Abstract
The COVID-19 pandemic has introduced a global public health threat unparalleled in our history. The most severe cases are marked by ARDS attributed to microvascular thrombosis. Hypercoagulability, resulting in a profoundly prothrombotic state, is a distinct feature of COVID-19 and is accentuated by a high incidence of fibrinolysis shutdown. The aims of this review were to describe the manifestations of fibrinolysis shutdown in COVID-19 and its associated outcomes, review the molecular mechanisms of dysregulated fibrinolysis associated with COVID-19, and discuss potential implications and therapeutic targets for patients with severe COVID-19.
Abbreviations and Acronyms: DIC, disseminated intravascular coagulation; INR, international normalized ratio; LY30, lysis at 30 minutes; MCF, maximum clot firmness; ML, maximum lysis; PAI-1, plasminogen activator inhibitor 1; ROTEM, rotational thromboelastometry; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TAFI, thrombin activatable fibrinolysis inhibitor; TEG, thrombelastography; tPA, tissue plasminogen activator; VTE, venous thromboembolism
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was discovered in a cluster of patients with pneumonia of unknown origin in December 2019 in Wuhan, China.1 The SARS-CoV-2 virus was subsequently identified as the causative pathogen for COVID-19, a clinical syndrome characterized initially by fever, cough, and progression to ARDS.2 Severe disease will develop in 5% to 16% of patients, who will require a prolonged ICU stay,3 , 4 and 50% to 70% of those patients will require mechanical ventilation.4 , 5 The overall mortality rate for COVID-19 is 1% to 5%; however, this incidence increases to 22% to 64% in patients who progress to ARDS.4, 5, 6
COVID-19 was declared a global pandemic by the WHO in March 2020 and has rapidly become the largest public health emergency in modern times.7 As of February 18, 2021, there were 110 million confirmed cases and 2.5 million confirmed deaths from COVID-19 worldwide.8
Shortly after the start of the COVID-19 pandemic, it became increasingly clear that this disease was associated with a frequent and oftentimes severe coagulopathy that was augmented in nonsurvivors of the illness. In fact, in the first large and comprehensive evaluation of coagulation function in patients with COVID-19, Tang and colleagues9 found that 71% of nonsurvivors exhibited disseminated intravascular coagulation (DIC), as defined by the International Society on Thrombosis and Haemostasis standards.10 However, it has also become apparent that these patients exhibit a unique hypercoagulable phenotype of DIC with a propensity toward thrombosis rather than a bleeding diathesis.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 It is now apparent that patients with COVID-19 rarely progress to a state of overt DIC, as defined by the International Society on Thrombosis and Haemostasis.22 Rather, the coagulopathy associated with COVID-19 involves a different pathophysiology. As a result, therapeutic anticoagulation has been considered a potential component of the overall management of patients with COVID-19; although this strategy is clinically intuitive, groups like the International Society on Thrombosis and Haemostasis DIC subcommittee have issued pragmatic guidance on the subject, given a lack of high-level evidence to support therapeutic anticoagulation in all patients affected by COVID-19.23
Anticoagulation with heparin addresses only 1 component of an otherwise very complex coagulation cascade. Coagulation is a tightly balanced process with thrombotic and fibrinolytic pathways constantly working against each other to favor neither thrombosis nor hemorrhage during normal physiologic conditions. The importance of the balance of thrombosis and fibrinolysis has been appreciated since the 1940s.24 This highly regulated and dynamic system exhibits a maladaptive response in various disease states, promoting either excessive thrombosis, a bleeding diathesis, or a combination of both. The fibrinolytic pathway is an integral component of this response and has been shown to become profoundly dysregulated in a variety of pathologic conditions, including trauma25, 26, 27, 28 and sepsis.29 Fibrinolysis shutdown, a relative hypofibrinolytic state induced by or acting as a marker of severe disease, is associated with poor outcomes, including ARDS, multisystem organ failure, and mortality.25, 26, 27, 28, 29 Viscoelastic testing, using thrombelastography (TEG) and rotational thromboelastometry (ROTEM), is a whole-blood assay superior to conventional coagulation tests (eg prothrombin time, aPTT, and international normalized ratio) that provides a global assessment of coagulation function, including the cellular participants, in addition to both the intrinsic and extrinsic pathways. Recently, fibrinolysis shutdown has been identified in COVID-19 patients using both TEG and ROTEM and has been associated with poor outcomes, including venous thromboembolism (VTE) and other thrombotic events.30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Therefore, our objectives were to describe the manifestations of fibrinolysis shutdown in COVID-19 and its associated outcomes, review the molecular mechanisms of dysregulated fibrinolysis associated with COVID-19, and discuss potential implications and therapeutic targets for patients with severe COVID-19.
Thrombotic and Bleeding Complications in COVID-19-Associated Fibrinolysis Shutdown
Thrombotic complications are now recognized as leading causes of morbidity and mortality in COVID-19. These include VTE, ischemic stroke, MI, acute limb ischemia, and other macro- and microthrombotic complications, such as multiple organ failure. Between 30% and 80% of ICU patients with COVID-19 experience a thrombotic complication at some point during their disease course.44, 45, 46, 47 VTE is the most common thrombotic complication in patients with COVID-19, occurring in 15% to 85% of patients.12 A meta-analysis of 6 studies including 678 patients identified 5 risk factors for the development of VTE in patients with COVID-19.13 These include age, ICU admission, leukocytosis, lymphopenia, and elevated d-dimer. d-dimer, a marker of fibrinolysis and fibrin deposition, is consistently elevated in patients with severe COVID-19 and is a predictor of poor outcomes.9 , 47 , 48 Although d-dimer has been associated with fibrinolysis, d-dimers are a biomarker of clot formation and, in a patient with COVID-19, can represent unbridled clot formation.49, 50, 51 In fact, d-dimer levels in the first 7 days of disease and the rate of change of d-dimer levels have been shown to reliably predict VTE.52 Other thrombotic complications, including stroke and cerebral venous sinus thrombosis,14 , 15 MI,16, 17, 18 acute limb ischemia,19 acute kidney injury,20 and ischemic colitis,21 have been reported in patients with COVID-19. Mechanistically, the markedly elevated levels of d-dimer in patients with fibrinolysis shutdown might represent local thrombosis in the microvasculature (eg pulmonary and renal) that are not consistently captured on whole-blood assays. Inconsistencies in timing of sample measurements and within-patient variability in coagulation profiles make interpretation of these data even more complex.
Several groups have now studied the hypercoagulable state in patients with COVID-19 using viscoelastic testing, including both ROTEM and TEG. In a retrospective study of 40 ICU patients, Pavoni and colleagues32 identified decreased clot formation times and high clot strength (maximum clot firmness [MCF]) on ROTEM in the majority of their patients. Maximum lysis (ML) at 60 minutes was also decreased significantly, indicating fibrinolysis shutdown. A second study by this group comparing 20 COVID-19 ICU patients with 25 non-COVID ICU patients with pneumonia confirmed decreased clot formation times and increased MCF at ICU admission in patients with COVID-19, and persistent hypercoagulability at ICU day 5 and day 10.31 Deceased patients with COVID-19 had significantly lower ML at 60 minutes at all time points. In addition, among the 4 patients meeting criteria for fibrinolysis shutdown at ICU day 10, three patients died of pulmonary embolism and 1 patient died of multisystem organ failure,31 suggesting an association between fibrinolysis shutdown and thrombotic complications. In a retrospective study of 52 patients using TEG, Salem and colleagues41 reported a 31% incidence of hypercoagulability, as defined by maximum amplitude and α-angle. Lysis at 30 minutes (LY30) was exceedingly low in all patients with COVID-19, with a median LY30 of 0%, and was significantly lower in patients who experienced thrombotic complications.41
These findings have been corroborated by other groups as well. Collett and colleagues34 identified elevated clot amplitude at 10 minutes and MCF in at least 1 ROTEM pathway, with negligible ML, in all patients with COVID-19.34 In a prospective observational study of 19 ICU patients, Ibañez and colleagues35 identified increased MCF and decreased clot lysis in patients with COVID-19. One study so far identified hypercoagulability in patients with COVID-19 based on elevated MCF and shorter clot formation times; however, they found no difference in ML.53 The addition of exogenous tissue plasminogen activator (tPA) to viscoelastic tests has been shown to aid in identifying patients in fibrinolysis shutdown, and insensitivity to exogenous tPA is associated with a 5-fold higher mortality in trauma patients.54 To date, 3 studies using exogenous tPA in ROTEM samples have demonstrated a lack of sensitivity to tPA among patients with COVID-19.33 , 36 Finally, it appears that these hypercoagulable changes are persistent despite therapeutic anticoagulation. Tsantes and colleagues38 found significantly higher amplitude at 10 minutes and MCF with lower lysis index at 60 minutes in patients with COVID-19 admitted to the ICU on therapeutic anticoagulation compared with ICU patients without COVID-19, non-ICU patients with COVID-19, and healthy controls. Blasi and colleagues39 demonstrated similar results, including significantly elevated MCF and clot lysis time, in a cohort of 23 ICU patients with COVID-19 compared with healthy controls.
To date, 2 studies have focused specifically on the evaluation of fibrinolysis shutdown in patients with COVID-19. Using an EXTEM ML < 3.5% on ROTEM as the definition for fibrinolysis shutdown, Creel-Bulos and colleagues40 identified a 44% incidence of shutdown in a cohort of 25 ICU patients with COVID-19. Fibrinolysis shutdown was associated with a 73% incidence of thrombotic complications, including 7 deep vein thromboses, 3 pulmonary emboli, and 1 MI. Our group identified a 57% incidence of complete fibrinolysis shutdown with LY30 of 0% on TEG in 44 critically ill patients with COVID-19.30 Complete fibrinolysis shutdown predicted VTE with an area under the receiver operator characteristic curve of 0.742. In addition, d-dimer of ≥ 2,600 ng/mL predicted the need for dialysis and the combination of d-dimer ≥ 2,600 ng/mL and LY30 0% was associated with a 50% incidence of VTE. In patients with 0% lysis and D-dimer ≥ 2,600 ng/mL, the rates of requiring hemodialysis and thrombotic stroke were 80% and 30%, respectively.30 These findings were recently corroborated in a cohort of 40 ICU patients with COVID-19 using ROTEM, where combining ML with d-dimer levels predicted VTE risk with high sensitivity and specificity.55 Taken together, the available viscoelastic testing evidence provides compelling support for the existence of fibrinolysis shutdown in patients with COVID-19 and its association with thrombotic complications.
Despite the presence of fibrinolysis shutdown in a significant proportion of patients with COVID-19, bleeding complications, including hemorrhagic stroke56 and gastrointestinal bleeding,57 are not uncommon and deserve special consideration. A systematic review and meta-analysis of hospitalized patients with COVID-19 reported a 7.8% overall pooled incidence of bleeding with 3.9% comprising major bleeding complications.58 Patients on intermediate- or high-dose anticoagulation had the highest pooled incidence of bleeding complications at 21.4%.58 Others have reported similar findings. In a multicenter retrospective review of 400 patients with COVID-19, including 144 ICU patients, Al-Samkari and colleagues59 reported a 2.3% incidence of major bleeding in patients receiving unfractionated heparin or low-molecular-weight heparin at prophylactic doses. In addition to higher bleeding complications, therapeutic anticoagulation has also been shown to increase in-hospital mortality.60 However, because these patients were transitioned to therapeutic anticoagulation based on markers such as elevation in d-dimer, the argument could be made that the use of therapeutic anticoagulation and the subsequent higher observed mortality might be a marker of increased disease severity rather than a direct effect of therapeutic anticoagulation. Other large studies have found the use of anticoagulation to be associated with lower rates of mortality and intubation while maintaining similar rates of bleeding complications.61 Recently, a multiple platform randomized controlled trial spanning 4 continents investigating the effect of therapeutic anticoagulation in patients with COVID-19 suspended enrollment in critically ill patients for futility and likely increased bleeding complications in this subgroup (ClinicalTrials.gov Identifier: NCT04505774).62 This underscores the necessity of thoroughly studying an intervention for benefit, as well as potential risks, even during a pandemic, as treatments that might seem intuitive can in fact be harmful. These diametrically conflicting results emphasize the necessity for monitoring the coagulation status of hospitalized COVID-19 patients.
Bleeding complications in patients with fibrinolysis shutdown have been well-described in other populations.63 Trauma patients in fibrinolysis shutdown with elevated d-dimers have been reported to require more transfusions than patients with low d-dimers.64, 65, 66 As with trauma- and sepsis-induced coagulopathy, the coagulopathy caused by COVID-19 is likely multifactorial, has primary and secondary components, and is affected by timing of infection and resuscitation.67 Although the particular mechanisms behind COVID-19-associated coagulopathy are currently poorly understood, they likely involve many of the same features common to other well-described coagulopathies. A potential mechanism that has not received as much attention in COVID-19 could be related to hyperfibrinogenemia from an acute-phase response, which demonstrates a step-wise increase in prolonged clotting time with reptilase.68 Animal viral disease associated with hyperfibrinogenemia has also been associated with mucosal bleeding and respiratory disease.69 Fibrin formation has a feedback mechanism to reduce thrombin generation.70 In addition, when thrombin is bound to fibrin its activity is modified.71 More recently, it has also been proposed that thrombin can be trapped by fibrin networks,72 and other fibrinogen-binding molecules can impair thrombin generation.73 This interaction is further complicated by the local environment in which cellular factors and the geographic location for thrombin generation can promote bleeding vs clotting.74 Therefore, it is not surprising that patients can manifest with a mixed phenotype of clotting and bleeding at the same time when the coagulation and fibrinolytic systems have been pushed to extremes. Understanding the local environment driving intracranial bleeding and mucosal bleeding in COVID-19 compared with micro- and macrovascular thrombosis is an important future area of research.
Thrombosis and Fibrinolysis: Molecular Mechanisms in COVID-19
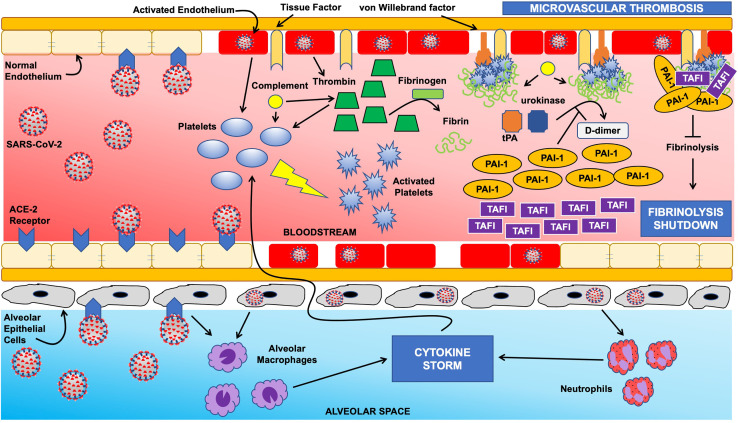
The tightly regulated balance between thrombosis and fibrinolysis is clearly disrupted in COVID-19. This coagulation dysregulation is intimately associated with the host immune response to viral infection. Recent proteomic work has shown a considerable dysregulation in coagulation factor function and increased antifibrinolytic activity as a function of elevated interleukin-6 levels.75 A comprehensive review of the crosstalk between inflammation and coagulation is outside the scope of this review (see Whyte and colleagues37). In this section, we focus on mechanisms of fibrinolytic dysfunction in COVID-19 (Fig. 1 ).
Figure 1.
Schematic of fibrinolysis shutdown mechanisms in COVID-19. The normal homeostasis between coagulation and fibrinolysis is severely disrupted in COVID-19. Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via the angiotensin converting enzyme 2 (ACE-2) receptor on endothelial cells results in activation of the endothelium, which in turn augments platelet activation and thrombin generation. Endothelial injury leads to exposure of tissue factor and von Willebrand factor, initiating the coagulation cascade, with the end result of platelet- and fibrin-rich thrombus on the endothelial surface. These mechanisms are further accentuated by a massive influx of cytokines (ie “cytokine storm”) as a result of alveolar macrophage and neutrophil activation secondary to viral invasion via ACE-2 receptors on alveolar epithelial cells. Normally, endothelial fibrin accumulation is prevented from progressing to microvascular thrombosis via the fibrinolytic agents tissue plasminogen activator (tPA) and urokinase; however, infection with SARS-CoV-2 results in overwhelming levels of plasminogen activator inhibitor 1 (PAI-1) and thrombin activatable fibrinolysis inhibitor (TAFI) with the net result of fibrinolysis shutdown.
ARDS is one of the most dramatic and devastating complications of COVID-19. It is well-known that ARDS from other causes is associated with a local hypercoagulable state within the lungs, leading to abnormal fibrin deposition and microthrombi development as an end result.76, 77, 78, 79 Similar pathologic findings have since been described in deceased patients with COVID-19 and ARDS.80, 81, 82, 83 This hypercoagulable state is mediated, at least in part, by tissue injury and inflammation resulting in increased levels of tissue factor production by alveolar macrophages and epithelial cells,84 leading to thrombin generation and fibrin deposition.37 Another significant mechanism contributing to hypercoagulability in COVID-19 is endothelialitis.85, 86, 87, 88, 89 Postmortem analyses have demonstrated that SARS-CoV-2 infects vascular endothelial cells directly, likely via the angiotensin converting enzyme 2 receptor, resulting in the presence of viral inclusion bodies in the lungs, liver, kidneys, and small intestine.90 , 91 Endothelial invasion by SARS-CoV-2 leads to disruption of the normal protective endothelial glycocalyx, as evidenced by increased shedding of glycocalyx proteins and decreases in the anticoagulant heparanase-2, resulting in a transition from the normally anticoagulant state during homeostasis to a relative prothrombotic state.85 , 92 , 93 In patients with COVID-19, plasmin might actually potentiate this hypercoagulable state by increasing the virulence of the virus,94 resulting in more immune-mediated tissue injury and higher levels of circulating tissue factor and thrombin generation. Clinical studies have corroborated these findings. Bouck and colleagues95 described increased thrombin generation potential and endogenous plasmin potential in patients with COVID-19 compared with those with sepsis from other causes. A separate study measured thrombin generation potential and anti-Xa levels in 48 ICU patients with COVID-19 on anticoagulation and found thrombin generation potential within the reference range, despite elevated anti-Xa levels.33 As the authors point out, this suggests either a hypercoagulable state not affected by heparin therapy or heparin resistance; however, they measured antithrombin levels as well and these were within the reference range, indicating that a hypercoagulable state despite anticoagulation was more likely. High median evoked thrombin potential values have been described in patients with COVID-19 up to 1 week after ICU admission.49
The local hypercoagulable state in the pulmonary alveoli in ARDS is further exacerbated by an impaired fibrinolytic response primarily mediated by overexpression of plasminogen activator inhibitor 1 (PAI-1) from endothelial cells and activated platelets.93 , 96 , 97 We believe these mechanisms lead to a state of fibrinolysis shutdown in these patients, which, coupled with increased thrombin generation, lead to poor outcomes, including ARDS and other markers of microvascular thrombosis in other patient populations.25, 26, 27, 28 Although PAI-1 is likely the most potent antifibrinolytic mediator, data from patients with interstitial lung disease have also identified elevated thrombin activatable fibrinolysis inhibitor (TAFI) and protein C inhibitor (also known as PAI-3) levels in the alveolar space.37 , 98 These findings were also noted during the related SARS-CoV epidemic in 2002.37 Markedly elevated PAI-1 levels have now been reported in patients with COVID-19 as well, with some reports describing levels up to 4-fold higher in patients with COVID-19 compared with controls.39 , 49 This elevation in PAI-1 levels in COVID-19 can be exacerbated by elevated levels of circulating angiotensin II. It is now known that the SARS-CoV-2 virus infects via the angiotensin converting enzyme 2 receptor, resulting in elevated levels of circulating angiotensin II, given saturation of its endogenous receptor. These elevated levels of angiotensin II can subsequently increase stimulation of PAI-1 production by endothelial cells.37 , 99 , 100 In addition to higher PAI-1 levels, Nougier and colleagues33 found markedly elevated circulating tPA and TAFI levels in patients with COVID-19. Despite elevated tPA, these patients were hypofibrinolytic, suggesting that the higher levels of PAI-1 and TAFI likely overwhelm the capabilities of tPA, leading to microvascular fibrin deposition.
In summary, it is clear that the hypercoagulable state seen in patients with COVID-19 is multifactorial and complex. Increased thrombin generation potential mediated by virus-induced tissue injury and expression of tissue factor leads to profound hypercoagulability, which is exacerbated by a significant state of fibrinolysis shutdown, mediated by overexpression of PAI-1 and TAFI. This overexpression overwhelms the local capabilities of tPA and urokinase, despite elevated circulating tPA levels and increased plasmin generation potential.37 , 93 , 95 The balance between coagulation and fibrinolysis is lost in patients with COVID-19. However, knowledge of these mechanisms allows for the development of treatment strategies targeted at this maladaptive response to potentially improve patient outcomes.
Therapeutic Implications of Fibrinolysis Shutdown in COVID-19
As stated previously, there are several potential therapeutic targets within the coagulation system in patients with COVID-19. In fact, there are currently more than 50 studies evaluating various anticoagulant and antifibrinolytic strategies listed on ClinicalTrials.gov. We believe that a regimen with promise is an antifibrinolytic strategy, given the available evidence of fibrinolysis shutdown in this patient population. The use of antifibrinolytics for ARDS is not a new concept. In fact, Hardaway and colleagues101 reported on the use of streptokinase in patients with severe ARDS in 2001. In this phase I clinical trial, they found dramatic improvements in oxygen requirements in patients with severe ARDS without significant bleeding events. Although these findings are promising, the results should be interpreted with caution, given the modest sample size of only 20 patients in a mixed cohort of trauma and sepsis, which explains why the use of antifibrinolytic agents is not currently standard of care in the management of ARDS. However, there have been several experimental models that also suggest a benefit of fibrinolytic therapy in ARDS.102
The use of tPA has since been proposed for patients with severe ARDS from COVID-19.103 We believe the potential benefit in patients with COVID-19 will result from modifying the profound hypercoagulable/hypofibrinolytic response. To date, preliminary results show improvement in PaO2/FiO2 ratios, although these responses have been transient in some, suggesting the potential need for re-dosing, higher dosing, or the addition of anticoagulant therapy in addition to tPA.11 , 104, 105, 106 The study protocol107 has since been modified to include some of these changes and is currently underway. We expect the results to be available in early 2021 (ClinicalTrials.gov Identifier: NCT04357730). Although a significant impact on patient outcomes might be expected108 based on preliminary data, it is again critical to rigorously study these interventions in large randomized trials before implementing them in the clinical realm to ensure benefit and, more importantly, no harm.
Several other potential therapies targeting fibrinolysis and endothelial dysfunction in patients with COVID-19 have been proposed or are currently underway. A complete analysis of the merits of each of these therapies is outside the scope of this review; however, suggested interventions include combination atorvastatin, l-arginine, folic acid, nicorandil, and nebivolol for endothelial dysfunction (ClinicalTrials.gov Identifier: NCT04631536); tranexamic acid (ClinicalTrials.gov Identifiers: NCT04550338, NCT04338074, NCT04338126, and NCT04390217); tissue factor inhibitors (ClinicalTrials.gov Identifier: NCT04655586); and tenectaplase (ClinicalTrials.gov Identifiers: NCT04505592 and NCT04558125).
Conclusions
The COVID-19 pandemic has arguably become the largest public health threat in recent history with profound morbidity and mortality. In addition to severe ARDS, COVID-19 is associated with multiple other hypercoagulable events, including VTE, stroke, MI, and multisystem organ failure. Clinical data have demonstrated a significant hypercoagulable state with fibrinolysis shutdown as a central component, mediated by elevated circulating levels of antifibrinolytic factors like PAI-1 and TAFI. Therapeutic strategies leveraging these findings, including the use of fibrinolytic agents, are currently being investigated and show promise for improving patient outcomes.
Author Contributions
Study conception and design: Meizoso, HB Moore, EE Moore
Acquisition of data: Meizoso, EE Moore
Analysis and interpretation of data: Meizoso, HB Moore, EE Moore
Drafting of manuscript: Meizoso
Critical revision: Meizoso, HB Moore, EE Moore
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: DrsHBMoore and EE Moore have received grant support from Haemonetics and Instrumentation Laboratories; have patents pending related to coagulation and fibrinolysis diagnostics and therapeutic fibrinolytics; and are co-founders with stock options in Thrombo Therapeutics, Inc. Dr EE Moore has received grant support from Stago, Hemosonics, and Diapharma. Dr Meizoso has nothing to declare.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization WHO Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at:
- 8.Johns Hopkins University Coronavirus COVID-19 global cases. https://coronavirus.jhu.edu/map.html Available at:
- 9.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor F.B., Jr., Toh C.H., Hoots W.K., et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 11.Wang J., Hajizadeh N., Moore E.E., et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribes A., Vardon-Bounes F., Memier V., et al. Thromboembolic events and Covid-19. Adv Biol Regul. 2020;77:100735. doi: 10.1016/j.jbior.2020.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loomba R.S., Aggarwal G., Villarreal E.G., et al. Factors associated with deep venous thrombosis in patients infected with coronavirus disease 2019: a meta-analysis. Blood Coagul Fibrinolysis. 2021;32:23–28. doi: 10.1097/MBC.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 14.Fraiman P., Godeiro C., Jr., Moro E., et al. COVID-19 and cerebrovascular diseases: a systematic review and perspectives for stroke management. Front Neurol. 2020;11:574694. doi: 10.3389/fneur.2020.574694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxley T.J., Mocco J., Majidi S., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colling M.E., Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. 2020;25:471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harari R., Bangalore S., Chang E., Shah B. COVID-19 complicated by acute myocardial infarction with extensive thrombus burden and cardiogenic shock. Catheter Cardiovasc Interv. 2020 May 19 doi: 10.1002/ccd.28992. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan K.H., Lim S.L., Damati A., et al. Coronavirus disease 2019 (COVID-19) and ischemic colitis: An under-recognized complication. Am J Emerg Med. 2020;38 doi: 10.1016/j.ajem.2020.05.072. 2758.e1–2758.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Great Lakes Hemophilia Foundation COVID-19 and coagulopathy: frequently asked questions. https://glhf.org/covid-19-and-coagulopathy-frequently-asked-questions/ Available at:
- 23.Thachil J., Juffermans N.P., Ranucci M., et al. ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost. 2020;18:2138–2144. doi: 10.1111/jth.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macfarlane R.G., Biggs R. Fibrinolysis; its mechanism and significance. Blood. 1948;3:1167–1187. [PubMed] [Google Scholar]
- 25.Moore H.B., Moore E.E. Temporal changes in fibrinolysis following injury. Semin Thromb Hemost. 2020;46:189–198. doi: 10.1055/s-0039-1701016. [DOI] [PubMed] [Google Scholar]
- 26.Moore H.B., Moore E.E., Gonzalez E., et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–817. doi: 10.1097/TA.0000000000000341. discussion 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore H.B., Moore E.E., Liras I.N., et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222:347–355. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meizoso J.P., Karcutskie C.A., Ray J.J., et al. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg. 2017;224:575–582. doi: 10.1016/j.jamcollsurg.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt F.C.F., Manolov V., Morgenstern J., et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9:19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright F.L., Vogler T.O., Moore E.E., et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193–203 e1. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavoni V., Gianesello L., Pazzi M., et al. Derangement of the coagulation process using subclinical markers and viscoelastic measurements in critically ill patients with coronavirus disease 2019 pneumonia and non-coronavirus disease 2019 pneumonia. Blood Coagul Fibrinolysis. 2021;32:80–86. doi: 10.1097/MBC.0000000000000971. [DOI] [PubMed] [Google Scholar]
- 32.Pavoni V., Gianesello L., Pazzi M., et al. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50:281–286. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nougier C., Benoit R., Simon M., et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost. 2020;18:2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collett L.W., Gluck S., Strickland R.M., Reddi B.J. Evaluation of coagulation status using viscoelastic testing in intensive care patients with coronavirus disease 2019 (COVID-19): an observational point prevalence cohort study. Aust Crit Care. 2021;34:155–159. doi: 10.1016/j.aucc.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibañez C., Perdomo J., Calvo A., et al. High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? J Thromb Thrombolysis. 2021;51:308–312. doi: 10.1007/s11239-020-02226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss E., Roux O., Moyer J.D., et al. Fibrinolysis resistance: a potential mechanism underlying COVID-19 coagulopathy. Thromb Haemost. 2020;120:1343–1345. doi: 10.1055/s-0040-1713637. [DOI] [PubMed] [Google Scholar]
- 37.Whyte C.S., Morrow G.B., Mitchell J.L., et al. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsantes A.E., Frantzeskaki F., Tsantes A.G., et al. The haemostatic profile in critically ill COVID-19 patients receiving therapeutic anticoagulant therapy: an observational study. Medicine (Baltimore) 2020;99(47) doi: 10.1097/MD.0000000000023365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blasi A., von Meijenfeldt F.A., Adelmeijer J., et al. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J Thromb Haemost. 2020;18:2646–2653. doi: 10.1111/jth.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Creel-Bulos C., Auld S.C., Caridi-Scheible M., et al. Fibrinolysis shutdown and thrombosis in a COVID-19 ICU. Shock. 2021;55:316–320. doi: 10.1097/SHK.0000000000001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem N., Atallah B., El Nekidy W.S., et al. Thromboelastography findings in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020 Oct 4:1–5. doi: 10.1007/s11239-020-02300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hightower S., Ellis H., Collen J., et al. Correlation of indirect markers of hypercoagulability with thromboelastography in severe coronavirus 2019. Thromb Res. 2020;195:69–71. doi: 10.1016/j.thromres.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranucci M., Sitzia C., Baryshnikova E., et al. Covid-19-associated coagulopathy: biomarkers of thrombin generation and fibrinolysis leading the outcome. J Clin Med. 2020;9:3487. doi: 10.3390/jcm9113487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nahum J., Morichau-Beauchant T., Daviaud F., et al. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima W.G., Barra A., Brito J.C.M., Nizer W.S.C. D-dimer serum levels as a biomarker associated for the lethality in patients with coronavirus disease 2019: a meta-analysis. Blood Coagul Fibrinolysis. 2020;31:335–338. doi: 10.1097/MBC.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 49.Hardy M., Michaux I., Lessire S., et al. Prothrombotic disturbances of hemostasis of patients with severe COVID-19: a prospective longitudinal observational study. Thromb Res. 2020;197:20–23. doi: 10.1016/j.thromres.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keragala C.B., Medcalf R.L., Myles P.S. Fibrinolysis and COVID-19: a tale of two sites? J Thromb Haemost. 2020;18:2430–2432. doi: 10.1111/jth.15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adam S.S., Key N.S., Greenberg C.S. D-dimer antigen: current concepts and future prospects. Blood. 2009;113:2878–2887. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- 52.Creel-Bulos C., Liu M., Auld S.C., et al. Trends and diagnostic value of D-dimer levels in patients hospitalized with coronavirus disease 2019. Medicine (Baltimore) 2020;99(46) doi: 10.1097/MD.0000000000023186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spiezia L., Boscolo A., Poletto F., et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore H.B., Moore E.E., Huebner B.R., et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg. 2017;83:1014–1022. doi: 10.1097/TA.0000000000001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruse J.M., Magomedov A., Kurreck A., et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. 2020;24:676. doi: 10.1186/s13054-020-03401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dogra S., Jain R., Cao M., et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mauro A., De Grazia F., Lenti M.V., et al. Upper gastrointestinal bleeding in COVID-19 inpatients: Incidence and management in a multicenter experience from Northern Italy. Clin Res Hepatol Gastroenterol. 2020 Aug 14 doi: 10.1016/j.clinre.2020.07.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiménez D., Garcia-Sanchez A., Rali P., et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. 2021;159:1182–1196. doi: 10.1016/j.chest.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Musoke N., Lo K.B., Albano J., et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. 2020;196:227–230. doi: 10.1016/j.thromres.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nadkarni G.N., Lala A., Bagiella E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Institutes of Health. NIH ACTIV trial of blood thinners pauses enrollment of critically ill COVID-19 patients. Available at: https://www.nih.gov/news-events/news-releases/nih-activ-trial-blood-thinners-pauses-enrollment-critically-ill-covid-19-patients. Accessed Febuary 9, 2021.
- 63.Moore H.B., Moore E.E., Neal M.D., et al. Fibrinolysis shutdown in trauma: historical review and clinical implications. Anesth Analg. 2019;129:762–773. doi: 10.1213/ANE.0000000000004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gall L.S., Vulliamy P., Gillespie S., et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg. 2019;269:1184–1191. doi: 10.1097/SLA.0000000000002733. [DOI] [PubMed] [Google Scholar]
- 65.Cardenas J.C., Wade C.E., Cotton B.A., et al. TEG lysis shutdown represents coagulopathy in bleeding trauma patients: analysis of the PROPPR cohort. Shock. 2019;51:273–283. doi: 10.1097/SHK.0000000000001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomez-Builes J.C., Acuna S.A., Nascimento B., et al. Harmful or physiologic: diagnosing fibrinolysis shutdown in a trauma cohort with rotational thromboelastometry. Anesth Analg. 2018;127:840–849. doi: 10.1213/ANE.0000000000003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore H.B., Gando S., Iba T., et al. Defining trauma-induced coagulopathy with respect to future implications for patient management: communication from the SSC of the ISTH. J Thromb Haemost. 2020;18:740–747. doi: 10.1111/jth.14690. [DOI] [PubMed] [Google Scholar]
- 68.Van Cott E.M., Smith E.Y., Galanakis D.K. Elevated fibrinogen in an acute phase reaction prolongs the reptilase time but typically not the thrombin time. Am J Clin Pathol. 2002;118:263–268. doi: 10.1309/WUB3-72JT-E50M-EU8J. [DOI] [PubMed] [Google Scholar]
- 69.Braun U., Thur B., Weiss M., Giger T. [Bovine virus diarrhea/mucosal disease in cattle--clinical findings in 103 calves and cattle] Schweiz Arch Tierheilkd. 1996;138:465–475. [PubMed] [Google Scholar]
- 70.Naski M.C., Shafer J.A. Alpha-thrombin within fibrin clots: inactivation of thrombin by antithrombin-III. Thromb Res. 1993;69:453–465. doi: 10.1016/0049-3848(93)90234-f. [DOI] [PubMed] [Google Scholar]
- 71.Weitz J.I., Hudoba M., Massel D., et al. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelley M., Leiderman K. A Mathematical model of bivalent binding suggests physical trapping of thrombin within fibrin fibers. Biophys J. 2019;117:1442–1455. doi: 10.1016/j.bpj.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vu T.T., Stafford A.R., Leslie B.A., et al. Histidine-rich glycoprotein binds fibrin(ogen) with high affinity and competes with thrombin for binding to the gamma'-chain. J Biol Chem. 2011;286:30314–30323. doi: 10.1074/jbc.M111.253831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolberg A.S., Campbell R.A. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apher Sci. 2008;38:15–23. doi: 10.1016/j.transci.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D'Alessandro A., Thomas T., Dzieciatkowska M., et al. Serum proteomics in COVID-19 patients: altered coagulation and complement status as a function of IL-6 level. J Proteome Res. 2020;19:4417–4427. doi: 10.1021/acs.jproteome.0c00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bone R.C., Francis P.B., Pierce A.K. Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med. 1976;61:585–589. doi: 10.1016/0002-9343(76)90135-2. [DOI] [PubMed] [Google Scholar]
- 77.Ware L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 78.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 79.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 80.Tian S., Hu W., Niu L., et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carsana L., Sonzogni A., Nasr A., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rapkiewicz A.V., Mai X., Carsons S.E., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bastarache J.A., Wang L., Geiser T., et al. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax. 2007;62:608–616. doi: 10.1136/thx.2006.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mosleh W., Chen K., Pfau S.E., Vashist A. Endotheliitis and endothelial dysfunction in patients with COVID-19: Its role in thrombosis and adverse outcomes. J Clin Med. 2020;9:1862. doi: 10.3390/jcm9061862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24:353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vrints C.J.M., Krychtiuk K.A., Van Craenenbroeck E.M., et al. Endothelialitis plays a central role in the pathophysiology of severe COVID-19 and its cardiovascular complications. Acta Cardiol. 2020 Nov 19 doi: 10.1080/00015385.2020.1846921. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J., Tecson K.M., McCullough P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. 2020;21:315–319. doi: 10.31083/j.rcm.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 90.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stahl K., Gronski P.A., Kiyan Y., et al. Injury to the endothelial glycocalyx in critically ill patients with COVID-19. Am J Respir Crit Care Med. 2020;202:1178–1181. doi: 10.1164/rccm.202007-2676LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwaan H.C., Lindholm P.F. The central role of fibrinolytic response in COVID-19—a hematologist's perspective. Int J Mol Sci. 2021;22:1283. doi: 10.3390/ijms22031283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100:1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bouck E.G., Denorme F., Holle L.A., et al. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol. 2021;41:401–414. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grau G.E., de Moerloose P., Bulla O., et al. Haemostatic properties of human pulmonary and cerebral microvascular endothelial cells. Thromb Haemost. 1997;77:585–590. [PubMed] [Google Scholar]
- 97.MacLaren R., Stringer K.A. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy. 2007;27:860–873. doi: 10.1592/phco.27.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fujimoto H., Gabazza E.C., Hataji O., et al. Thrombin-activatable fibrinolysis inhibitor and protein C inhibitor in interstitial lung disease. Am J Respir Crit Care Med. 2003;167:1687–1694. doi: 10.1164/rccm.200208-905OC. [DOI] [PubMed] [Google Scholar]
- 99.Coccheri S. COVID-19: the crucial role of blood coagulation and fibrinolysis. Intern Emerg Med. 2020;15:1369–1373. doi: 10.1007/s11739-020-02443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaughan D.E., Lazos S.A., Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardaway R.M., Harke H., Tyroch A.H., et al. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67:377–382. [PubMed] [Google Scholar]
- 102.Liu C., Ma Y., Su Z., et al. Meta-analysis of preclinical studies of fibrinolytic therapy for acute lung injury. Front Immunol. 2018;9:1898. doi: 10.3389/fimmu.2018.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moore H.B., Barrett C.D., Moore E.E., et al. Is there a role for tissue plasminogen activator (tPA) as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome (ARDS)? J Trauma Acute Care Surg. 2020;88(6):1–2. doi: 10.1097/TA.0000000000002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barrett C.D., Oren-Grinberg A., Chao E., et al. Rescue therapy for severe COVID-19-associated acute respiratory distress syndrome with tissue plasminogen activator: a case series. J Trauma Acute Care Surg. 2020;89:453–457. doi: 10.1097/TA.0000000000002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poor H.D., Ventetuolo C.E., Tolbert T., et al. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Clin Transl Med. 2020;10(2):e44. doi: 10.1002/ctm2.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goyal A., Saigal S., Niwariya Y., et al. Successful use of tPA for thrombolysis in COVID related ARDS: a case series. J Thromb Thrombolysis. 2021;51:293–296. doi: 10.1007/s11239-020-02208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moore H.B., Barrett C.D., Moore E.E., et al. STudy of Alteplase for Respiratory failure in SARS-Cov2/COVID-19: study design of the phase IIa STARS trial. Res Pract Thromb Haemost. 2020;4:984–996. doi: 10.1002/rth2.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choudhury R., Barrett C.D., Moore H.B., et al. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: a Markov decision analysis. World J Emerg Surg. 2020;15:29. doi: 10.1186/s13017-020-00305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]