Abstract
The use of bioactive compounds and probiotic bacteria against the viral diseases in human is known for a long time. Anti-viral, anti-inflammatory and anti-allergic properties of bioactive compounds and bacteria with probiotic properties in respiratory viral diseases may have significance to enhance immunity. This review highlights some of the important bioactive compounds and probiotic bacteria, suggesting them as a ray of hope in the milieu of the COVID-19 management.
Keywords: Probiotics, Bioactive compounds, COVID, Food, Metabolites
1. Background
The bioactive compounds with nutraceutical properties are found in various plants as well as in foods, including vegetables, fruits, whole grains, nuts & oils [1]. These bioactive compounds hold curative potential against disorders related to the inflammatory state and oxidative stress [2]. The bioactive compounds have the ability to modulate functions of receptors, enzymes like inhibition and induction, as well as have antioxidant properties [3]. The epidemiological studies observe that the increased consumption of food having bioactive compounds like phytochemicals, vitamins, and most importantly, the phenolic compounds, carotenoids and flavonoids, hints to a profoundly positive effect on human health. This could also diminish the risk of many deadly diseases like heart strokes, heart diseases, cancer, cataracts, respiratory issues, age-related functional decadence, diabetes and Alzheimer's [4].
In addition to bioactive compounds, probiotic microorganisms can confer enormous health benefits. The three broadly studied probiotic bacteria are Saccharomyces, Lactobacillus and Bifidobacterium [5]. Probiotics uphold defense mechanisms, including both adaptive as well as the innate immune response by modulating the function of macrophages, dendritic cells, B-lymphocytes & T-lymphocytes. One such immune-modulatory mechanism is by activating the toll-like receptors. The two main elemental mechanisms of the action of probiotic are either by the signaling pathways in the cells of the host or the gene expression regulation [6].
Severe Acute Respiratory Syndrome-Coronavirus-2 or ‘SARS-CoV-2’ is the viral strain responsible for the widespread damage of human lives worldwide, causing the novel coronavirus disease (COVID-19). This, being declared a pandemic by the World Health Organization (WHO), needs high containment [7]. It has been observed that the affected individuals with high immunity have a high recovery rate.
Due to human-to-human transmission of SARS-CoVID, there is a rapid increase in cases and mortality rates worldwide. Sample extracted from patients with pneumonia have shown enveloped single-stranded RNA-type beta-coronavirus with genome sequences sharing 79.5% sequence identity to severe acute respiratory syndrome-related coronaviruses (SARS-CoV) [8] [9] [10]. Besides, the spike (S) protein of SARS-CoV-2 and SARS-CoV enters human alveolar epithelial cells through binding angiotensin-converting enzyme 2 (ACE2) receptor [12]. Since there is no confirmed treatment strategy for the pandemic, the bioactive compounds and probiotics can thus, be of great help to improve immunity [11], [12]. This review provides an insight into the function of probiotics and bioactive compounds in various anti-viral properties that may aid to curb infections related to COVID by their various clinically proven properties.
2. Bioactive compounds and COVID-19 infection
Herbs and plants have been used as medicine to cure and prevent diseases by restraining its onset at the root [13]. Various plants such as Curcuma longa, Ocimum sanctum, Azadirachta indica, Elettaria cardomomum, Syzygium aromaticum, Viola odorata, Momordica charantia, etc., possess enormous medicinal and therapeutic properties [13]. Metabolites derived from plants provide health benefits beyond the basic nutritional value. Secondary metabolites, also known as bioactive compounds, act as potential therapeutics that may have an impact on pro-inflammatory state, oxidative stress, etc. [14]. Bioactive compounds such as terpenoids, alkaloids, flavanoids, phenolic acids, saponins, possess anti-microbial, anti-viral, anti-cancerous, anti-inflammatory, anti-allergic, anti-diabetic properties [15]. Bioactive compounds modulate metabolic processes and thus denote favorable therapeutic properties such as antioxidant effect, inhibition of receptor activities, inhibition or induction of enzymes, and induction and inhibition of gene expression [14]. Various in-vitro studies on bioactive compounds have shown positive results in enhancing immune functions like immune-modulating properties, including enhanced cell-mediated immunity, human leukocyte antigen (HLA) molecular expression, increased antibody production and macrophage mobility [16].
Globally, respiratory viral infections are the major root of morbidity and mortality. The juvenile viral infection leads to acute illness and can be linked with the development of wheezing and asthma in later life as there may be a reduced regulation of the immune response in infants, leading to increased immune pathology [17]. Influenza virus, coronavirus, adenovirus, respiratory syncytial virus, and rhinovirus are the most commonly known respiratory viruses. The ubiquitous nature of respiratory viruses, along with their advanced potential of spreading among human populations, ensure their occurrence among persons of all ages, the immune-competent and the immune-compromised [18]. These viral infections may be asymptomatic or lethal. In respiratory tract infections, both RNA, as well as DNA based viruses, can cause infections via viral replication with or without the symptoms. Herbal extracts containing bioactive compounds are effective in treating viral respiratory ailments. A study on the extract of Lycoris radiate, Artemisia annua, Pyrosia lingua and Lindera aggregate shows significant inhibition effect of SARS-CoV strain [19]. The inhibition effect was estimated by a dose-dependent manner in which EC50 value showed optimized concentration required to inhibit the effect of SARS-CoV strain [19]. Similarly, another study explained the importance of phyto-compounds against anti-SARS-CoV [20].
Bioactive compound glycyrrhizin (triterpene glycoside glycyrrhizic acid) of licorice roots have antiviral as well as an anti-inflammatory effect against SARS-CoV [21]. Further, bioactive components in Chinacea purpurea, Geranium sanguineum, Glycyrrhiza uralensis F., Cistus incanus L., Ephedrae herba, etc., have also shown effective immune response during the prognosis of viral infection [22]. For the last 20 years, well-known two major outbreaks worldwide targeted the respiratory system leading to remarkable mortality among people with the weak immune system, smokers and aged people [23]. In order to build up the immune system, the intake of food rich in vitamins such as vitamin C, vitamin D, vitamin A, etc., is essential. Some fat-soluble compounds present such vitamins include retinoic acid, retinol and β-carotene, play a very crucial role in boosting up the immune function and are known to lower the susceptibility to infection [23]. Vitamin D has been found showing promising results in managing the COVID. It has been found that higher serum levels of 25-hydroxyvitamin D significantly improve the clinical condition of the patient and even helped to mitigate the worse outcomes and conversely, the low levels were related to the worse outcomes [24]. The exact mechanism behind this effect is still unknown, but many other mechanisms proposed for vitamin D in reducing the viral infections might be due to the induction of cathelicidins and defensins, where these compounds decrease the rate of viral replication by increasing the concentration of anti-inflammatory cytokines and lowering the concentration of pro-inflammatory cytokines [25].
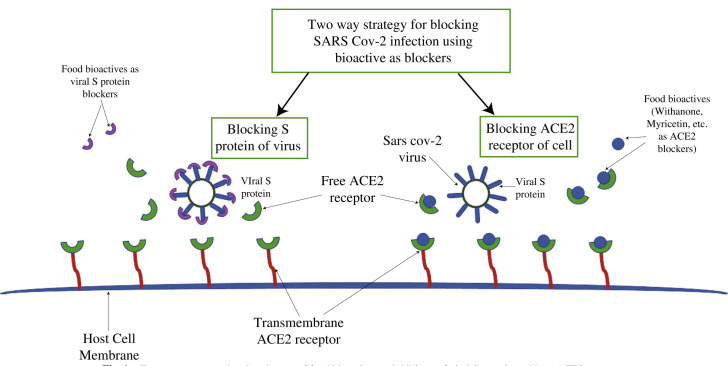
Plant's secondary metabolites have been reported to have potent antiviral activity. Nowadays, in silico molecular docking techniques and other computational techniques have made it possible to understand the interaction between receptor proteins and bioactive compounds that act as a ligand [26]. Studies have shown the relevance of the ACE2 receptor in SARS-CoV infection both in vitro [27] and in vivo experiments [28]. The viral entry is promoted by the binding of S protein of the viral membrane with the ACE2 transmembrane receptor, followed by its downregulation [29]. The upregulation of the ACE2 receptor provides a protective effect against SARS-CoV-2. So, dietary intake of some specific food bioactives that upregulate ACE2 expression may play an essential role in combating the severe outcomes of the disease [30]. The two-way strategy that can be adopted to stop the entry of SARS-CoV2 infection is either blocking the S protein of the viral membrane or tissue-specific or localized blocking of the ACE2 receptor. For this, several inhibitors of natural origin, such as food bioactive, can be used as blocking agent (Fig. 1 ). During in silico examination on some bioactive natural compounds presnt in the fruit and leaf extract of Anthocephalus Cadamba, it has been found that oleanic acid, cadambine, chlorogenic acid, ursolic acid, Isovallesiachotamine, D-myo-inositol, Vincosamide-N-oxide, Vallesiachotamine and pentyle ester has been appeared to be a potential inhibitor for COVID-19 [31]. Bioactive compounds like kaempferol, demethoxycurcumin, catechin, gingerol, quercetin, luteolin-7-glucoside, naringenin, apigenin-7-glucoside, oleuropein, curcumin, epicatechin-gallate, zingerol, and allicin act as potential inhibitor models for SARS-CoV-2 Mpro [32]. Mpro is essential for proteolytic maturation of the coronavirus and acts as a potential target for the inhibition of coronavirus replication. It has been analyzed that the protein sequence of SARS-CoV-2 Mpro and the COVID-109 Mpro have a 96% similarity [26]. Hence these bioactive natural compounds or their structural analogs may be explored as an anti-COVID19 drug agent.
Fig. 1.
Two-way strategy showing the use of food bioactives as inhibitors of viral S protein and host ACE2 receptor.
Ayurveda, over the years, has screened thousands of plant sources in order to combat many diseases and enhancing the immune system of the body. Recently on March 26, 2020, the Indian Council of Medical Research (ICMR), Govt of India, has approved the use of hydroxychloroquine for prophylactic treatment of COVID-19 infection. It has been found that ashwagandha, tulsi, giloy has antiviral potential to combat COVID-19. Withania somnifera (Ashwagandha) has phytochemical Withanone, which can affect RBD and host ACE2 receptor complex by interrupting the electrostatic interactions between them, thereby blocking or weakening the viral entry into the human body and further decreasing its effectivity [33].
Scutellarein Phseudolysimachion longifoiium and myricetin Aglaia perviridis display antiviral activity against SARS-CoV, by helicase inhibition [34]. In that same study, it was found that these two bioactive compounds were able to inhibit the helicase activity by ATPase activity. Moreover, this inhibition was virus-specific as the same bioactive compounds were not able to inhibit the hepatitis C virus ATPase activity. In that same study, modeling analysis of these bioactives depicts that they bind specifically to the ATP binding pocket of the helicase enzyme, thereby inhibiting its activity, which was a significant finding concerning the targeted inhibition of SARS-CoV. Various studies highlighting the role of bioactive compounds against viral infections, which may have implications in providing resistance against infections related to COVID-19, are stated in Table 1 .
Table 1.
Plausible bioactive compounds that may assist in the management of COVID infection.
| Bioactive compound | Plant | Reference |
|---|---|---|
| Glycyrrhizin | Licorice radiate | [21] |
| Astaxanthin | Haematococcus pluvialis (algae) | [35] |
| Urosolic acid | Anthocephalus cadamba | [31] |
| D-myo-inositol | Anthocephalus cadamba | [31] |
| Withanone | Withania somnifera | [33] |
| Scutellarein | Phseudolysimachion longifoiium | [34] |
| Myricetin | Aglaia perviridis | [34] |
3. Probiotics and COVID-19 infection
The relationship between the microbiota and diseases investigates that the human intestinal bacteria have the capability to regulate the host immune system, not only in healthy individuals but also in affected individuals. It has been divulged that synanthropic bacteria and their metabolites regulate the immune system and immensely affect mucosal immunity by interacting with TLRs, T regulatory lymphocytes, cytokines, chemokines and the expression of the nuclear transcription factor (NF-κB) [36], [37]. The epithelial and immune cells are directly influenced by bacterial metabolites, especially short-chain fatty acids, which effected pattern recognition receptors (PRR) by an activating of NF-κB, TNF-α and it decreased stimulation of PRR [38]. The various experiments on animal models suggest the role of intestinal microbiota for maintaining antiviral respiratory immunity. An experiment demonstrates that the intake of antibiotics decreases resistance towards intranasal influenza A virus infection by increasing the viral load, decreasing the IgG and IgA level and also by deactivating the CD4+ and CD8+ T cell versus the animals without antibiotics [39]. An antibiotic suppressed antiviral immune response is reinstated by administering of TLR ligands (commensal bacterial peptidoglycans) [39]. This confirms that the received signal is transmitted from the lower gastrointestinal tract to the mucosa of other biotopes like the respiratory tract to amplify the protection against infection. Thus, it gives an idea that the probiotic has immune-modulatory activity and its application for the treatment or prevention of various diseases like acute respiratory infection caused by viruses. The potency of probiotics is to enhance both the innate and acquired immunity and also reduce the gastrointestinal infection [40] and upper respiratory tract infections [41] [42]. Probiotics are live microorganisms that enhance the immune system and provide health benefits for the host [43], they are ingested as fermented food while paraprobiotics or ghost probiotics have immune-modulatory potential beyond their viability [44]. Two classes of probiotics bacteria, namely Bifidobacteria and lactic acid bacteria, are used for a long time and are mostly present in the fermented foods and probiotic supplement confers their safety record of not causing any disease through translocation. These are known to be present on all human mucosal surfaces naturally [45]. Various studies have demonstrated the clinical potential of probiotics and following are some of the probiotic bacteria with potential clinical roles.
3.1. Lactobacillus rhamnosus (LGG)
An intranasal administration of the LGG strain shows the preventive effect against rhinovirus infection [46]. LGG was administered against gastroenteritis infection i.e., rotavirus-induced diarrhea shows the positive result by improving the intestinal permeability [47]. The immunomodulatory effects of probiotic mechanisms are not entirely understood. Although, LGG enhanced the innate and adaptive immune responses, especially against gastrointestinal pathogens, by increasing the IgG level in serum and secretory IgA against respiratory pathogens such as rotavirus [47].
3.2. Lactobacillus casei Shirota (LcS)
In athletes, the administration of LcS strain decreased plasma Epstein–Barr virus and cytomegalovirus antibody titers [48]. However, the potential of LcS remains controversial; an effective mechanism was investigated wherein LcS strain was regulating the activity of natural killer (NK) cells, the first-line defense against viral infection [49].
3.3. Lactobacillus paracasei (L. casei 431)
An oral or intranasal administration of L. casei 431 strain was reported to have the capability to reduce the upper respiratory infection, although, in healthy individuals, it showed no outcome on the immune response to influenza vaccination [50]. L. casei 431 strain was demonstrated to regulate the immune system by increasing the IgG, IgG1, and IgG3 levels in plasma as well as secretory IgA level in saliva [51]. Although, it induces the innate viral defense mechanism and decreases inflammation.
3.4. Lactobacillus casei strain DN-114 001 (DN-114)
The administration of the DN-114 strain significantly reduced the frequency and incidence of acute diarrhea in healthy individuals aged 6–24 months [52].
3.5. Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 (R-1)
In elderly people, the consumption of fermented yogurt with R-1 was found to an increase in NK cell activity and decreased the risk of common cold [53]. Other studies found that R-1 and its released polysaccharides enhanced the immune system by increasing the activity of NK cells and also prevent the respiratory infections caused by influenza viruses [53] [54].
3.6. Lactobacillus plantarum L-137
In mice, the administration of heat-killed HK L-137 showed an immunomodulatory effect, enhancing the innate and acquired immune responses by increasing the production of type 1 IFNs and interleukin (IL)-12 [55] [56]. The intake of HK L-137 significantly lowered the UTRI symptoms; the duration and severity of medication showed negative correlations with the duration of HK L-137 intake [55]. The administration of HK L-137 strain showed an increase in concanavalin A-induced proliferation of peripheral blood mononuclear cells (PBMCs). However, IFN-β production in serum was not significantly increased [57].
3.7. Enterococcus faecalis FK-23
The effect of paraprobiotic FK-23 (Enterococcus faecalis strain FK-23) was reported that decreased alanine aminotransferase (ALT) levels in HCV-positive individuals but did not reduce viral load [58]. However, the mechanism behind that was still unclear; they suggested that reduction in ALT level by FK-23 strain might alter the microbiota in HCV patients [58].
3.8. Saccharomyces boulardii
The oral administration of Saccharomyces boulardii shortened the duration of diarrhea in acute rotavirus gastroenteritis in children, but the mechanism was not available [59].
3.9. Lactococcus lactis ssp. lactis JCM 5805
Production of a large quantity of IFNs by plasmacytoid dendritic cells (pDCs) plays an essential role in antiviral immunity. The administration of L. lactis JCM5805 strain was found to increase the activity of human pDCs among healthy volunteers, especially in those who showed low pDCs activity, and it also significantly reduced common cold symptoms [60].
The administration of L. lactis JCM5805 significantly reduces cough, fever and symptoms of influenza-like illness by enhancing the IFN-α-mediated response against the influenza virus [61].
3.10. Bifidobacterium longum MM-2
Bifidobacterium is a probiotic bacteria that contribute to the major component of the intestinal microflora. It is known to modulate the intestinal immune system. Studies have shown that Bifidobacterium longum MM-2 which was isolated from healthy human contribute to anti-influenza virus activity in the murine model, where oral administration of bacterium for 17 days results in the improved clinical symptoms, mortality, suppressed inflammation in the lower respiratory tract, reduced cell death and suppressed the level of the pro-inflammatory cytokine in the bronchoalveolar lavage fluid [62].
3.11. Bifidobacterium longum BB536
Similar to the study mentioned above for the bifidobacterium on murine model, a study has been carried out on the mice model against the influenza virus infection and it has been seen that mice administered orally with Bifidobacterium longum BB536 showed improved symptoms, less reduction of body weight and inhibited proliferation of virus in the lungs as compared to the control group [63].
3.12. Bifidobacterium animalis ssp. lactis (BB-12®)
A randomized, double-blind placebo study was conducted on 211 human subjects. In this study, once-daily minimal oral colony-forming units in the form of capsules of BB-12® and diary drink containing L. Casei 431® or matching placebo was given to the subjects for 6 weeks, and after 2 weeks all the subjects were given the influenza vaccine. It has been found that subjects administered with dietary supplementation of BB-12® and L. Casei 431® lead to the increased adaptive immune response to the influenza vaccine [64].
3.13. Bifidobacterium longum SPM1205, and SPM1206
This study was carried out on Caco-2 cell lines and neonatal mouse model; these probiotic bacteria inhibited the human rotavirus strain ‘Wa’ infection in the Caco-2 cell line. Moreover, these bacterial strains also inhibited the replication of the virus in the neonatal mouse model. The gene expression study carried out in the same study revealed the increased level of INF-α and INF-β as compared to that of the controls. This suggests that these strains were found to be inhibiting the replication of the rotavirus in both in-vitro and in-vivo experiments [65].
3.14. Bifidobacterium longum SP 07/3 and B. bifidum MF 20/5
In a study that was carried out in 2005, a randomized, double-blind controlled trial was done where 479 healthy adults in the age group of 18-67 years were recruited. In this study, the subjects were given the daily dose of vitamins, minerals with or without probiotic bacteria were given in the form of tablets for 3 months and then during the episodes of the common cold, participants recorded their symptoms daily. It was found that the fever was found to be for the shorter duration in the probiotic treated group and there was an increase in the number of suppressor T-cells (CD8+) and helper T-cells (CD4+) in the probiotic group. Overall, reduced episodes and severity of symptoms by 2 days were observed in the probiotic group than the control group [66].
4. Mechanism for the action of probiotics to inhibit Viral Infections and relevance to COVID
The supplementation with a combination of Streptococcus thermophilus and Bifidobacterium bifidum decreased the occurrence of viral infection [67], an effect that has been established in a subsequent study [68], indicating inhibition of viral replication by probiotic bacteria. The probiotic strains were not administered to the respiratory tract directly. So, at this site, direct inhibition may appear impossible. However, lungs have their microbiota and a gut-lung connection through host-microbe, microbe-microbe, and immune interactions, that may influence the course of respiratory infections [69]. An imbalance in the microbial communities of the gastrointestinal and respiratory tracts are associated with respiratory tract infections [70], [71]. This dysbiosis may change immune function and lead to secondary infections. The intestinal dysbiosis associated with COVID that causes inflammation and a weaker response to pathogens was reported in China [72], [73]. The case exists that restore gut homeostasis by probiotic strains [74]. It is useful that the gut lung axis could influence by probiotic strains that are orally administered. Some probiotic strains can migrate from the gut to various other sites, such as the breast, to treat mastitis [75]. At various mucosal sites i.e., lungs, the immune response can be influenced by the gut microbiome [Baud]. The balance between proinflammatory and immunoregulatory cytokines are modified by probiotic strains that allow viral clearance. This might be relevant to prevent acute respiratory distress syndrome (ARDS), a significant subject associated with COVID-19. However, probiotic strains increase the integrity of tight junctions, through enhancing butyrate, a fuel for colonocytes that may reduce SARS-Cov-2 invasion. While various mechanisms have been tested on the new SARS-CoV-2 virus, this approach should not be negate, mainly the effects of probiotics against other coronavirus strains have been reported [76], [77], [78], [79]. The oral administration of Lactobacillus acidophilus CMCC878 in mice, started 24 h after pulmonary inoculation of Staphylococcus aureus and Pseudomonas aeruginosa reduced systemic inflammation and lung damage [80].
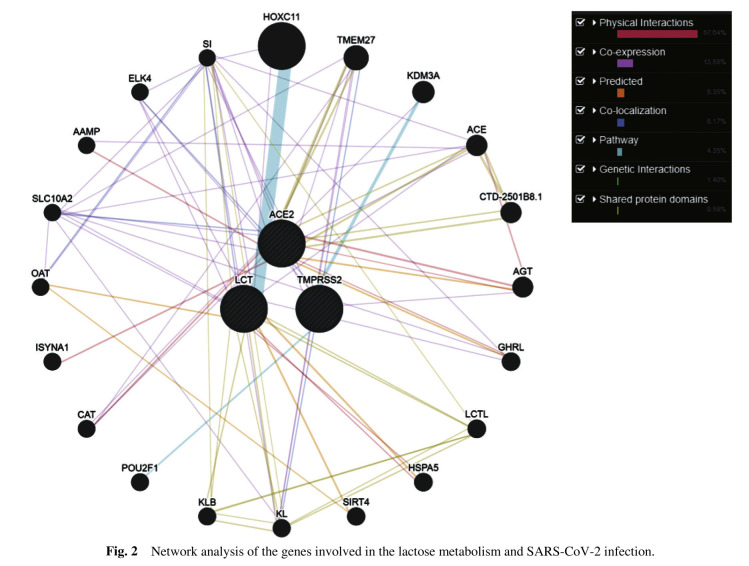
The above-stated studies have explained the potential of probiotics against viral infections; these bacteria also play an important role in the modulation of the immune system of the host. Studies have elucidated the role of probiotic bacteria in many respiratory diseases infection, especially respiratory viral diseases. Since coronavirus emerged as an outbreak in recent times globally, it has been found that the individuals who are elderly and are immune-compromised were observed to be at higher risk of infection and death [81]. This depicts that individuals with altered immune responses or individuals who have a weak immune system are prone to infection. Considering these facts, probiotics may provide promising results against SARS-CoV-2. As discussed earlier several studies on the animal model provided evidence for the role of probiotic bacteria in disease management. Similarly, another study showed that the strain Bifidobacterium bifidum helps in the modulation of the innate and humoral immune response in influenza infective mice [82] and in case of the respiratory syncytial virus in mice model prophylactic potential of probiotic strain Lactobacillus gasseri SBT2055 was observed as an antiviral activity and was found helpful in decreasing the elevated pro-inflammatory cytokines generated in response against virus infection [83]. Studies have also shown that yogurt substituted with specific probiotic strains may prevent the respiratory tract infection in older adults by inducing T-cell mediated immune response [84]. Studies that have been carried out on the role of probiotics in the management of SARS-CoV-2 infection specifically, but few studies have been carried out globally showing promising results regarding the use of probiotics in enhancing immune system, protection against various respiratory infections (Table 2 ). Moreover, with the use of antibiotics and antiviral medications in the treatment of COVID-19 it has been observed that the patients with the dysbiosis of the gut microbiota found to be suffering from diarrhea wherein such condition probiotic may act as a therapeutic target for the management of the disease [85]. National Health Commission of China (NHCC) approved the use of probiotics for the treatment of the patients suffering from intestinal dysbiosis due to the COVID-19 infection and to prevent the secondary bacterial infections that may occur [85], [86]. It is interesting to know that amongst the Asian countries, India, Korea, Japan, Indonesia, Nepal, Sri Lanka are the highest probiotic consumers [87]. Relating this fact with the COVID-19 situation, all these probiotic consuming countries are able to contain the pandemic to some extent, or we can say that there are less severity and mortality rate because of COVID-19. There might be many factors that may have contributed to this condition, but probiotic intake may also be an important factor and taking into consideration all the studies mentioned above probiotic intake on a regular basis increases the immunity of the individuals that may also help in controlling the severity of this pandemic to some extent. Furthermore, a recent review explained the possible relation of gut microbiota with the SARS-CoV-2, infections has been found to more severe in elderly and immuno-compromised individuals and interestingly dysbiosis of gut microbiota was implicated to these individuals suggesting a link between gut microbiota and SARS CoV-2. This adds to the link that supplementing such individuals with probiotics may help in managing the severity of the diseases by the immunomodulation property of the probiotic bacteria [88]. Similarly, another study showed the relation of gut microbiota with COVID-19 [86]. Considering the importance of a balanced diet/nutrition along containing required micronutrients and supplemented with probiotics may help in the management and prevention of COVID-19 [89]. Two types of probiotic bacteria, i.e., Lactobacilli and Bifidobacteria are considered to be non-pathogenic, but it cannot be concluded that they can be used as a treatment strategy against COVID-19 without full evaluation and confirmation through experimental studies [90]. Additionally, the association of probiotics and the immune system might be due to genetic interplay between different genes that have a role in maintaining mucosal health and the genes that are involved in the entry of the virus into the host cell. In order to observe the interaction, the network analysis was performed using the GeneMANIA server [91], ACE2 and TMPRSS2 genes that are involved in SARS CoV-2 infection [92], [93] and the genes involved in lactose metabolism like LCT [94], [95] (Fig. 2 ). Interestingly, all three genes were found to be interacting with each other directly and indirectly via different interactions (Fig. 2). After observing the interaction, we mined the literature and observe that ACE2 expression observed in lungs, epithelial cells, ileum, colon [96], and in epithelial cells of mucus membranes [97]. TMPRSS2 has also been observed in co-expression with ACE2 in mucosal cells of different organs [98]. Yunshan et al. [99] observe the role of the LCT gene in maintaining mucosal health in an animal model by improving the growth of probiotic bacteria. Lactase also aids in regulating the innate mucosal immune system. The role of the above-stated genes in mucosal health and entry of the virus in the human system may have a role in the severity of SARS CoV-2 infection. It could be possible that the individuals with good mucosal health may found affected asymptomatically with SARS CoV-2 due to poor expression of ACE2 along with TMPRSS2 and improved expression of LCT gene may prevent virus to enter into the host cells. This could be the reason that, due to same level of exposure of infection among different individuals, they may have different levels of severity of infection and varied recovery rate (especially in countries where intake of probiotics is high). Use of probiotics has opened up a ray of hope in the management of this pandemic and more studies in future may provide an evidence to use probiotics in controlling such pandemic.
Table 2.
Plausible strains of probiotic bacteria that may assist in reducing the COVID infection.
| Probiotic Bacteria | Effectiveness | References |
|---|---|---|
| L. reuteri DSM 17938 | Protection against upper respiratory tract infection | [100] |
| L. reuteri ATCC | Protect short term sick-leave caused by respiratory | [101] |
| L. casei | Protection against upper respiratory tract infection | [102] |
| Lactobacillus rhamnosus GG | Protection against severity of cold symptoms | [103] |
| Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 | Improve mucosal immune function | [104] |
| Lactobacillus paracasei | ||
| Lactobacillus plantarum HEAL9 | Reduce the respiratory infection during cold and strengthen the immune | [105] |
| Lactobacillus paracasei 8700:2 | ||
| Lactobacillus paracasei MCC1849 | Improve secretion of antigen-specific IgA | [106] |
| Lactobacillus casei (DN-114 001) | Protection against upper respiratory tract infection | [107] |
| Lactobacillus plantarum L-137 | Protection against upper respiratory tract infection | [108] |
| Enterococcus faecalis FK-23 | Stabilizing the integrity of the alveolar-capillary barrier | [109] |
| Saccharomyces boulardii | Reduce cytokines inflammation and systemic inflammatory response in lung injury | [110] |
| Bifidobacterium animalis(Bb12) | Influence NK- and T-cell function in upper respiratory tract infection | [111] |
| Bifidobacterium lactisB94 | Protection against acute respiratory tract infections | [112] |
| Lactococcus lactisJCM5805 | Induce anti-viral response against respiratory viral infection via enhancing lung immune response through activation of Plasmacytoid dendritic cells | [113] |
Fig. 2.
Network analysis of the genes involved in the lactose metabolism and SARS-CoV-2 infection.
5. Conclusion
In the review, we tried to provide an insight into/about the role of bioactive compounds and probiotics against the symptomatic complications associated with the COVID. We also highlight that bioactive compounds and probiotics have an immune-modulatory role by regulating innate and acquired immunity. Keeping in view the above stated effectiveness and exiguous scientific literature available on the issue, it is pertinent to examine/probe the said effectiveness against COVID infection in detail.
Declaration of Competing Interest
All the authors declare no conflict of interest.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd
References
- 1.Kris-Etherton P.M., Hecker K.D., Bonanome A., et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002;113(Suppl 9B):71S–88S. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 2.Siriwardhana N., Kalupahana N.S., Cekanova M., et al. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013;24(4):613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Carbonell-Capella J.M., Buniowska M., Esteve M.J., et al. Effect of Stevia rebaudiana addition on bioaccessibility of bioactive compounds and antioxidant activity of beverages based on exotic fruits mixed with oat following simulated human digestion. Food Chem. 2015;184:122–130. doi: 10.1016/j.foodchem.2015.03.095. [DOI] [PubMed] [Google Scholar]
- 4.Hassimotto N.M., Genovese M.I., Lajolo F.M. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J. Agric. Food Chem. 2005;53(8):2928–2935. doi: 10.1021/jf047894h. [DOI] [PubMed] [Google Scholar]
- 5.Yan F., Polk D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011;27(6):496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behnsen J., Deriu E., Sassone-Corsi M., et al. Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med. 2013;3(3):a010074. doi: 10.1101/cshperspect.a010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zu Z.Y., Jiang M.D., Xu P.P., et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020;296(2):200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu A.P., Peng Y.S., Huang B.Y., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCutcheon A.R., Roberts T.E., Gibbons E., et al. Antiviral screening of British Columbian medicinal plants. J. Ethnopharmacol. 1995;49(2):101–110. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlietinck A.J., Vanden Berghe D.A. Can ethnopharmacology contribute to the development of antiviral drugs? J. Ethnopharmacol. 1991;32(1–3):141–153. doi: 10.1016/0378-8741(91)90112-q. [DOI] [PubMed] [Google Scholar]
- 13.Setlur A.S., Naik S.Y., Skariyachan S. Herbal lead as ideal bioactive compounds against probable drug targets of Ebola virus in comparison with known chemical analogue: a computational drug discovery perspective. Interdiscip Sci. 2017;9(2):254–277. doi: 10.1007/s12539-016-0149-8. [DOI] [PubMed] [Google Scholar]
- 14.Santos D.I., Saraiva J.M.A., Vicente A.A., et al. Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds. Elsevier; 2019. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. p. 23-54. [DOI] [Google Scholar]
- 15.Segneanu A.E., Velciov S.M., Olariu S., et al. Bioactive molecules profile from natural compounds, in amino acid—new insights and roles in plant and animal. Intech Open. 2017:209–228. doi: 10.5772/interchopen.68643. [DOI] [Google Scholar]
- 16.Boon A.C.M., Vos A.P., Garaus Y.M.F., et al. In vitro effect of bioactive compounds on influenza virus specific B-and T-cell responses. Scand. J. Immunol. 2002;55(1):24–32. doi: 10.1046/j.1365-3083.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 17.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couch R.B., Englund J.A. Respiratory viral infections in immunocompetent and immunocompromised persons. Am. J. Med. 1997;102(3):2–9. doi: 10.1016/S0002-9343(97)00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S.Y., Chen C., Zhang H.Q., et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen C.C., Kuo Y.H., Jan J.T., et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50(17):4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 21.Hoever G., Baltina L., Michaelis M., et al. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48(4):1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- 22.Chon H. Elsevier; 2012. Medicinal herbs and plant extracts for influenza: bioactivity, mechanism of anti-influenza effects, and modulation of immune responses, in Studies in Natural Products Chemistry. p. 305-323. [DOI] [Google Scholar]
- 23.Galanakis C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods. 2020;9(4):523. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alipio M., Vitamin D. 2020. supplementation could possibly improve clinical outcomes of patients infected with Coronavirus-2019 (COVID-19) Available at SSRN 3571484. [DOI] [Google Scholar]
- 25.Grant W.B., Lahore H., McDonnell S.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tallei T.E., Tumilaar S.G., Niode N.J., et al. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: a molecular docking study. Prepr. 2020 doi: 10.20944/preprints202004.0102.v3. 2020040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W., Moore M., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuba K., Imai Y., Rao S., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gheblawi M., Wang K., Viveiros A., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klaus W.L., Yukiko N. Food bioactives, micronutrients, immune function and COVID-19. J. Food Bioactive. 2020;10 doi: 10.31665/JFB.2020.10222. [DOI] [Google Scholar]
- 31.A.K. Mishra, S.P. Tewari, In silico screening of some naturally occurring bioactive compounds predicts potential inhibitors against SARS-COV-2 (COVID-19) protease. arXiv preprint arXiv:2004.01634, (2020).
- 32.Khaerunnisa S., Kurniawan H., Awaluddin R., et al. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Prepr. 2020:1–14. doi: 10.20944/preprints202003.0226.v1. [DOI] [Google Scholar]
- 33.Balkrishna A., Pokhrel S., Singh J., et al. Withanone from Withania somnifera may inhibit novel Coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. Research Square. 2020 doi: 10.21203/rs.3.rs-17806/v1. [DOI] [Google Scholar]
- 34.Yu M.S., Lee J., Lee J.M., et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talukdar J., Dasgupta S., Nagle V., et al. COVID-19: potential of microalgae derived natural astaxanthin as adjunctive supplement in alleviating cytokine storm. Prepr. 2020:3579738. doi: 10.2139/ssrn.3579738. [DOI] [Google Scholar]
- 36.Perez-Lopez A., Behnsen J., Nuccio S.P., et al. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016;16(3):135–148. doi: 10.1038/nri.2015.17. [DOI] [PubMed] [Google Scholar]
- 37.Goto Y., Uematsu S., Kiyono H. Epithelial glycosylation in gut homeostasis and inflammation. Nat. Immunol. 2016;17(11):1244–1251. doi: 10.1038/ni.3587. [DOI] [PubMed] [Google Scholar]
- 38.Samuelson D.R., Welsh D.A., Shellito J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichinohe T., Pang I.K., Kumamoto Y., et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guandalini S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011;45(Suppl):S149–S153. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 41.Park M.K., NGO V., Kwon Y.M., et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8(10):e75368. doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehtoranta L., Pitkäranta A., Korpela R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect Dis. 2014;33(8):1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill C., Guarner F., Reid G., et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 44.Taverniti V., Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6(3):261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isolauri E., Kirjavainen P., Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut. 2002;50(Suppl 3):III54–III59. doi: 10.1136/gut.50.suppl_3.iii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumpu M., Kekkonen R.A., Korpela R., et al. Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Benef. Microbes. 2015;6(5):631–639. doi: 10.3920/BM2014.0164. [DOI] [PubMed] [Google Scholar]
- 47.Sindhu K.N.C., Sowmyanarayanan T.V., Paul A., et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2014;58(8):1107–1115. doi: 10.1093/cid/ciu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleeson M., Bishop N.C., Struszczak L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: a placebo-controlled, randomized trial. Eur. J. Appl. Physiol. 2016;116(8):1555–1563. doi: 10.1007/s00421-016-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Puyenbroeck K., Hens N., Coenen S., et al. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am. J. Clin. Nutr. 2012;95(5):1165–1171. doi: 10.3945/ajcn.111.026831. [DOI] [PubMed] [Google Scholar]
- 50.Jespersen L., Taronw I., Eskesen D., et al. Effect of Lactobacillus paracasei subsp. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Am. J. Clin. Nutr. 2015;101(6):1188–1196. doi: 10.3945/ajcn.114.103531. [DOI] [PubMed] [Google Scholar]
- 51.Rizzardini G., Eskesen D., Calder P.C., et al. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br. J. Nut. 2012;107(6):876–884. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 52.Pedone C.A., Arnaud C.C., Postaire E.R., et al. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int. J. Clin. Pract. 2000;54(9):568–571. [PubMed] [Google Scholar]
- 53.Nagai T., Makino S., Ikegami S., et al. Effects of oral administration of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 and its exopolysaccharides against influenza virus infection in mice. Int Immunopharmacol. 2011;11(12):2246–2250. doi: 10.1016/j.intimp.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Makino S., Sato A., Goto A., et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016;99(2):915–923. doi: 10.3168/jds.2015-10376. [DOI] [PubMed] [Google Scholar]
- 55.Hirose Y., Murosaki S., Yamamoto Y., et al. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J. Nutr. 2006;136(12):3069–3073. doi: 10.1093/jn/136.12.3069. [DOI] [PubMed] [Google Scholar]
- 56.Arimori Y., Nakamura R., Hirose Y., et al. Daily intake of heat-killed Lactobacillus plantarum L-137 enhances type I interferon production in healthy humans and pigs. Immunopharmacol Immunotoxicol. 2012;34(6):937–943. doi: 10.3109/08923973.2012.672425. [DOI] [PubMed] [Google Scholar]
- 57.Hirose Y., Yamamoto Y., Yoshikai Y., et al. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J. Nutr. Sci. 2013;6;2:e39. doi: 10.1017/jns.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oo K.M., Lwin A.A., Kyaw Y.Y., et al. Safety and long-term effect of the probiotic FK-23 in patients with hepatitis C virus infection. Biosci. Microbiota. Food Health. 2016;35(3):123–128. doi: 10.12938/bmfh.2015-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grandy G., Medina M., Soria R., et al. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis. 2010;25:253. doi: 10.1186/1471-2334-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugimura T., Jounai K., Ohshio K., et al. Immunomodulatory effect of Lactococcus lactis JCM5805 on human plasmacytoid dendritic cells. Clin. Immunol. 2013;149(3):509–518. doi: 10.1016/j.clim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Sugimura T., Takahashi H., Jounai K., et al. Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br. J. Nutr. 2015;114(5):727–733. doi: 10.1017/S0007114515002408. [DOI] [PubMed] [Google Scholar]
- 62.Kawahara T., Takahashi T., Oishi K., et al. Consecutive oral administration of Bifidobacterium longum MM-2 improves the defense system against influenza virus infection by enhancing natural killer cell activity in a murine model. Microbiol Immunol. 2015;59(1):1–12. doi: 10.1111/1348-0421.12210. [DOI] [PubMed] [Google Scholar]
- 63.Iwabuchi N., Xiao J.Z., Yaeshima T., et al. Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol. Pharm. Bull. 2011;34(8):1352–1355. doi: 10.1248/bpb.34.1352. [DOI] [PubMed] [Google Scholar]
- 64.Rizzardini G., Eskesen D., Calder P.C., et al. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012;107(6):876–884. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
- 65.Kang J.Y., Lee D.Y., Ha N.J., et al. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J. Microbiol. 2015;53(11):796–803. doi: 10.1007/s12275-015-5302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Vrese M., Winkler P., Rautenberg P., et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin. Nutr. 2005;24(4):481–491. doi: 10.1016/j.clnu.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Saavedra J.M., Bauman N.A., Oung I., et al. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344(8929):1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez-Ochoa G., Flores-Mendoza L.K., Icedo-Garcia R., et al. Modulation of rotavirus severe gastroenteritis by the combination of probiotics and prebiotics. Arch. Microbiol. 2017;199(7):953–961. doi: 10.1007/s00203-017-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enaud R., Prevel R., Ciarlo E., et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front. Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar V., Baruah K., Nguyen D.V., et al. Phloroglucinol-mediated Hsp70 production in crustaceans: protection against Vibrio parahaemolyticus in Artemia franciscana and Macrobrachium rosenbergii. Front. Immunol. 2018;9:1091. doi: 10.3389/fimmu.2018.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sencio V., Barthelemy A., Tavares L.P., et al. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020;30(9):2934–2947. doi: 10.1016/j.celrep.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 72.Gao Q.Y., Chen Y.X., Fang J.Y. 2019 novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2020;21(3):125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu K., Cai H.L., Shen Y.H., et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Journal of Zhejiang University (medical science) 2020;49(1):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Pierro F. A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SARS-CoV-2. Minerva. Med. 2020;111(3):281–283. doi: 10.23736/s0026-4806.20.06570-2. [DOI] [PubMed] [Google Scholar]
- 75.Getahun H., Gunneberg C., Granich R., et al. HIV infection—associated tuberculosis: the epidemiology and the response. Clin. Infect Dis. 2010;50(Supplement_3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 76.Chai W.D., Burwinkel M., Wang Z.Y., et al. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch. Virol. 2013;158(4):799–807. doi: 10.1007/s00705-012-1543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y.S., Liu Q., Jiang Y.L., et al. Surface-displayed porcine IFN-λ3 in Lactobacillus plantarum inhibits porcine enteric coronavirus infection of porcine intestinal epithelial cells. J. Microbiol. Biotechnol. 2020;30(4):515–525. doi: 10.4014/jmb.1909.09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar R.V.J., Seo B.J., Mun M.R., et al. Putative probiotic Lactobacillus spp. from porcine gastrointestinal tract inhibit transmissible gastroenteritis coronavirus and enteric bacterial pathogens. Trop Anim. Health Prod. 2010;42(8):1855–1860. doi: 10.1007/s11250-010-9648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang K., Ran L., Yan T., et al. Anti-TGEV miller strain infection effect of Lactobacillus plantarum supernatant based on the JAK-STAT1 signaling pathway. Front Microbiol. 2019;10:2540. doi: 10.3389/fmicb.2019.02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shoaib A., Xin L., Xin Y. Oral administration of Lactobacillus acidophilus alleviates exacerbations in Pseudomonas aeruginosa and Staphylococcus aureus pulmonary infections. Pak. J. Pharm. Sci. 2019;32:1621–1630. [PubMed] [Google Scholar]
- 81.Ceribelli A., Motta F., De Santis M., et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J. Autoimmun. 2020;109:102442. doi: 10.1016/j.jaut.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahooti M., Abdolalipour E., Salehzadeh A., et al. Immunomodulatory and prophylactic effects of Bifidobacterium bifidum probiotic strain on influenza infection in mice. World J. Microbiol. Biotechnol. 2019;35(6):91. doi: 10.1007/s11274-019-2667-0. [DOI] [PubMed] [Google Scholar]
- 83.Eguchi K., Fujitani N., Nakagawa H., et al. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pu F.F., Guo Y., Lin M., et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open-label trial. Clin. Interv. Aging. 2017;12:1223–1231. doi: 10.2147/cia.s141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.D’Amico F., Baumgart D.C., Danses S., et al. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin. Gastroenterol. Hepatol. 2020;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao A.T., Tong Y.X., Gao C., et al. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J. Clin. Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raghuwanshi S., Misra S., Bisen P. Indian perspective for probiotics: a review. Indian J. Dairy Sci. 2015;68(3):195–205. [Google Scholar]
- 88.Dhar D., Mohanty A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jayawardena R., Sooriyaarachchi P., Chourdakis M., et al. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab. Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mak J.W., Chan F.K., Ng S.C. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol. Hepatol. 2020;5(7):644–645. doi: 10.1016/s2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franz M., Rodriguez H., Lopes C., et al. GeneMANIA update 2018. Nucleic Acids Res. 2018;46(W1):W60–W64. doi: 10.1093/nar/gky311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walls A.C., Park Y.J., Tortorici M.A., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.058. 281-292 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arnold J.W., Simpson J.B., Roach J., et al. Prebiotics for lactose intolerance: variability in galacto-oligosaccharide utilization by intestinal Lactobacillus rhamnosus. Nutrients. 2018;10(10):1517. doi: 10.3390/nu10101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jarvela I., Torniainen S., Kolho K.L. Molecular genetics of human lactase deficiencies. Ann. Med. 2009;41(8):568–575. doi: 10.1080/07853890903121033. [DOI] [PubMed] [Google Scholar]
- 96.Zou X., Chen K., Zou J.W., et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu H., Zhong L., Deng J.X., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H., Kang Z.J., Gong H.Y., et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69(6):1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- 99.He Y., Tang Y., Peng M., et al. Influence of Debaryomyces hansenii on bacterial lactase gene diversity in intestinal mucosa of mice with antibiotic-associated diarrhea. PLoS One. 2019;14(12):e0225802. doi: 10.1371/journal.pone.0225802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gutierrez-Castrellon P., Lopez-Velazquez G., Diaz-Garcia L., et al. Diarrhea in preschool children and Lactobacillus reuteri: a randomized controlled trial. Pediatrics. 2014;133(4):e904–e909. doi: 10.1542/peds.2013-0652. [DOI] [PubMed] [Google Scholar]
- 101.Tubelius P., Stan V., Zachrisson A. Increasing work-place healthiness with the probiotic Lactobacillus reuteri: a randomised, double-blind placebo-controlled study. Environ. Health. 2005;4:25. doi: 10.1186/1476-069x-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pimentel-Nunes P., Soares J.B., Roncon-Albuquerque R., et al. Toll-like receptors as therapeutic targets in gastrointestinal diseases. Expert Opin. Ther. Targets. 2010;14(4):347–368. doi: 10.1517/14728221003642027. [DOI] [PubMed] [Google Scholar]
- 103.Liu S., Hu P.W., Du X.X., et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr. 2013;50(4):377–381. doi: 10.1007/s13312-013-0123-z. [DOI] [PubMed] [Google Scholar]
- 104.Yamamoto Y., Fujino K., Saruta J., et al. Effects of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on the IgA flow rate of saliva in elderly persons residing in a nursing home: a before-after non-randomised intervention study. Gerodontol. 2017;34(4):479–485. doi: 10.1111/ger.12296. [DOI] [PubMed] [Google Scholar]
- 105.Ahren I.L., Berggren A., Teixeira C., et al. Evaluation of the efficacy of Lactobacillus plantarum HEAL9 and Lactobacillus paracasei 8700:2 on aspects of common cold infections in children attending day care: a randomised, double-blind, placebo-controlled clinical study. Eur. J. Nutr. 2020;59(1):409–417. doi: 10.1007/s00394-019-02137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arai S., Iwabuchi N., Takahashi S., et al. Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS One. 2018;13(6):e0199018. doi: 10.1371/journal.pone.0199018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guillemard E., Tondu F., Lacoin F., et al. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010;103(1):58–68. doi: 10.1017/s0007114509991395. [DOI] [PubMed] [Google Scholar]
- 108.Hirose Y., Yamanoto Y., Yoshikai Y., et al. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J. Nutr. Sci. 2013;2:e39. doi: 10.1017/jns.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fukada K., Fujikura D., Nakayama Y., et al. Enterococcus faecalis FK-23 affects alveolar-capillary permeability to attenuate leukocyte influx in lung after influenza virus infection. Springerplus. 2013;2(1):269. doi: 10.1186/2193-1801-2-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karen M., Yuksel O., Akyürek N., et al. Probiotic agent Saccharomyces boulardii reduces the incidence of lung injury in acute necrotizing pancreatitis induced rats. J. Surg. Res. 2010;160(1):139–144. doi: 10.1016/j.jss.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 111.Meng H., Lee Y., Ba Z., et al. Consumption of Bifidobacterium animalis subsp. lactis BB-12 impacts upper respiratory tract infection and the function of NK and T cells in healthy adults. Mol. Nutr. Food Res. 2016;60(5):1161–1171. doi: 10.1002/mnfr.201500665. [DOI] [PubMed] [Google Scholar]
- 112.Jungersen M., Wind A., Johansen E., et al. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12(®) Microorganisms. 2014;2(2):92–110. doi: 10.3390/microorganisms2020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jounai K., Sugimura T., Ohshio K., et al. Oral administration of Lactococcus lactis subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PLoS One. 2015;10(3):e0119055. doi: 10.1371/journal.pone.0119055. [DOI] [PMC free article] [PubMed] [Google Scholar]