This editorial refers to ‘Classical monocyte transcriptomes reveal significant anti-inflammatory statin effect in women with chronic HIV’ by E. Ehinger et al., pp. 1166–1177.
Atherosclerosis, the underlying cause of myocardial infarction, ischaemic stroke and limb ischaemia, is a chronic inflammatory disease characterized by the progressive accumulation of lipoproteins and leucocytes in the arterial vessel wall.1 Monocytes, precursors of macrophages, contribute critically to all phases of atherosclerosis and the outcome of its complications. Upon production and release from the bone marrow, inflammatory blood monocytes, characterized as Ly6Chigh in mice and CD14++CD16− in humans,2 fuel atherosclerosis lesion formation by binding to activated endothelium, migrating into the vessel wall, and differentiation into macrophages.3 Circulating CD14++CD16− blood monocytes numbers correlate closely with disease progression and the cardiovascular event rate in patients.4
About 40 years after the observation of the first cases of acquired immunodeficiency syndrome (AIDS), 1.7 million people become newly infected with the disease-causing human immunodeficiency virus (HIV) every year.5 Of 38 million people living with HIV, 26 million are accessing antiretroviral therapy (ART). While CD4+ T cells represent the primary target of HIV, myeloid cells can also be infected by HIV.6 In patients with undetectable plasma virus loads under ART, blood monocytes are reservoirs for replication-competent HIV.7 Patients with persistent HIV infection, despite effective viral suppression under ART, display a more than two-fold increased risk for cardiovascular disease.8 The increased prevalence of atherosclerosis in people living with HIV has been associated with blood monocyte CD11b and CX3CR1 surface marker abundance9 and their potential to form disease-promoting foam cells ex vivo.10
In their article entitled ‘Classical monocyte transcriptomes reveal significant anti-inflammatory statin effect in women with chronic HIV’, Ehinger et al11 report the results of whole transcriptome sequencing (RNA-Seq) performed on classical blood monocytes (CM) obtained from participants of the Women’s Interagency HIV Study (WIHS). The investigators took advantage of this unique resource by selecting blood samples from a total of 92 study participants, which were equally divided into four study cohorts based on HIV status (H+ or H−) and the presence of carotid artery plaque as assessed by ultrasound examination (C+ or C−): H−C−, H+C−, H−C+, and H+C+. Cryopreserved blood samples were processed in a standardized fashion and CM were flow-sorted as live CD3− CD19− CD56− CD66b− CD14++ CD16−, before each CM sample was subjected to bulk RNA-Seq (Figure 1).
Figure 1.
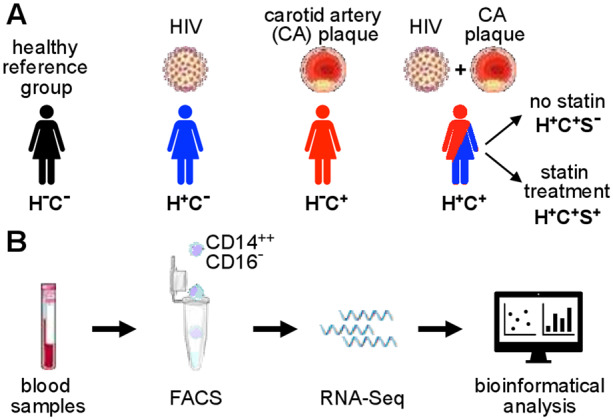
Schematic illustration of study participant groups and classical monocyte transcriptome analysis. (A) A total of 92 participants of the WIHS were selected based on HIV status (H− or H+) and the presence of carotid artery plaque as assessed by ultrasound examination (C− or C+). Within the group with both HIV and carotid artery plaque (H+C+), a subgroup analysis was performed based on statin treatment (S− or S+). (B) From previously frozen blood samples, classical monocytes were isolated by fluorescence-activated cell sorting. Whole transcriptome analysis was performed (RNA-Seq) followed by bioinformatical analysis.
The authors found significantly altered gene expression patterns in CM from both H+C− and H−C+ cohorts when compared to H−C−, the control group. Displaying high consistency within each group and clearly outweighing down-regulated genes, 204 and 179 genes were significantly overexpressed in CM from H+C− and H−C+, respectively. Enabled by their resourceful study design, Ehinger et al. found striking similarities between these two groups, with 42 genes up-regulated in both groups, including pro-inflammatory cytokines like Ιnterleukin 1α and Interleukin 6 (IL6). Accordingly, subsequent pathway and gene regulation analysis prominently featured IL6, High Mobility Group Box protein 1 (HMGB1), and NF-κB signalling in H+C− and H−C+ groups in a comparable fashion. This compelling concordance in blood monocyte transcriptomes found in patients with either HIV infection or early stages of atherosclerosis points towards a shared myeloid cell activation pattern. The fact that 91% of HIV patients without carotid artery plaque (H+C−) were on ART and 61% had undetectable HIV loads illustrates that the pro-inflammatory circulating monocyte phenotype is persistent under effective pharmacological suppression of virus replication.
Next, the investigators compared the transcriptomes of CM from study participants with HIV and carotid artery plaque (H+C+) to the H−C− reference group. Unexpectedly, the authors found only modest changes in CM gene expression with just 9 differentially regulated genes in H+C+ compared to H−C−. As 43% of the H+C+ cohort received statin medication (compared to only 22% in the H−C+ group), Ehinger et al. hypothesized that statin treatment may be a potential confounder. Indeed, a comparison of H+C+ study participants without statin use (H+C+S−) to the H−C− control group revealed 118 significantly overexpressed genes. Upstream regulatory pathway analysis showed activation of pro-inflammatory pathways like IL6, HMGB1, and NF-κB signalling as previously seen in samples from H+C− and H−C+ patients. Of note, the pattern of up-regulated genes in CM from H+C+S− was overall distinct from the two other disease groups, but also overlapped with a panel of 27 genes that were significantly increased in all three cohorts (H+C−, H−C+, and H+C+S−). In contrast to samples from patients with HIV and atherosclerosis without statin use (H+C+S−), CM from H+C+ study participants with ongoing statin treatment showed no relevant gene expression changes when compared to the H−C− reference group, further arguing in favour of statin medication as a main confounder.
Finally, the authors identified a gene cluster, comprising the cytokine CXCL2 and Tissue Inhibitor of Metalloproteinases 1, among others, which was up-regulated in CM from H+C+S+ when compared to H+C+S−. Overexpression of these genes, referred to as cluster 9, has previously been associated with acute cardiovascular events like acute myocardial infarction and plaque rupture, suggesting a potentially lower event risk in the statin-treated subgroup with HIV and atherosclerosis.
In their thoughtful gene expression study on human blood monocytes, the authors shed light on inflammatory myeloid cells at the intersection of infection and cardiovascular disease. Over the past years, we have learned that modifiable lifestyle factors, including unhealthy diet, psycho-social stress, sleep deprivation, and lack of physical activity, negatively impact atherosclerosis and cardiovascular event risk by triggering inflammatory myeloid cell abundance.12 Growing evidence suggests that this concept can be expanded to viral infections like influenza and hepatitis C.13,14 The striking similarities in gene expression of circulating monocytes from patients with either chronic HIV infection or early-stage atherosclerosis, as reported by Ehinger et al., point towards a common activation pattern of myeloid cells. Of note, this finding emerged from whole transcriptome analysis, preventing investigator bias towards established mechanisms. Inflammatory monocyte activation was lacking in patients with HIV and atherosclerosis (H+C+) on statin treatment, indicating anti-inflammatory action of statins in this cohort. As there was no relevant difference in blood cholesterol levels between H+C+ patients with or without statin use, this effect might be assigned to pleiotropic statin effects, potentially targeting endothelial cells as well as leucocytes.15 End-point oriented clinical studies will ultimately have to resolve the question if statin treatment can lower the burden of cardiovascular disease in HIV patients.16
Conflict of interest: M.N. has been a paid consultant or received research support from Takeda, Novartis, GSK, Medtronic, Verseaux, Sigilon, Alnylam, IFM Therapeutics, and Molecular Imaging Inc. The authors declare no conflicts of interest, financial or otherwise.
Funding
This work was funded in part by federal funds from the National Institutes of Health (HL142494, HL139598 and HL125428) and the MGH Research Scholar Program.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874. [DOI] [PubMed] [Google Scholar]
- 2. Geissmann F, Jung S, Littman DR.. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 3. Swirski FK, Nahrendorf M, Libby P.. Mechanisms of myeloid cell modulation of atherosclerosis. Microbiol Spectr 2016;4:27726819. DOI:10.1128/microbiolspec.MCHD-0026-2015 [DOI] [PubMed] [Google Scholar]
- 4. Berg KE, Ljungcrantz I, Andersson L, Bryngelsson C, Hedblad B, Fredrikson GN, Nilsson J, Björkbacka H.. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet 2012;5:122–131. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. Global HIV and AIDS Statistics - 2020 Fact Sheet. www.unaids.org/en/resources/fact-sheet (5 February 2021, date last accessed).
- 6. Wong ME, Jaworowski A, Hearps AC.. The HIV reservoir in monocytes and macrophages. Front Immunol 2019;10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sonza S, Mutimer HP, Oelrichs R, Jardine D, Harvey K, Dunne A, Purcell DF, Birch C, Crowe SM.. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS 2001;15:17–22. [DOI] [PubMed] [Google Scholar]
- 8. Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, Rajagopalan S, Kottilil S, Nair H, Newby DE, McAllister DA, Mills NL.. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018;138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westhorpe CL, Maisa A, Spelman T, Hoy JF, Dewar EM, Karapanagiotidis S, Hearps AC, Cheng WJ, Trevillyan J, Lewin SR, Sviridov D, Elliott JH, Jaworowski A, Dart AM, Crowe SM.. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol 2014;92:133–138. [DOI] [PubMed] [Google Scholar]
- 10. Angelovich TA, Trevillyan JM, Hoy JF, Wong ME, Agius PA, Hearps AC, Jaworowski A.. Monocytes from men living with HIV exhibit heightened atherogenic potential despite long-term viral suppression with antiretroviral therapy. AIDS 2020;34:513–518. [DOI] [PubMed] [Google Scholar]
- 11. Ehinger E, , Ghosheh Y, , Pramod A B, , Lin J, , Hanna D B, , Mueller K, , Durant C P, , Baas L, , Qi Q, , Wang T, , Buscher K, , Anastos K, , Lazar J M, , Mack W J, , Tien P C, , Cohen M H, , Ofotokun I, , Gange S, , Heath S L, , Hodis H N, , Tracy R P, , Landay A L, , Kaplan R C, , Ley K. Classical Monocyte Transcriptomes Reveal Significant Anti-Inflammatory Statin Effect in Women with Chronic HIV. Cardiovasc Res 2020; 32658258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schloss MJ, Swirski FK, Nahrendorf M.. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ Res 2020;126:1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yedlapati SH, Khan SU, Talluri S, Lone AN, Khan M, Khan MS, Navar AM, Gulati M, Johnson HM, Baum SJ, Michos ED.. Effects of influenza vaccine on mortality and cardiovascular outcomes Ii patients with cardiovascular disease: a systematic review and meta-analysis. Circulation 2020;142:A13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babiker A, Jeudy J, Kligerman S, Khambaty M, Shah A, Bagchi S.. Risk of cardiovascular disease due to chronic hepatitis C infection: a review. J Clin Transl Hepatol 2017;5:1–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oesterle A, Laufs U, Liao JK.. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017;120:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grinspoon SK, Fitch KV, Overton ET, Fichtenbaum CJ, Zanni MV, Aberg JA, Malvestutto C, Lu MT, Currier JS, Sponseller CA, Waclawiw M, Alston-Smith B, Cooper-Arnold K, Klingman KL, Desvigne-Nickens P, Hoffmann U, Ribaudo HJ, Douglas PS; REPRIEVE Investigators. Rationale and design of the randomized trial to prevent vascular events in HIV (REPRIEVE). Am Heart J 2019;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


