Abstract
Background and Aims
Routine screening for colorectal cancer is typically recommended until age 74 years. Although it has been proposed that screening stop age could be determined based on sex and comorbidity, less is known about the impact of screening history. We investigated the effects of screening history on selection of optimal age to stop screening.
Methods
We used the microsimulation model MISCAN-Colon to estimate harms and benefits of screening with biennial faecal immunochemical tests by sex, comorbidity status, and screening history. The optimal screening stop age was determined based on incremental number needed for 1 additional life-year per 1000 screened individuals compared to threshold provided by stopping screening at 76 years in the average-health population with perfect screening history (attended all required screening, diagnostic and follow-up tests) to biennial faecal immunochemical testing from age 50 years.
Results
For persons of age 76 years, 157 women and 108 men with perfect screening history would need to be screened to gain 1 life-year per 1000 screened individuals. Previously unscreened women with no comorbid conditions and no history of screening could undergo an initial screening through 90 years, whereas unscreened males could undergo initial screening through 88 years, before this balance is reached. As screening adherence improved or as comorbidities increased, the optimal age to stop screening decreased to a point that, regardless of sex, individuals with severe comorbidities and perfect screening history should stop screening at age 66 years or younger.
Conclusions
Based on the harm-benefit balance, optimal stop age for colorectal cancer screening ranges from 66 years for unhealthy individuals with perfect screening history to 90 years for healthy individuals without prior screening. These findings can be used to assist patients and clinicians in making decisions about screening participation.
Keywords: colon cancer, detection, precision medicine, patient outcomes
Short (Lay) Summary
The balance of harms and benefits for colorectal cancer screening is highly dependent on personal risk factors. Decisions to participate in screening should take into consideration an individual’s screening history and level of comorbidity.
Introduction
Colorectal cancer (CRC) screening guidelines typically recommend screening for CRC in individuals at average risk between the ages of 50–74 years.1 However, screening recommendations based solely on age do not consider the heterogeneity of the population and ignore other factors that play a role in the determination of harms and benefits of screening. Risk of CRC, for example, is affected by several factors including family history, sex, screening history, lifestyle, and comorbidity status.
Although some guidelines have recently suggested that screening could be offered to those aged over 74 depending on screening history and comorbidity2, 3 there is little practical guidance on how to implement this. Previous studies investigating the impact of comorbidity on screening stop-age were conducted in a setting of opportunistic colonoscopy screening, or assumed regular adherence to faecal immunochemical test (FIT) screening,4–6 however this ignores the complexity and varied nature of screening history. In this analysis, we aimed to address this gap in knowledge.
By using microsimulation modelling, we investigated the impact that age, sex, comorbidity status, and screening history have on the possible benefits and harms of CRC screening. We used this information to determine the optimal age to stop screening for CRC and therefore provide recommendations for a more personalized approach to CRC screening cessation.
Methods
We used the Microsimulation Screening Analysis-Colon (MISCAN-Colon) model to estimate the harms and benefits of undergoing one more screen by sex, age, comorbidity status, and screening history. The harms and benefits for each cohort were then compared to the average-health population, with perfect prior screening since age 50, having one more screen at age 74 and 76 years of age. Optimal age to stop screening was considered to be the age where the harm-benefit-ratio fell within this range.
MISCAN-Colon
MISCAN-Colon is a well-established microsimulation model for CRC developed at the Department of Public Health at Erasmus University Medical Centre (Rotterdam, the Netherlands).7 The structure, underlying assumptions and data sources used to calibrate the model are described in detail in the Supplementary Methods. Briefly, the model simulates a large population of individuals from birth to death, first without and then with screening for CRC. As each simulated person ages, one or more adenomas may arise and some can progress in size from small (≤5 mm) to medium (6 to 9 mm) to large (≥ 10 mm). Medium and large adenomas can develop into preclinical cancer and subsequently progress through stage I to IV. During each stage, symptoms may present and CRC may be diagnosed. Survival after a clinical diagnosis is determined by the person’s age, the stage at diagnosis, and the location of the cancer.8
The introduction of screening may alter the simulated life histories through detection and removal of adenomas, which may prevent some cancer cases, or through detection of cancers at an earlier stage with more favourable survival. MISCAN-Colon quantifies the effectiveness, harms and costs of screening by comparing all simulated life histories with screening with the corresponding life histories without screening.
MISCAN-Colon was calibrated to match CRC incidence and stage distribution in Canada using incidence data from the Canadian cancer registry in 2001, which was prior to the introduction of population-based screening.9 Additional model assumptions can be found in Table 1 and the Supplementary Methods.
Table 1:
Model Inputs: Test characteristics and participation assumptions associated with colorectal cancer screening.
| TEST CHARACTERISTICS | |
| Specificity and sensitivity of FITa | |
| Specificity (per person) | 96.7% |
| Sensitivity adenoma 1–5mm | 0.0% |
| Sensitivity adenoma 6–9mm | 17.6% |
| Sensitivity adenoma 10+ mm | 34.0% |
| Sensitivity cancer long before clinical diagnosis | 29.5% |
| Sensitivity cancer shortly before clinical diagnosisb | 66.0% |
| Specificity and sensitivity of colonoscopyc,d | |
| Specificity | 86% |
| Sensitivity adenoma 1–5mm | 75% |
| Sensitivity adenoma 6–9mm | 85% |
| Sensitivity adenoma 10+ mm | 95% |
| Sensitivity preclinical cancer | 95% |
| Complication of colonoscopye | |
| Fatal perforationf | 0.0074% |
| Bleedingg | 0.1640% |
| Perforationg | 0.0850% |
| Otherh | 0.3310% |
| PARTICIPATION | |
| Participation in previous screening episodes | |
| No prior screening | 0% |
| Some prior screening | 25% |
| Reasonable prior screening | 50% |
| Most prior screening | 75% |
| Perfect prior screening | 100% |
| Colonoscopy 10 years prior | 0% |
| Colonoscopy 15 years prior | 0% |
| Participation in current screening episode | 100% |
| Participation with diagnostic colonoscopyi | |
| Males | 79% |
| Females | 78% |
| Participation in surveillanceJ | 80% |
Abbreviations: FIT = faecal immunochemical test
Specificity and sensitivity of FIT derived from data from the Dutch colorectal cancer screening program15 and were adjusted to an overall positivity of 7.5% which equated to a cut-off level of 23 μg Hb/g feces
We assume that faecal screening is more sensitive in cancers towards the end of the occult bleeding period as they progress towards becoming symptomatic (i.e. visible bleeding) and clinically detectable23
Specificity for colonoscopy is based on Schroy et al, 2013.20 The lack of specificity with endoscopy reflects the detection of non-adenomatous lesions, which, in the case of colonoscopy leads to unnecessary polypectomy, which is associated with an increased risk complications
Sensitivity of colonoscopy for the detection of adenomas and CRC within the reach of the endoscope was obtained from a systematic review on miss rates observed in tandem colonoscopy studies19
Complications are conditional on polypectomy, and we assume that polypectomy is only performed if colonoscopy is positive
Risk of dying from colonoscopy were based on Canadian literature.22 A death was attributed to colonoscopy if it occurred within 30 days following an index colonoscopy.
Complications of colonoscopy were based on Canadian literature.21, 22 A complication is considered as individuals who were admitted to hospital with colonoscopy related events during the 30 days following the index colonoscopy
Other events include post-polypectomy syndrome, cardiac events, syncope/hypotension, gastrointestinal symptoms, splenic/hepatic hematoma, fall/injury, thrombophlebitis, hyponatremia, oesophageal variceal haemorrhage, and various other symptoms
The participation with diagnostic colonoscopy after a positive faecal test is taken from Cancer Quality Council of Ontario13 and is the same for all screening scenarios except under the assumption of perfect adherence to screening
The participation rate for colonoscopy surveillance was assumed to be 80%, based on data from US clinical practice 14 and is the same for all screening scenarios except under the assumption of perfect adherence to screening where we assume 100% adherence to surveillance
Setting
We assumed screening occurred in the Canadian setting. There is no national CRC screening program in Canada. Cancer screening is funded, organised and delivered at the provincial level and may co-exist with opportunistic screening. We therefore considered screening was taking place within an organised CRC screening program, commencing at age 50 years utilising biennial FIT, with opportunistic screening with colonoscopy.
We assumed that after a positive FIT result, a diagnostic colonoscopy was offered. Adenomas identified at screening or diagnostic colonoscopies were removed and the individual entered colonoscopy surveillance at intervals dependent on adenoma findings according to the surveillance recommendations from Ontario.10 It was assumed that surveillance stopped at 85 years of age.
Population
In the base-case analysis, we simulated 728 different cohorts of 10 million individuals varying them by sex, age (66, 68, …, 88, 90 years), comorbidity status (no, low, moderate, severe; Table 2), and screening history with FIT (no, some, reasonable, most or perfect prior screening) or colonoscopy (10 or 15 years prior). Simulated individuals were followed until death.
Table 2:
Overview of comorbidity levels and associated conditions.
| CONDITIONS INCLUDEDa | |
|---|---|
| No comorbid conditions | None of the conditions listed for mild, moderate or severe |
| Low comorbid conditions | Myocardial infarction (MI), ulcer or rheumatologic disease |
| Moderate comorbid conditions | Peripheral vascular disease, cerebrovascular disease paralysis, diabetes, or combinations of mild conditions (with or without diabetes) |
| Severe comorbid conditions | AIDS, Chronic Obstructive Pulmonary Disease, cirrhosis, chronic hepatitis, chronic renal failure, dementia, congestive heart failure, or combinations of at least one moderate condition (except diabetes) with any mild or moderate condition |
Abbreviations: AIDS = acquired immune deficiency syndrome
Comorbid conditions previously specified in Lansdorp and colleagues, 20144
Comorbidity Condition Specific Lifetables
To develop Canadian specific comorbidity life tables, we took hazard ratios from comorbidity specific life tables from the United States11 compared to the average life table, and applied these ratios to the 2010–2012 Canadian life tables12 (Supplementary Figures 1a–f). We assumed that comorbid conditions influenced non-cancer life expectancy but did not influence cancer risk, progression, treatment, survival or complications.
Screening History
As adherence to screening varies, we assessed five prior screening scenarios with FIT: 1) no prior screening; 2) some prior screening; 3) reasonable prior screening; 4) most prior screening; 5) perfect prior screening. In the no prior screening scenario, we assumed that no CRC screening of any kind had occurred. Then, screening participation was increased stepwise by 25%, until perfect prior participation was achieved (Table 1). We considered adherence to screening to be randomly assigned across the lifespan and not dependent on participation in the previous screening round. In addition, we assessed screening with colonoscopy 10 and 15 years prior to the investigated stop-age.
Attendance at diagnostic colonoscopy was assumed to be 79% for males and 78% for females based on observed rates in Ontario in 2015,13 and if adenomas were diagnosed and removed, we assumed 80% adherence to surveillance guidelines.14 This was altered in the perfect prior screening scenario, where we assumed that individuals had perfect adherence to diagnostic colonoscopy and any subsequent surveillance. To provide estimates for harms and benefits for a person considering screening, we assumed 100% participation in the screening, diagnostic and surveillance tests for the current screening episode.
Test Characteristics of FIT and Colonoscopy
We used the test characteristics of OC-Sensor (Eiken Chemical Co, Tokyo, Japan) based on data from the Dutch CRC screening program (Table 1).15 Although Canadian provinces use different tests (including OC-Sensor and NS-Prime (Alfesa Pharma, Osaka, Japan)), FITs with similar positivity rates have been shown to perform similarly.16, 17 We considered an overall positivity of 7.5% which equated to a cut-off level of 23 micrograms of haemoglobin per gram of faeces (μg Hb/g faeces) (Table 1). The FIT characteristics were adjusted to take into account the effect of systematic false-positive and false-negative results (that is, individuals without adenomas who test positive and adenomas do not bleed).18
The test characteristics of colonoscopy were based on a systematic review of polyp miss rates in tandem colonoscopy studies.19 The lack of specificity of colonoscopy reflects the detection of hyperplastic polyps, which are not cancer precursors.20 Complications of colonoscopy, including bleeding, perforation and death, were based on Canadian literature.21, 22
Analyses and Outcomes
For each cohort, we compared the harms and benefits of participating in FIT screening versus no further screening at their current age, considering sex, comorbidity status and screening history. The benefits of screening are provided as life years gained (LYG) and cancer deaths prevented (CDP) per 1000 males or females of a given age. Harms are expressed as the number of: i) colonoscopies; ii) complications from colonoscopy; iii) false-positive test results (i.e. negative diagnostic colonoscopies after positive FIT results); and iv) over-diagnosed cancer cases (i.e. cancers that would not have caused symptoms during a person’s lifetime). The balance between harms and benefits is presented as the incremental number needed to screen per life-year gained (NNS/LYG). We also provide details on the incremental number needed to screen per CDP and the incremental number of colonoscopies per LYG and per CDP.
Reference scenario
To identify the optimal age to stop screening, we first established an acceptable balance of harms and benefits based on current screening recommendations (acceptable threshold) and then determined a threshold where the balance was no longer considered acceptable (upper threshold). To do this, we simulated a cohort of individuals aged 74 and 76 years with average health and life expectancy, who had perfectly adhered to biennial FIT screening, diagnostic and surveillance colonoscopies from age 50. We used this threshold because it is currently recommended and thus deemed acceptable.
The acceptable threshold was determined by assessing the harms and benefits FIT screening at age 74 compared to stopping screening at age 72. The upper threshold was determined by similarly evaluating a cohort of 76 year olds, undergoing screening at age 76 compared to stopping screening at age 74. For each comorbidity level and screening history, the optimal age to stop screening was considered to be the age where the harm-benefit-ratio fell within the range between the acceptable and upper thresholds. Where no value or two values fell within the range, the age closest to the acceptable threshold was chosen.
Sensitivity Analyses
To assess the generalisability of our results, we conducted several sensitivity analyses to assess the impact of alternative screening histories. In the first instance we assessed historical screening using the less sensitive Hemoccult II,23 a guaiac faecal occult blood test (gFOBT, Supplementary Methods Table 2), with FIT administered in the current screening episode. Secondly, we assessed historical screening where FIT was administered in the current and previous two screening episodes, but prior to that gFOBT was administered.
We also assessed scenarios of annual screening with FIT and 10-yearly screening with colonoscopy in accordance with practice in the US. Finally, we assessed the base case screening scenario using a FIT with a lower cut-off (15 μg Hb/g faeces) and therefore a higher overall positivity rate (9%). In these scenarios the acceptable thresholds were adjusted accordingly.
Results
Threshold range to stop screening
Screening 1000 females with average health and life expectancy at age 74 years (compared to stopping screening at 72 years), under the assumption of perfect prior screening with FIT since age 50, gained 6.9 LYs and prevented 0.9 CRC deaths (Table 3). In addition, there were 17.3 false-positive test results, 35.9 colonoscopies, 0.3 over-diagnosed cases of CRC and 0.1 complications of colonoscopy. Under these assumptions, 145 needed to be screened to gain one life year (acceptable threshold). An additional screen at age 76 (compared to stopping at 74 years) yielded fewer benefits thereby increasing the NNS/LYG to 157 (upper threshold (Figure 1a)). Screening in males followed the same pattern as in females, however, in general the gains in life years were higher, resulting in an acceptable and upper NNS/LYG threshold of 94 and 108 respectively (Table 3, Figure 1b).
Table 3:
Reference scenario: harms and benefits of screening 1000 males and females with average health under the assumption of perfect prior screening adherence to biennial faecal immunochemical testing from age 50 years until age 74 or 76, and the balance between those harms and benefits.a
| BENEFITSb | HARMSb | BALANCEb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Life-years gained,nc | Cancer deaths prevented, n | Falsepositive tests, nd | Overdiagnosed cases, n | Colonoscopies, n | Complications of colonoscopy, n | NNS/LYG | NNS/CDP | NNSc/LYG | NNSc/CDP | |
| FEMALES | ||||||||||
| Screening to age 74e | 6.9 | 0.9 | 17.3 | 0.3 | 35.8 | 0.1 | 145 | 1141 | 5.2 | 40.8 |
| Screening to age 76f | 6.4 | 0.9 | 19.0 | 0.3 | 37.6 | 0.1 | 157 | 1147 | 5.9 | 43.1 |
| MALES | ||||||||||
| Screening to age 74e | 10.6 | 1.5 | 15.3 | 0.6 | 47.4 | 0.2 | 94 | 659 | 4.5 | 31.3 |
| Screening to age 76f | 9.2 | 1.4 | 17.4 | 0.7 | 50.3 | 0.2 | 108 | 690 | 5.4 | 34.7 |
Abbreviations: FIT = faecal immunochemical test; NNS/CDP = number needed to screen to prevent one CRC death; NNS/LYG = number needed to screen to gain one life year; NNSc/CDP = number needed to scope to prevent one CRC death; NNSc/LYG = number needed to scope to gain one life year
Note: These results are used to inform the acceptable and upper threshold to determine the optimal age to stop screening.
In this analysis we used a FIT positivity of 7.5% which equated to a cut-off level of 23 μg Hb/g. The FIT characteristics were adjusted to take into account the effect of systematic false-negative results18
Results are per 1000 persons screened
One life year gained per 1000 persons corresponds with 0.365 days gained per person
A false-positive test is defined as a negative colonoscopy after a positive FIT
Compared to stopping screening at age 72 years
Compared to stopping screening at age 74 years
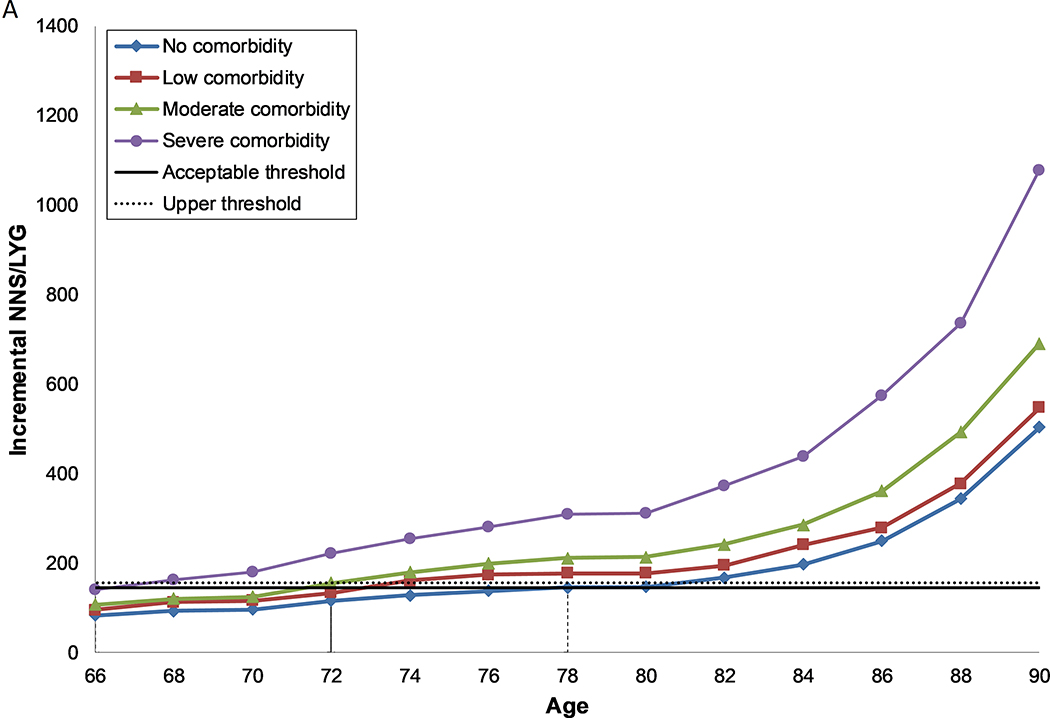
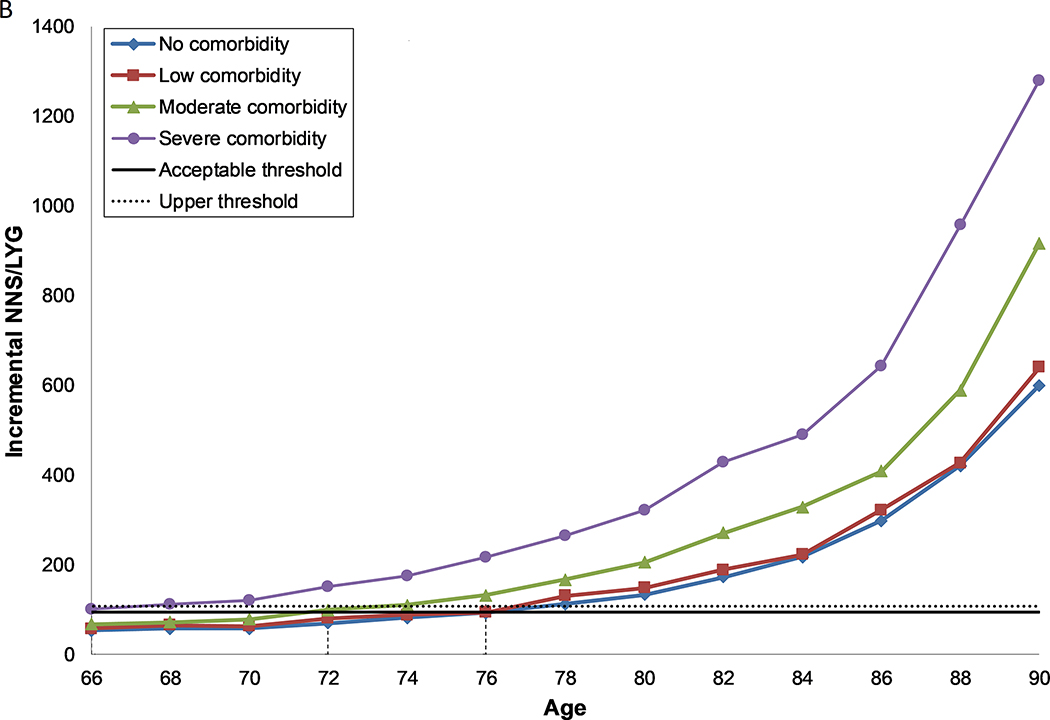
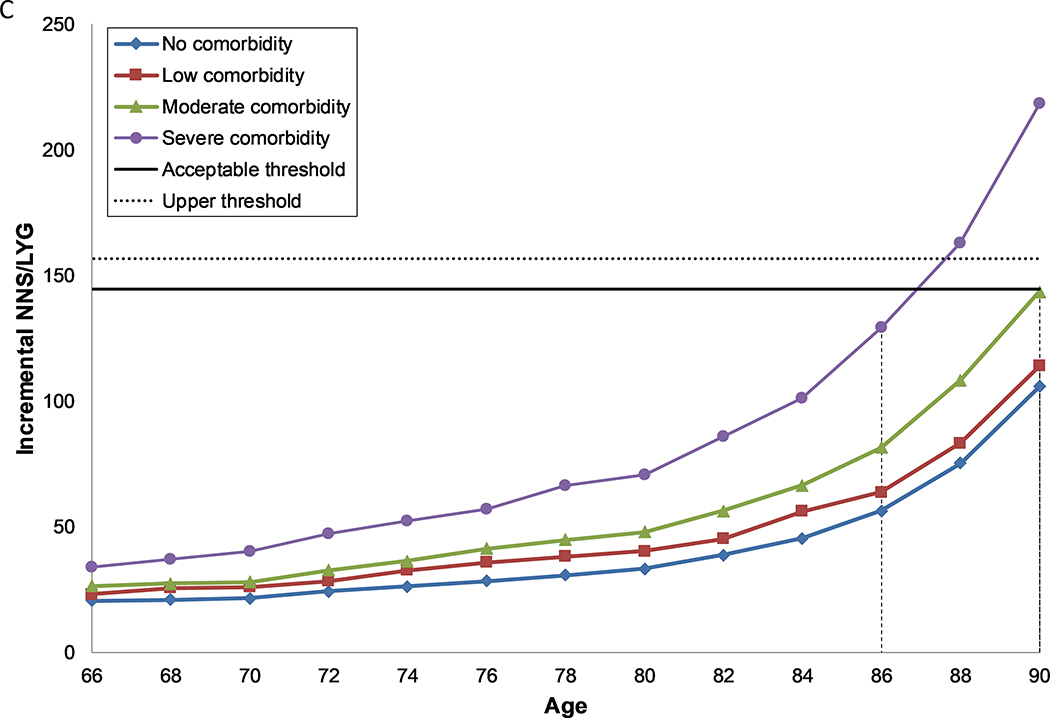
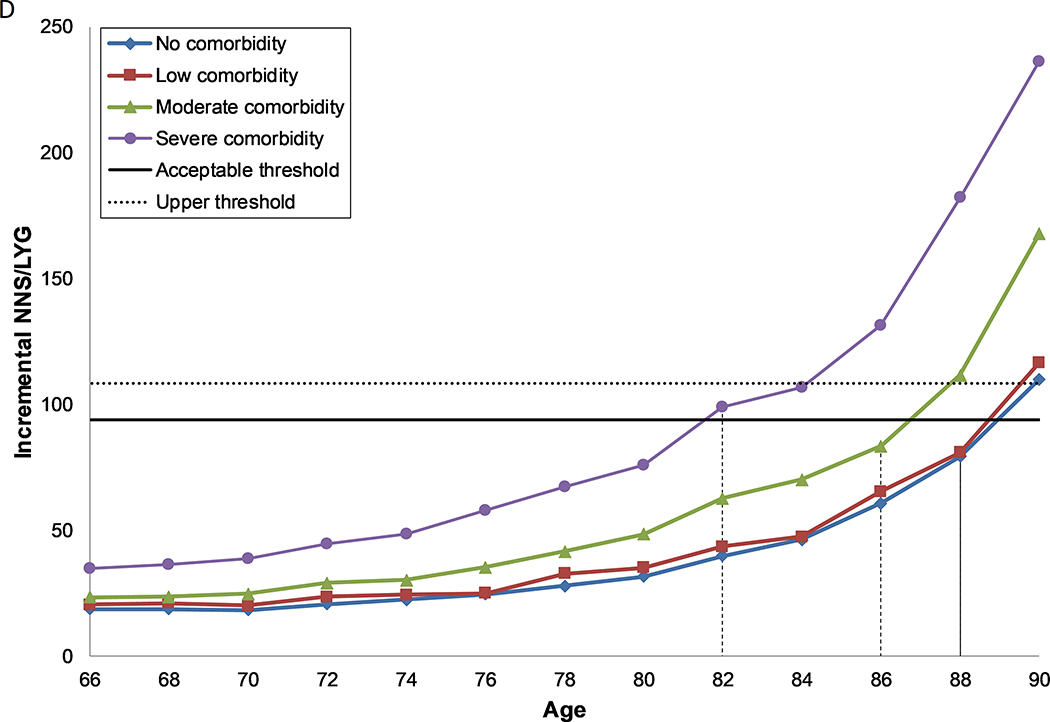
Figure 1: Number needed to screen per life year gained by age and comorbidity level, for females (a, c) and males (b, d) with perfect prior FIT screening (a, b) and no prior screening (c, d).
A. Females, under the assumption of perfect prior screening with FIT
B. Males, under the assumption of perfect prior screening with FIT
C. Females, under the assumption of no prior screening with FIT
D. Males, under the assumption of no prior screening with FIT
Abbreviations: NNS/LYG = number needed to screen to gain one life year
Each line represents the number needed to screen per life year gained over the ages 66 to 90 for each level of comorbidity. The solid horizontal line represents the threshold for the number needed to screen per life year gained for screening in the average health population until the age of 74 (acceptable threshold). The dashed line represents the threshold for the number needed to screen per life year gained for screening in the average health population until the age of 76 (upper threshold). The recommended CRC screening stop-age is defined by this range. Where two ages fall within the threshold range, the lowest of the two values is selected. Where no values fall within the threshold range, the age closest to the lowest level is selected. The vertical dashed lines indicate the age for each comorbidity group where screening provides a balance of harms and benefits similar to those aged 74 with average health.
Screening based on sex, comorbidity, and screening history
Compared to perfectly screened 74 year old women with average health, those with no comorbid conditions enjoyed greater benefits (7.8 LYG and 1.0 CDP). Although harms were similar, they experienced slightly less (0.2) over-diagnosed CRCs (Supplementary Table 1a). This resulted in a more favourable balance between the harms and benefits (NNS/LYG: 129 (Figure 1a)). As comorbidity increased, the harms of screening also increased while the benefits decreased, worsening the harm-benefit ratio as indicated by the increased NNS/LYG (Supplementary Table 1a–g).
Women aged 74 years with no comorbid conditions and without prior screening, yielded substantially greater benefits (38.1 LYG and 4.9 CDP) than females aged 74 with average health and perfect prior screening due to their increased risk and longer life-expectancy. This resulted in a substantially lower NNS/LYG (26.0) in this group (Figure 1c, Supplementary Table 1e). However, this group also experienced a noteworthy increase in harms. For example, there was 60% increase in the number of false-positive tests, a more than four-fold increase in the number of colonoscopies and over-diagnosed CRC cases and a more than six-fold increase in the number of complications of colonoscopy. As adherence to prior screening improved, both the harms and benefits of screening decreased, however benefits decreased to a greater extent, which resulted in an increase in the NNS/LYG (Supplementary Table 1b–f, Supplementary Figures 2a–e). In general, for females with a colonoscopy 10 years prior, the harms outweighed the benefits at or before the age of 74 years (Supplementary Table 1f). For females with a colonoscopy 15 years prior, the harms outweighed the benefits at or after the age of 74 years except for those with severe comorbidities (Supplementary Table 1g).
Screening in males followed the same pattern as in females, although in general they experienced both greater harms and greater benefits. However, as the benefits increased to a greater extent, the NNS/LYG was lower (Figures 1b and d, Supplementary Tables 2a–g, Supplementary Figures 3a–e).
Age of last screen based on sex, comorbidity, and screening history
Males and females without comorbidities who had previously been screened with FIT and those with no or some prior FIT screening, regardless of comorbidity status, could screen past the recommended stop-age, with age of last screen ranging from 76–90 years (Table 4). Those with severe comorbid conditions should consider having a last screening episode before the recommended stop-age (66–70 years), unless they had no, some or reasonable prior screening, in which case they should continue to screen up to or past the recommended stop-age (74–86 years).
Table 4:
Suggested age of last screening episode for colorectal cancer based on the number needed to screen to gain one life year, by sex, comorbidity status and prior screening with biennial faecal immunochemical testing or colonoscopy. The faecal immunochemical test had a positivity of 7.5% (23 μg Hb/g faeces).
| Screening Historya / Comorbidity statusb | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Low | Mod | Sev | No | Low | Mod | Sev | |
| Perfect Prior Screening with FIT | 78 | 72 | 72 | 66 | 76 | 76 | 72 | 66 |
| Most Prior Screening with FIT | 84 | 82 | 78 | 70 | 80 | 80 | 76 | 70 |
| Reasonable Prior Screening with FIT | 86 | 84 | 82 | 76 | 82 | 82 | 78 | 74 |
| Some Prior Screening with FIT | 88 | 86 | 86 | 80 | 84 | 84 | 82 | 78 |
| No Prior Screening | 90 | 90 | 90 | 86 | 88 | 88 | 86 | 82 |
| Colonoscopy 10 years prior | 74 | 68 | 66 | <66 | 73 | 72 | 69 | <66 |
| Colonoscopy 15 years prior | 83 | 76 | 74 | 68 | 80 | 78 | 75 | 66 |
Abbreviations: FIT = faecal immunochemical test; Mod = moderate; Sev = severe
Key: Blue – stop screening later than recommended in guidelines; Green – stop screening in line with guidelines; Red – stop screening earlier than recommended in guidelines
Detailed descriptions of screening history are found in Table 1. In brief, perfect prior screening assumes 100% attendance in prior screening rounds, most prior screening assumes 75% attendance in prior screening rounds, reasonable prior screening assumes 50% attendance in prior screening rounds, some prior screening assumes 25% attendance in prior screening rounds and no prior screening assumes no attendance in prior screening rounds. For colonoscopy we assume screening occurred 10 and 15 years prior to the investigated stop-age.
Detailed descriptions of comorbid conditions are found in Table 2. In brief there are four categories: no comorbidity, low comorbidity, moderate comorbidity and severe comorbidity
For those who had a colonoscopy 10 years prior to the investigated stop-age, regardless of sex or comorbidity, the last screening episode should occur at or before the age of 74 years. While for those who had a colonoscopy 15 years ago, screening stop-age was dependent on both sex and comorbidity status and ranged from 66–83 years (Table 4).
Sensitivity analyses
Our results were robust to alterations in screening history with biennial gFOBT and FIT: the pattern of age of last screening remained the same as in the base-case scenario, however, the ages were slightly older (Supplementary Tables 3a–c, Table 5). For annual FIT screening, the stop-ages were slightly lower. For colonoscopy screening, screening should stop earlier than the recommended screening stop-age for those with severe comorbidities and at or just after the recommended screening stop-age for those with no comorbidities (Supplementary Table 4).
Discussion
According to our analysis, several groups may benefit from screening past the recommended stop-age. For example, individuals without comorbidity, those who are screening naive or who had a colonoscopy 15 years ago and are without severe comorbidities could undergo screening until between 76–90 years of age. Screening these individuals after the recommended stop-age presents an opportunity to reduce their risk of CRC and maximise the benefits of screening while maintaining an appropriate balance of harms. For others, such as those with severe comorbidity and most to perfect prior FIT screening, screening could stop earlier than currently recommended. This earlier than recommended stop-age also applies to those with a colonoscopy 10 years prior, except for females without comorbidity. Continued screening in these individuals provides fewer benefits and increases unnecessary harms and burden compared to the average health population.
Our results are in line with previous findings that individuals with lower comorbidity and less intensive screening history will benefit from screening past the recommended stop-age.4, 5 However, our investigation builds on previous research by more comprehensively assessing the impact of screening history on optimal age to stop screening. This approach is more in keeping with “real life”, where exposure to prior colorectal tests may be quite varied.
There are four noteworthy limitations to this investigation. First, rates of participation in prior screening were determined a priori and do not necessarily reflect what is happening in practice. More accurate data on actual patterns of adherence would be a useful addition. Second, by assuming that the population was at average risk for CRC, we did not consider the probable variation in risk. CRC risk is affected by genetic profile, family history, lifestyle factors (such as smoking and obesity)24, 25 and comorbid conditions (i.e. diabetes increases risk26). As these factors are likely to affect both the harms and benefits of screening, we believe they should be incorporated into future research. Third, our life tables came from a statistical analysis of administrative data provided by SEER and included a broad ranges of diseases such as AIDS which may seem less relevant than other diseases. However, that statistical analysis showed that having AIDS resulted in a higher probability of dying. Furthermore, the life tables do not include mortality for cancers, therefore our results may underestimate other-cause mortality rates and the harm-benefit ratio, but not the comparisons of the comorbid condition groups to the average health population. Finally, we did not include quality of life in our outcome measures. However, the purpose of this analysis was to separately assess the harms, burden and benefits of screening to allow individuals to make their own decisions about screening participation. Had we presented this, we would expect similar results as the thresholds would also have shifted.
Notwithstanding these limitations, there are several important implications of this investigation. Firstly, using MISCAN-Colon, a well-established, validated model for CRC screening,7 we have developed a complex algorithm incorporating age, sex, comorbidity status, and screening history that allows for a comprehensive assessment of individuals within the population. In addition, by using life tables, which were based on administrative data and personal factors such as age, sex, comorbidity, and screening history, which are generally found in health administrative data, in the future screening participation recommendations could potentially be automated for use in the clinical setting (for example in a clinical decision support system).
Our results provide detailed guidance for clinicians and patients when discussing screening participation. For example, if a clinician was meeting with a 72 year old female patient who has cardiovascular disease (considered as a moderate level of comorbidity) and who has participated 50% of prior screening rounds, our results indicate that she could participate in another screening round, as the benefits still outweigh the harms. However, for a male patient aged 74 with chronic obstructive pulmonary disease (a severe comorbid condition) who has previously participated in 75% of screening episodes, our results indicate that the benefits of screening may no longer outweigh the potential harms, and he should consider stopping screening at this time. This guidance is based on the metric of NNS/LYG. However, should clinicians prefer to use another metric, they are available in the Supplementary Tables. For example, using the balance of NNSc/CDP, our 72-year-old female noted above may consider stopping screening because this balance of harms and benefits is no longer favourable. Decisions to participate in screening should depend on individual patient preferences and our results help to facilitate this decision-making in an informed way. Furthermore, they can assist policy makers who are designing or updating existing CRC screening programs and guidelines, and addresses concerns that this evidence has been lacking for FIT-based screening programs.27
Conclusion
There is a growing body of evidence highlighting the benefits of personalising screening to optimise benefits and reduce harms. By providing reliable information about the possible benefits, harms and burden of screening, our results facilitate an evidence-based approach for formulating guidelines and making informed decisions about screening participation. Our research suggests that varying screening stop-age from <66 to 90 years depending on age, sex, comorbidity status and screening history is a more efficient approach and results in better patient outcomes than one that is based on age alone. These results may assist patients and clinicians to make informed decisions about screening participation and could be used to inform future CRC screening program guidelines.
Supplementary Material
What you Need to Know.
BACKGROUND AND CONTEXT:
Routine screening for colorectal cancer is typically recommended until age 74 years. Screening stop age might be determined based on sex, comorbidities, and screening history.
FINDINGS:
Based on the harm–benefit balance, the optimal stop age for colorectal cancer screening ranges from 66 years for unhealthy individuals with perfect screening history to 90 years for healthy individuals without prior screening.
IMPLICATIONS FOR PATIENT CARE:
An individual’s comorbidities, sex, and results from previous screening can be used to select the optimal stop age for colorectal cancer screening.
Acknowledgments
Grant Support
This research benefitted from our participation in the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) (grant number: U01-CA199335) and was supported by research funding from Cancer Care Ontario.
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. DRC and ILV had full access to all the data and DRC had final responsibility for the decision to submit for publication. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding bodies.
Conflict of Interest Statement
All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: DRC, SKN and ILV declare grants from National Cancer Institute Cancer Intervention and Surveillance Modeling Network (CISNET) and JT declares grants and personal fees from Cancer Care Ontario.
Abbreviations
- CDP
cancer deaths prevented
- CRC
colorectal cancer
- FIT
faecal immunochemical test
- gFOBT
guaiac faecal occult blood test
- LYG
life years grained
- MISCAN-Colon
Microsimulation Screening Analysis-Colon
- NNS
number needed to screen
- μg Hb/g faeces
Micrograms of haemoglobin per gram of faeces
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637–49. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Task Force on Preventive Health Care, Bacchus CM, Dunfield L, et al. Recommendations on screening for colorectal cancer in primary care. CMAJ 2016;188:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 4.Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med 2014;161:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hees F, Saini SD, Lansdorp-Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology 2015;149:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hees F, Habbema JD, Meester RG, et al. Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis. Ann Intern Med 2014;160:750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeve F, Boer R, van Oortmarssen GJ, et al. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res 1999;32:13–33. [DOI] [PubMed] [Google Scholar]
- 8.Rutter CM, Johnson EA, Feuer EJ, et al. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst 2013;105:1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statistics Canada. Table 103–0550 - New cases for ICD-O-3 primary sites of cancer (based on the July 2011 CCR tabulation file), by age group and sex, Canada, provinces and territories, annual, CANSIM (database). [Internet]. Statistics Canada; 2011. [Available from: http://www5.statcan.gc.ca/cansim/a01?lang=eng]. [Google Scholar]
- 10.Cancer Care Ontario. ColonCancerCheck (CCC) Recommendations for Post-Polypectomy Surveillance [Internet]. Cancer Care Ontario,; 2019. [Available from: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/38506].
- 11.Cho H, Klabunde CN, Yabroff KR, et al. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med 2013;159:667–76. [DOI] [PubMed] [Google Scholar]
- 12.Statistics Canada. Life Tables, Canada, Provinces and Territories 2010 to 2012: Statistics Canada; 2013. [updated May 2016. Available from: http://www.statcan.gc.ca/pub/84-537-x/84-537-x2016006-eng.htm].
- 13.Cancer Quality Council of Ontario. Colorectal Cancer Screening Follow-Up: Cancer Quality Council of Ontario; 2016. [Available from: http://www.csqi.on.ca/by_patient_journey/screening/colorectal_screening_follow_up/].
- 14.Colquhoun P, Chen HC, Kim JI, et al. High compliance rates observed for follow up colonoscopy post polypectomy are achievable outside of clinical trials: efficacy of polypectomy is not reduced by low compliance for follow up. Colorectal Dis 2004;6:158–161. [DOI] [PubMed] [Google Scholar]
- 15.Toes-Zoutendijk E, van Leerdam ME, Dekker E, et al. Real-Time Monitoring of Results During First Year of Dutch Colorectal Cancer Screening Program and Optimization by Altering Fecal Immunochemical Test Cut-Off Levels. Gastroenterology 2017;152:767–775 e2. [DOI] [PubMed] [Google Scholar]
- 16.Grobbee EJ, van der Vlugt M, van Vuuren AJ, et al. A randomised comparison of two faecal immunochemical tests in population-based colorectal cancer screening. Gut 2017;66:1975–1982. [DOI] [PubMed] [Google Scholar]
- 17.Catomeris P, Baxter NN, Boss SC, et al. Effect of Temperature and Time on Fecal Hemoglobin Stability in 5 Fecal Immunochemical Test Methods and One Guaiac Method. Arch Pathol Lab Med 2018;142:75–82. [DOI] [PubMed] [Google Scholar]
- 18.van der Meulen MP, Lansdorp-Vogelaar I, van Heijningen EM, et al. Nonbleeding adenomas: Evidence of systematic false-negative fecal immunochemical test results and their implications for screening effectiveness-A modeling study. Cancer 2016;122:1680–8. [DOI] [PubMed] [Google Scholar]
- 19.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006;101:343–50. [DOI] [PubMed] [Google Scholar]
- 20.Schroy PC 3rd, Coe A, Chen CA, et al. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med 2013;159:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilsden RJ, Dube C, Heitman SJ, et al. The association of colonoscopy quality indicators with the detection of screen-relevant lesions, adverse events, and postcolonoscopy cancers in an asymptomatic Canadian colorectal cancer screening population. Gastrointest Endosc 2015;82:887–94. [DOI] [PubMed] [Google Scholar]
- 22.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 2008;135:1899–1906, 1906 e1. [DOI] [PubMed] [Google Scholar]
- 23.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, et al. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer 2009;115:2410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson CM, Wei C, Ensor JE, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013;24:1207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunlop MG, Tenesa A, Farrington SM, et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42,103 individuals. Gut 2013;62:871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–87. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins M Colorectal cancer screening is cost-effective in the elderly who have had less intense prior screening, high baseline risk of colorectal cancer and less comorbidities. Evidence Based Medicine 2016;21:182–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.