Abstract
Background
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is associated with a high fatality rate (34%), which is higher in the presence of co-morbidities. The aim of the current study was to assess the clinical course and the outcome in hematological or oncological malignancy cases, diagnosed with MERS-CoV.
Methods
This is a case series of hematological /oncological cases, diagnosed with MERS-CoV, in a tertiary care setting in 2015. The cases were identified based on the World Health Organization (WHO) MERS-CoV case definition. The demographic, clinical, and outcome data were retrieved from the patients’ medical charts and electronic health records.
Results
In total, nine hematological or oncological cases were identified, diagnosed with MERS-CoV. The baseline malignant condition was hematological malignancy in seven patients, as well as colon cancer and osteosarcoma in one patient each. Six (67%) patients were male. The median age was 65 years (range 16–80 years). Co-morbidities included chronic kidney disease (n = 3.33%), diabetes mellitus (n = 3.33%), and hypertension (n = 2.22%). The presenting symptoms were shortness of breath (n = 6.66%), fever (n = 5.55%), cough (n = 2.22%), and diarrhea (n = 2.22%). Chest x-rays indicated bilateral infiltrates in 6 patients (66%). The PCR (polymerase chain reaction) test was repeated in six patients to confirm the diagnosis. The mortality rate was 100%, and the median time to death was 26 days (range 15–77 days).
Conclusion
MERS-CoV infection in this small cohort of hematology or oncology patients has a 100% mortality rate, regardless of the status of the underlying disease. The confirmation of the diagnosis may require repeated testing. Additional studies are required to verify the findings and to elucidate the disease pathogenesis in cancer patients.
Abbreviations: SARS, Acute Respiratory Distress Syndrome; MERS-CoV, Middle East Respiratory Syndrome Coronavirus; WHO, World Health Organization; SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; RT-PCR, Reverse Transcriptase Polymerase Chain Reaction; WBC, White Blood Cells
Keywords: Middle East Respiratory Syndrome, Infection, Malignancy, Mortality
Introduction
Middle East Respiratory Syndrome Coronavirus (MERS-CoV) is a β-coronavirus [1]. The first case was reported in Saudi Arabia in September 2012, and the virus spread to other Arab countries, including the United Arab Emirates, Jordan, Kuwait, Yemen, Lebanon, Oman, and Qatar [2]. According to the 2019 World Health Organization (WHO) report, 2494 laboratory-confirmed cases have been identified in 27 countries. The fatality rate is high in MERS-CoV cases (n = 858, 34.4%) [3]. The majority of the cases occurred in Saudi Arabia, with a case fatality rate of 37.1% [4]. The majority (75%) of the cases diagnosed with MERS-CoV had at least one co-morbidity [5].
Several countries reported MERS-CoV spreading through zoonotic transmission from dromedary camels [[6], [7], [8]]. The clinical presentation is comparable with Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) [3]. The symptoms in mild cases are low-grade fever, runny nose, sore throat, and aching muscles. Severe cases progress to acute respiratory distress syndrome [9,10]. In Korea, the MERS-CoV cases presented atypically [11]. The hospital outbreaks were due to human-to-human transmission [5].
The MERS-CoV diagnosis is confirmed with a Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) assay of a respiratory specimen [10,11]. Immunocompromised individuals are prone to MERS-CoV infection, with a higher mortality rate [5]. The current paper describes the clinical course and the outcomes of nine hematological or oncological cases diagnosed with MERS-CoV, admitted to the intensive care unit (ICU) in a tertiary care hospital in Saudi Arabia.
Methods
In total, 130 patients were diagnosed with MERS-CoV in June–September 2015, hospital outbreak [12]. The design of the study was a retrospective case series analysis of nine hematology or oncology patients diagnosed with MERS-CoV in the year 2015. The study was conducted at a tertiary care hospital in, Riyadh, Saudi Arabia. Ethical approval was obtained from the Institutional Review Board (IRB), with the approval number (RC16/095).
MERS-CoV case definition
The cases were identified based on a prior diagnosis of a hematological or solid organ neoplasm, with a confirmed MERS-CoV infection. The patients presented with acute respiratory illness, a thoracic image suggestive of pneumonia and acute respiratory distress syndrome (SARS), and a confirmation done with RT-PCR assays targeting upstream of the E gene and the open-reading frame gene 1a.
A case was considered confirmed MERS-CoV if the laboratory confirmation of the RT-PCR was available. A probable case had a febrile respiratory illness with clinical, radiological, or histopathological signs, but an inconclusive RT-PCR test [13]. In the case of a negative result, the test was repeated by the treating physician.
The patients were followed-up from admission until death. The data included patient demographic characteristics, co-morbidities, underlying diagnosis, history of contact with a MERS-CoV patient, presenting symptoms, exposure to camel urine or milk, laboratory, RT-PCR, length of hospital or ICU stay, radiological data, and the outcome. The relevant data were extracted from the electronic medical records. The laboratory data were assessed at multiple time points (day 1, 7, 14, 21, 28). A white blood cell (WBC) count lower than 4.0 × 109/L was considered as leukopenia and a platelet count less than 140 × 109 as thrombocytopenia. The liver enzymes were deemed elevated if they were more than twice the range of the upper reference limit.
The variables gender, primary disease diagnosis, co-morbidities, disease stage, presenting symptoms, chest x-ray findings, and type of specimen are displayed as frequency and percentage. The age, length of stay in the ward, and time to death are summarized as median and range. The repeated samples and the clinical course of the disease are displayed in figures. The descriptive analysis were done with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Cohort characteristics
In total, in 2015 nine hematological or oncological patients had a confirmed diagnosis of MERS-CoV. All patients were exposed to the hospital environment during the 2015 hospital outbreak, and only one had a history of exposure to camels.
The majority of the patients (7/9) had a hematological malignancy, including chronic lymphocytic leukemia (CLL, n = 2), acute lymphocytic leukemia (B-ALL, n = 1), T cell ALL (n = 1), cutaneous T-cell lymphoma (n = 1), multiple myeloma (MM, n = 1), and primary myelofibrosis (PMF, n = 1). One patient was diagnosed with colon cancer and one with osteosarcoma. Six (67%) patients were male and the median age was 65 years, with the range 16–80 years. Half of the cohort (55%) was in a refractory disease stage when admitted. Four (44%) patients had no comorbid condition. Three (33%) patients had chronic kidney disease, three diabetes mellitus (33%), two hypertension (22%), and one was dialyzed. One patient had an allogeneic hematopoietic stem cell transplantation (HSCT) (Table 1 ).
Table 1.
Cohort characteristics.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Age in years | 16 | 27 | 33 | 41 | 65 | 75 | 77 | 78 | 80 |
| Gender | Male | Female | Male | Male | Male | Male | Female | Female | Male |
| Primary Disease | T cell -ALL | B-ALL | Cutaneous T-cell lymphoma | Metastatic Osteosarcoma Right fibula | MF | Recto-sigmoidal carcinoma | CLL | CLL | MM |
| Primary Disease Status | Relapse | Relapse | Refractory | – | Active | Refractory | Refractory | Refractory | Refractory |
| Immunosuppressants | FLAG | FLAG Clofarabine/Cyclophosphamide with Etoposide | CHOP GDP Hyper-CVAD part B |
Methotrexate | Ruxolitinib | Xeloda and Oxaliplatin | Ibrutinib | None | Carfilzomib and Dexamethasone |
| Co-morbidities | None | None | None | CKD | DM, CKD | None | DM, HTN | DM, HTN, Atrial fibrillation | IHD, CKD |
| Exposure to Camel milk/urine | No | No | No | No | No | No | No | Yes | No |
| HSCT | Allogeneic HSCT | No | No | – | No | – | No | No | No |
| Clinical Presentation | Fever, SOB | Fever, SOB | Fever, cough | SOB | Fever, cough, SOB | SOB, bloody diarrhea | Fever, diarrhea | Cough, fever | SOB, hemoptysis |
| Other Pathogens | None | None | Gram negative bacteremia | None | None | Enterococcus | None | None | None |
| Chest x-ray | Left lower lobe consolidation | Bilateral infiltrates | Bilateral infiltrates | Bilateral infiltrates and pleural effusion | Bilateral interstitial opacities | Left lower lobe consolidation /pleural effusion | Bilateral infiltrates | Right side consolidation /pleural effusion | Bilateral infiltrates |
| Ventilation | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Outcome | Died | Died | Died | Died | Died | Died | Died | Died | Died |
| Days post ICU admission | 7 | 6 | 6 | 23 | 6 | 19 | 7 | 1 | 19 |
Abbreviations: MF, Myelofibrosis; MM, Multiple Myeloma; CLL, Chronic lymphocytic leukemia; ALL, Acute lymphoblastic leukemia; HSCT, Hematopoietic stem cell transplantation; CKD, chronic kidney disease; IHD, ischemic heart disease; DM, Diabetes mellitus; HTN, Hypertension; SOB, Shortness of Breath; ICU, intensive care unit.
Clinical presentation
The lower respiratory tract was primarily involved, with the most prevalent presenting symptoms shortness of breath (n = 6.66%) and fever (n = 5. 55%). Only 2 patients (22%) coughed or had flu, and diarrhea was observed in 2(22%) (Table 1). The chest x-ray demonstrated bilateral infiltrates in 6 patients (67%) (Table 2 ).
Table 2.
Study Cohort Follow-up.
| Variables | n = 9 |
|---|---|
| Chest X-ray findings n (%)a | |
| Bilateral infiltrates | 6 (66.6) |
| Pleural effusion | 3 (33.3) |
| Consolidation | 2 (22.2) |
| Length of stay in ward (days) (median, range) | 16 (1–70) |
| Days stayed in ICU (median, range) | 7 (6–19) |
| Time taken to death (days) (median, range) | 26 (15–77) |
| Community exposure to infected person | 1 (11) |
| In-hospital during an outbreakb | 9 (100) |
| Type of specimen with positive PCR n (%) | |
| Tracheal Aspirate | 6 (67) |
| Nasopharyngeal swab | 2 (22) |
| Sputum | 1 (11) |
not mutually exclusive.
Referring to the outbreak period of mid-June to mid-September 2015.
Laboratory data
The laboratory parameters are summarized in Table 3 . The median WBC count reduced from 12.75 × 109/L on day 1 to 5.50 × 109/L on day 28. The median hemoglobin reduced from 99.5 gm/L on day 1 to 69 g m/L on day 28. The median neutrophil count increased from 16% on day 1 to62.5% on day 28.
Table 3.
Laboratory Results of the Study Cohort Overtime.
| Laboratory Parameters (median, range) | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| WBC (4.00–11.00 × 109/L) | 11 (4.7–31.3) | 7.95 (0.10–25.2) | 6.6-(0–25.3) | 5.45 (0.2−16.7) | 5.50 (0.40–24.9) |
| Neutrophils (2.00–7.50 × 109/L) | 19 (4.0−56.0) | 54.0 (5.0−54.0) | 59.5 (2.0−86) | 27.5 (8.0−47) | 62.5 (36−86) |
| Hemoglobin (120–160 gm/L) | 99.5 (68−157) | 82.5 (71−142) | 83 (72−120) | 72 (54−87) | 69 (63−102) |
| Platelet (150–400 × 109/L) | 52.0 (7.0−256) | 79.5 (14−168) | 46.0 (11−261) | 57.5 (2.0−378) | 74.0 (10.0−335) |
| AST (5–34 U/L) | 18.5 (10.0−77.0) | 34.0 (9.0−50.0) | 41.0 (8.0−65.0) | 33.0 (22.0−41.0) | 43.0 (5.0−246.0) |
| ALT (5–55 U/L) | 14.0 (9.0−240.0) | 19.0 (6.0−179.0) | 27.5 (17.0−139) | 26.0 (24.0−32.0) | 20.0 (6.0−32.0) |
| Alkaline phosphatase (40−15 U/L) | 114.5 (43−397) | 86.0 (81−307) | 183 (111−398) | 86 (86−320) | 119.5 (86−535) |
| Total Bilirubin (3.4–20.5 umol/L) | 8.8 (5.0−53.4) | 13.3 (9.8−37.2) | 14.5 (9.2−50.45) | 14.9 (8.9−17.9) | 18.05 (9.0−89.2) |
| PT (9.38–12.34 s) | 11.5 (10.7−16.4) | 11.4 (10.0−17.4) | 11.9 (9.40−41.0) | 14.1 (10.3−15.2) | 13.9 (10.9−16.7) |
| PTT (24.84–32.96 s) | 33.05 (21.2−39.9) | 29.2 (26.3−43.9) | 35.2 (22.2−69.2) | 35.0 (24.5−60.5) | 35.4 (24.4−50.5) |
| INR (0.80–1.20) | 1.06 (0.98−1.50) | 1.05 (0.92−1.60) | 1.09 (0.87−3.76) | 1.29 (0.95−1.39) | 1.28 (1.0−1.53) |
| Creatinine (50–98 mol/L) | 71 (37−156) | 52 (33−541) | 80 (30−322) | 51 (36−233) | 49 (38−191) |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; PT: prothrombin time; PTT: partial thromboplastin time; INR: international normalized ratio.
Treatment course
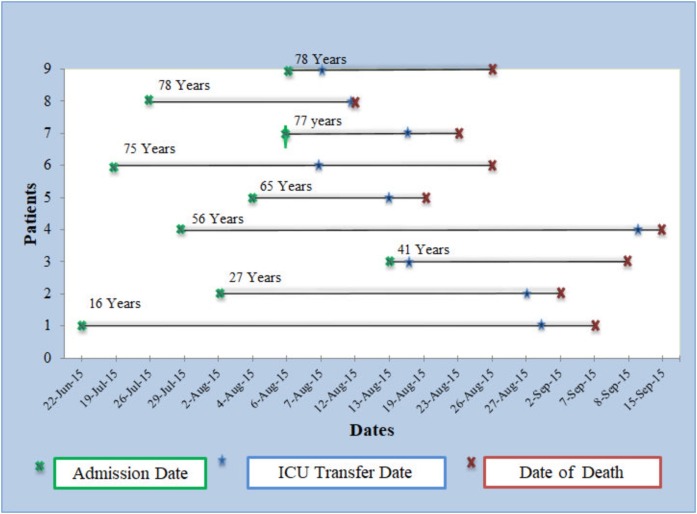
The patients’ disease course is presented in Fig. 1 . The length of stay in the ward was 16 days (range 1–70 days). The group of patients younger than 50 years stayed in the hospital longer than the group 50 years and older. The stay in ICU was shorter than the stay in the ward (Table 2). All patients required ICU admission and mechanical ventilation soon after the symptoms appeared. The management was mainly supportive, and antibiotics were used for bacterial co-infections, when required.
Fig. 1.
Patients’ disease course.
RT-PCR testing
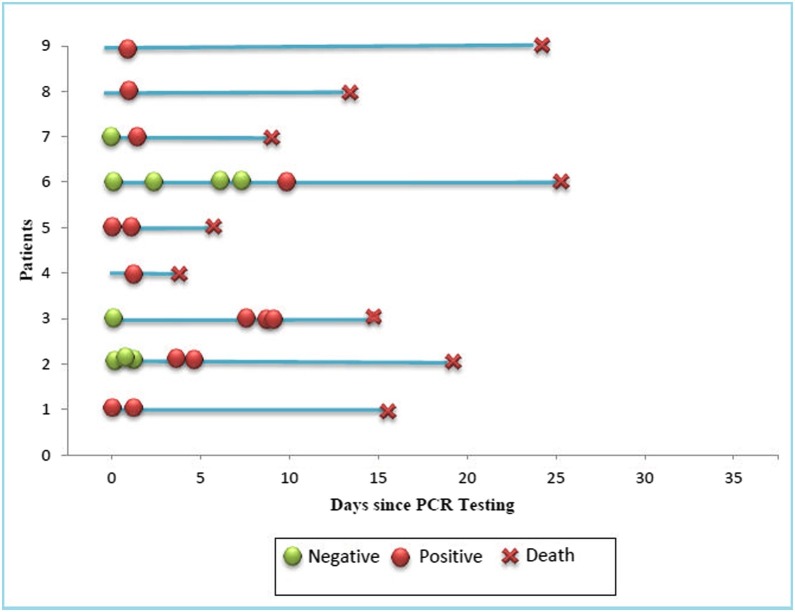
The specimen was primarily taken from tracheal aspirate (n = 6. 67%) (Table 2). The diagnosis was confirmed through RT-PCR testing (Fig. 2 ). Six patients had repeated RT-PCR testing to obtain a positive result.
Fig. 2.
Sequential RT-PCR results.
Outcome data
Two patients had central line related bacteremia, one gram-negative, and one enterococcus. The mortality rate was 100%. The median time to death was 26 days (range 15–77 days) (Table 3). The 30-day mortality was 55%.
Discussion
According to the 2018 WHO global summary and assessment of risk report, Saudi Arabia reported the majority of the MERS-CoV cases [14]. In this paper, we report nine hematological or oncological cases diagnosed with MERS-CoV in Saudi Arabia. All the patients presented with respiratory tract symptoms, including shortness of breath and cough, and some also had diarrhea [10,15,16]. A meta-analysis indicated that cough, fever, and shortness of breath were the most prevalent symptoms in patients diagnosed with MERS-CoV [17]. Multiple risk factors of acquiring MERS-CoV have been reported, including exposure to dromedary camels, diabetes mellitus, and cardiovascular disease [18]. In the current case series, only one patient was exposed to camels. Five patients had one co-morbidity, including diabetes mellitus, ischemic heart disease, or chronic kidney disease [14]. A systematic review reported that 50% of the patients diagnosed with MERS-CoV were diagnosed with diabetes and hypertension, and 30% with cardiac disease [17].
The mode of testing, with blood or body fluids, is important for the diagnosis. In the early phase, nucleic acid testing is diagnostic, however, antibodies are only detectable later in the disease course [19]. The diagnosis of MERS-CoV infection mainly depends on an RT-PCR assay of a respiratory specimen [20]. A RT-PCR was conducted for all the patients in the case series as it is the gold standard diagnostic test [21]. The samples were repeated at several time points based on the clinical judgement of the treating physician. The WHO recommends testing with upper and lower respiratory tract specimens. In our sample, a tracheal aspirate was done in 67%, and a nasopharyngeal swab in 22% [22].
In total, 130 cases were diagnosed with MERS-CoV during June–September 2015 hospital outbreak. Out of 130, 61(47%) had hospital acquired, while 26(20%) had community acquired MERS-CoV. The fatality rate was higher in hospital acquired infection 40/61(65.5%) compared with community acquired infection 11/26(42.3%) [12]. In the current case series, the overall mortality was 100%, however, the 30-day mortality was 55%, which is higher than reported by Ahmed et al., 28.3% in 660 MERS-CoV cases [23]. As reported, the mortality rate is higher in elderly patients (45.2%), compared to patients younger than 60 years (20%) [[23], [24], [25], [26], [27]]. In the current case series, the group aged 60–80 years died, on average, within 21 days (range 15–38 days). The mortality in hematological malignancy cases diagnosed with coronavirus disease 2019 (COVID-19) is 37% (198/536) [28]. However, in a recent case series with eleven HSCT patients, diagnosed with COVID-19, none required mechanical ventilation and there was no mortality [29]. In the current case series, only one patient had HSCT.
Clinical trials are ongoing to identify the optimal therapeutic management for MERS-CoV [30]. Human vaccine development remains a major challenge, but various vaccines against MERS-CoV have been developed and are being tested in clinical trials [31,32].
According to literature, MERS-CoV outbreaks were due to a lack of awareness of the infection, overcrowding, lack of isolation rooms, and inadequate infection control measures [33]. Infection control and prevention became more challenging with the COVID-19 pandemic.
Limitations
This case series is specific to MERS-CoV infection in hematology or oncology patients. The small sample size is a major limitation of the study, preventing the stratification of individuals between different clinical profiles, and precluding statistical analysis. The clinical findings cannot be generalized to patients diagnosed with malignancy and MERS-CoV. In addition, the retrospective nature of the data and the dependence on documentation for assessing the clinical presentation and outcomes, is a limitation of the study.
Conclusion
In conclusion, patients with MERS-CoV have high mortality rate in general. Patients with comorbidities are at a greater risk of mortality when developing MERS-CoV. In particular, we have observed a 100% mortality rate in hematology or oncology patients diagnosed with MERS-CoV, regardless of age and the underlying disease status. The clinical presentation is not distinctive and the confirmation of the diagnosis may require several respiratory samples. Additional studies are required to verify the findings and to elucidate the disease pathogenesis in cancer patients.
Authors’ contributions
AA: conceived the idea, designed the study, reviewed results, and the manuscript.
NAS: descriptive analysis, reviewed the results, created the tables and figures and drafted the manuscript.
HR, MA and MAM: wrote the study proposal, developed the data collection form, and collected the data.
MB, HS, KA, GG, MD, BA, MAZ, AO, and AAH: reviewed the manuscript.
Funding
No funding sources.
Competing interests
None declared.
Ethics approval and consent to participate
The study was approved by King Abdullah International Medical Research Center (KAIMRC), Institutional Review Board (RC16/095).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
None.
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Mustafa S., Balkhy H., Gabere M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J Infect Public Health. 2018;11:9–17. doi: 10.1016/j.jiph.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten C., Seilmaier M., Corman V.M., Hartmann W., Scheible G., Sack S. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis. 2013;13:745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . 2019. Middle East respiratory syndrome coronavirus (MERS-CoV) Update. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowotny N., Kolodziejek J. Middle East respiratory syndrome coronavirus (MERS-CoV) in dromedary camels, Oman, 2013. Eurosurveillance. 2014;19 doi: 10.2807/1560-7917.ES2014.19.16.20781. [DOI] [PubMed] [Google Scholar]
- 7.Hemida M.G., Elmoslemany A., Al-Hizab F., Alnaeem A., Almathen F., Faye B. Dromedary camels and the transmission of middle east respiratory syndrome coronavirus (MERS-CoV) Transbound Emerg Dis. 2017;64:344–353. doi: 10.1111/tbed.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiambi S., Corman V.M., Sitawa R., Githinji J., Ngoci J., Ozomata A.S. Detection of distinct MERS-Coronavirus strains in dromedary camels from Kenya, 2017. Emerg Microbes Infect. 2018;7:195. doi: 10.1038/s41426-018-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumla A., Hui D.S., Perlman S. Middle east respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K. Middle east respiratory syndrome. N Engl J Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.-H., Ko J.-H., Park G.E., Cho S.Y., Ha Y.E., Kang J.-M. Atypical presentations of MERS-CoV infection in immunocompromised hosts. J Infect Chemother. 2017;23:769–773. doi: 10.1016/j.jiac.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alenazi T.H., Al Arbash H., El-Saed A., Alshamrani M.M., Baffoe-Bonnie H., Arabi Y.M. Identified transmission dynamics of middle east respiratory syndrome coronavirus infection during an outbreak: implications of an overcrowded emergency department. Clin Infect Dis. 2017;65:675–679. doi: 10.1093/cid/cix352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . 2013. Global alert and response. Revised interim case definition for reporting to WHO—middle East respiratory syndrome coronavirus (MERS-CoV) [Google Scholar]
- 14.WHO MERS Global Summary and Assessment of Risk. 2018.
- 15.Garbati M.A., Fagbo S.F., Fang V.J., Skakni L., Joseph M., Wani T.A. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to MERS coronavirus or other causes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabi Y.M., Arifi A.A., Balkhy H.H., Najm H., Aldawood A.S., Ghabashi A. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 17.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sikkema R.S., Farag E.A.B.A., Himatt S., Ibrahim A.K., Al-Romaihi H., Al-Marri S.A. Risk factors for primary middle east respiratory syndrome coronavirus infection in camel workers in Qatar during 2013-2014: a case-control study. J Infect Dis. 2017;215:1702–1705. doi: 10.1093/infdis/jix174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niedrig M., Patel P., El Wahed A.A., Schädler R., Yactayo S. Find the right sample: a study on the versatility of saliva and urine samples for the diagnosis of emerging viruses. BMC Infect Dis. 2018;18:707. doi: 10.1186/s12879-018-3611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corman V.M., Eckerle I., Bleicker T., Zaki A., Landt O., Eschbach-Bludau M. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Eurosurveillance. 2012:17. doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- 21.Al Johani S., Hajeer A.H. MERS-CoV diagnosis: an update. J Infect Public Health. 2016;9:216–219. doi: 10.1016/j.jiph.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laboratory Testing for Middle East Respiratory Syndrome Coronavirus: Interim Guidance. 2018.
- 23.Ahmed A.E. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis. 2017;17:615. doi: 10.1186/s12879-017-2712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almekhlafi G.A., Albarrak M.M., Mandourah Y., Hassan S., Alwan A., Abudayah A. Presentation and outcome of Middle East respiratory syndrome in Saudi intensive care unit patients. Crit Care. 2016;20:123. doi: 10.1186/s13054-016-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feikin D.R., Alraddadi B., Qutub M., Shabouni O., Curns A., Oboho I.K. Association of higher MERS-CoV virus load with severe disease and death, Saudi Arabia. Emerg Infect Dis. 2014;2015(21):2029–2035. doi: 10.3201/eid2111.150764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi W.S., Kang C.-I., Kim Y., Choi J.-P., Joh J.S., Shin H.-S. Clinical presentation and outcomes of middle east respiratory syndrome in the Republic of Korea. Infect Chemother. 2016;48:118–126. doi: 10.3947/ic.2016.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alraddadi B., Bawareth N., Omar H., Alsalmi H., Alshukairi A., Qushmaq I. Patient characteristics infected with Middle East respiratory syndrome coronavirus infection in a tertiary hospital. Ann Thorac Med. 2016;11:128–131. doi: 10.4103/1817-1737.180027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passamonti F., Cattaneo C., Arcaini L., Bruna R., Cavo M., Merli F. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfadil Haroon, Momen Alnassani, Mahmoud Aljurf, Syed Osman Ahmed, Marwan Shaheen, Amr Hanbli NC and REF. COVID‐19 post hematopoietic cell transplant, a report of 11 cases from a single center. Mediterr J Hematol Infect Dis 2020;12:e2020070. https://doi.org/DOI 10.4084/mjhid.2020.070. [DOI] [PMC free article] [PubMed]
- 30.Arabi Y.M., Asiri A.Y., Assiri A.M., Aziz Jokhdar H.A., Alothman A., Balkhy H.H. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21:8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.2019. New MERS Coronavirus Vaccine Clinical Trial Starts in Saudi Arabia. [Google Scholar]
- 32.Alharbi N.K., Padron-Regalado E., Thompson C.P., Kupke A., Wells D., Sloan M.A. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. Vaccine. 2017;35:3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azhar E.I., Hui D.S.C., Memish Z.A., Drosten C., Zumla A. The middle east respiratory syndrome (MERS) Infect Dis Clin North Am. 2019;33:891–905. doi: 10.1016/j.idc.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.