This editorial refers to ‘Ketones can become the major fuel source for the heart but do not increase cardiac efficiency’ by K.L. Ho et al., pp. 1178–1187.
Interest in the myocardial effects of exogenous ketones in both healthy and disease states has grown significantly in recent years.1 The human myocardium requires 35 L of oxygen to pump approximately 7200 L of blood per day against systemic vascular resistance to produce the requisite kilogram quantities of adenosine triphosphate (ATP) daily.2 Therefore, the prospect of leveraging ketones as an alternative fuel source, particularly in heart failure where insulin resistance and down-regulation of fatty acid oxidation have been reported,1,3 has generated significant interest.4–7 However, the interplay of exogenous ketones on competing substrate oxidation rates and cardiac efficiency is not well characterized. Such information provides necessary insight going forward as the ketogenic diet and exogenous ketosis become increasingly popular.
Ho et al.8 present novel and interesting data to address these important aims. The authors exposed isolated working murine hearts to glucose (at a fixed concentration), palmitate (using two concentrations), and beta-hydroxybutyrate (using three increasing concentrations), with and without the addition of insulin. The two concentrations of palmitate allowed inferences to be drawn regarding myocardial substrate use in the presence of high fat states, such as seen with the ketogenic diet. Use of glucose not only served as a substrate comparison, but also provided a source for anaplerosis, which is necessary to the continued utilization of cataplerotic ketones.1 Interestingly, myocardial ketone oxidation increased with increasing concentrations of ketones, even at the highest level studied. These results indicate that ketone oxidation occurs commensurately to their availability. In these studies, increasing ketones did not appear to significantly hinder oxidation of competing substrates.
Notably, the increase in myocardial ketone oxidation and tricyclic acid (TCA) cycle activity with higher concentrations of exogenous ketones was not accompanied by increases in cardiac ATP content or cardiac work. Furthermore, oxygen consumption continued to increase, with a resulting decrease in cardiac efficiency. The uncoupling between reducing equivalents and ATP production rates led the authors to speculate on several possible mechanisms including the role mitochondrial uncoupling proteins and the mitochondrial permeability transition pore. It seems plausible, that in the absence of increased demand and/or activation of stress signalling pathways, mitochondrial uncoupling may prevent ATP generation in the setting of excess fuel (including ketones, lactate, or palmitate).
Importantly, the decrease in myocardial efficiency with increasing ketone delivery might only be relevant to healthy myocardium under non-stressful conditions, and extrapolation to disease states may be limited. While exogenous ketone delivery in healthy myocardium may result in a ‘ceiling effect’ of cardiac work in the absence of heightened cardiac demand, pathologic conditions, and metabolic dysregulation that constrain cardiac work may favour improved pump function when supplemental fuels, including ketones, are available. Thus, states such as heart failure may afford the greatest clinical benefit.1 Further proof-of-concept stems from the authors’ previous work in heart failure, whereby oxygen consumption and TCA activity matched cardiac work,9 and clinical models of heart failure mirror these results.7 Yet, mild discordance between pre-clinical and clinical models of ‘healthy’ myocardium exists, as patients with mild cardiovascular comorbidities and no overt heart failure also seem to also augment oxygen consumption proportionately to stroke work, preserving myocardial efficiency.7
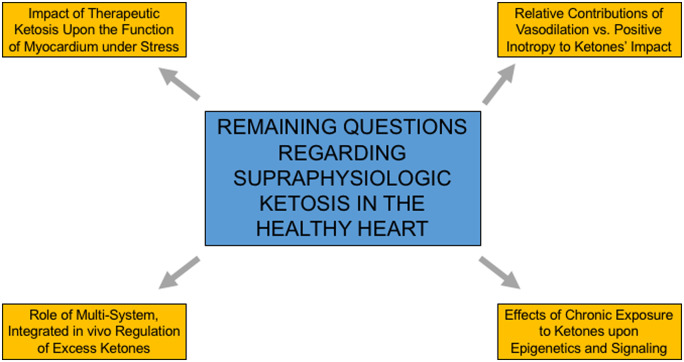
As with any novel data, this study generates more questions than it answers (Figure 1). For example, how does healthy myocardium respond in stressful situations to high concentrations of exogenous ketones with substrate oxidation and myocardial efficiency, considering benefit in exercise capacity has been observed with exogenous ketones?10 Is it possible that myocardial efficiency may normalize in this setting. Furthermore, how does the regulation of ketone metabolism and excretion influence these findings? At high levels of ketones, urinary excretion or exhalation of ketones may decrease myocardial exposure to supraphysiologic levels ketones, potentially acting as another safety mechanism to avoid unregulated ketone oxidation with resulting decreased myocardial efficiency. Do the potential vasodilatory effects of ketones actually augment cardiac work?1,7 Finally, the effects of chronic exposure of the myocardium to high concentrations of ketones (as may be seen with the ketogenic diet) on substrate competition are unknown, where transcriptional and epigenetics effects of ketones can add layers of complexity.
Figure 1.
Remaining questions regarding the effect of supraphysiologic ketosis in the healthy heart.
Therapeutic ketosis is a burgeoning field in cardiovascular disease, yet deeply in need of studies defining the effects of supraphysiologic ketone levels on myocardial metabolism. As such, we congratulate the authors for sharing their interesting findings that provide novel data on the complex interplay of ketones, competing substrate oxidation, and cardiac efficiency.
Disclosures
S.S. receives research grant support the National Institutes of Health (Training Grant 5-T32HL007843-23), the Doris Duke Charitable Foundation (Physician Scientist Fellowship Award 2020061), the Measey Foundation, Institute for Translational Medicine and Therapeutics (Junior Investigator Preliminary/Feasibility Grant Program award), and the American Society of Nuclear Cardiology (Institute for the Advancement of Nuclear Cardiology award) for work related to therapeutic ketosis. K.B.M. is supported by grants from the National Heart Lung and Blood Institute (U10 HL110338-07, R01 HL149891-01, and R01 HL151345-01).
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Selvaraj S, Kelly DP, Margulies KB.. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation 2020;141:1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ, American Heart Association Council on Basic Cardiovascular Sciences. Assessing cardiac metabolism: a scientific statement from the American Heart Association. Circ Res 2016;118:1659–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riehle C, Abel ED.. Insulin signaling and heart failure. Circ Res 2016;118:1151–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP.. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP.. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE.. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, Mellemkjaer S, Lassen TR, Pryds K, Bøtker HE, Wiggers H.. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ho KL, Karwi QG, Wagg C, Zhang L, Vo K, Altamimi T, Uddin GM, Ussher JR, Lopaschuk GD.. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc Res 2021;117:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD.. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 2019;115:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, King MT, Dodd MS, Holloway C, Neubauer S, Drawer S, Veech RL, Griffin JL, Clarke K.. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 2016;24:256–268. [DOI] [PubMed] [Google Scholar]