21:1541–5. [DOI] [PubMed] [Google Scholar]
- 28. Laney AS, Dollard SC, Jaffe HW, et al. Repeated measures study of human herpesvirus 8 (HHV-8) DNA and antibodies in men seropositive for both HHV-8 and HIV. AIDS 2004; 18:1819–26. [DOI] [PubMed] [Google Scholar]
- 29. Cannon MJ, Dollard SC, Black JB, et al. Risk factors for Kaposi’s sarcoma in men seropositive for both human herpesvirus 8 and human immunodeficiency virus. AIDS 2003; 17:215–22. [DOI] [PubMed] [Google Scholar]
- 30. Olp LN, Shea DM, White MK, Gondwe C, Kankasa C, Wood C. Early childhood infection of Kaposi’s sarcoma-associated herpesvirus in Zambian households: a molecular analysis. Int J Cancer 2013; 132:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.