Abstract
Aims
Anthracycline-induced cardiotoxicity (AIC) is a serious adverse effect among cancer patients. A central mechanism of AIC is irreversible mitochondrial damage. Despite major efforts, there are currently no effective therapies able to prevent AIC.
Methods and results
Forty Large-White pigs were included. In Study 1, 20 pigs were randomized 1:1 to remote ischaemic preconditioning (RIPC, 3 cycles of 5 min leg ischaemia followed by 5 min reperfusion) or no pretreatment. RIPC was performed immediately before each intracoronary doxorubicin injections (0.45 mg/kg) given at Weeks 0, 2, 4, 6, and 8. A group of 10 pigs with no exposure to doxorubicin served as healthy controls. Pigs underwent serial cardiac magnetic resonance (CMR) exams at baseline and at Weeks 6, 8, 12, and 16, being sacrifice after that. In Study 2, 10 new pigs received 3 doxorubicin injections (with/out preceding RIPC) and were sacrificed at week 6. In Study 1, left ventricular ejection fraction (LVEF) depression was blunted animals receiving RIPC before doxorubicin (RIPC-Doxo), which had a significantly higher LVEF at Week 16 than doxorubicin treated pigs that received no pretreatment (Untreated-Doxo) (41.5 ± 9.1% vs. 32.5 ± 8.7%, P = 0.04). It was mainly due to conserved regional contractile function. In Study 2, transmission electron microscopy (TEM) at Week 6 showed fragmented mitochondria with severe morphological abnormalities in Untreated-Doxo pigs, together with upregulation of fission and autophagy proteins. At the end of the 16-week Study 1 protocol, TEM revealed overt mitochondrial fragmentation with structural fragmentation in Untreated-Doxo pigs, whereas interstitial fibrosis was less severe in RIPC+Doxo pigs.
Conclusion
In a translatable large-animal model of AIC, RIPC applied immediately before each doxorubicin injection resulted in preserved cardiac contractility with significantly higher long-term LVEF and less cardiac fibrosis. RIPC prevented mitochondrial fragmentation and dysregulated autophagy from AIC early stages. RIPC is a promising intervention for testing in clinical trials in AIC.
Keywords: Cardiotoxicity, Anthracyclines, Cardio-oncology, Remote conditioning, Mitochondria, Magnetic resonance imaging
Graphical Abstract
1. Introduction
Anthracyclines, used alone or in combination with other approaches, remain the first line therapy for many forms of cancer. Anthracycline-induced cardiotoxicity (AIC) is a well-known side effect of these agents, limiting the total lifetime cumulative dose a patient can receive.1 The mechanisms by which anthracyclines generate cardiotoxicity seem to converge on damage to mitochondria.2
Several definitions of cardiotoxicity have been proposed, with a consensus conception of cancer therapy-related cardiac dysfunction as a reduction in left ventricular ejection fraction (LVEF) of 10 absolute points to a value below the normal range for the imaging technique.3–6 Severe AIC is associated with chronic heart failure (HF), which translates into increased mortality.6
Current treatment strategies for established AIC include standard HF therapies (beta-blockers and ACEs inhibitors, among others). Early intervention with these therapies is of limited effect, providing partial recovery of cardiac function in only some patients.7 With the exception of dexrazoxane, which reduced cardiac injury in a paediatric population,8 specific therapies able to prevent the development of AIC are lacking.8–10 Given the central role of mitochondria in AIC, several interventions targeting mitochondrial processes have been tested, including antioxidants, iron chelators, and cell therapy; however, despite promising preclinical results, these approaches have failed to demonstrate a clinical benefit.11
Remote ischaemic conditioning is the process whereby brief, reversible episodes of ischaemia and reperfusion in one vascular bed (e.g. an arm) confers a global protection that renders remote tissues and organs resistant to injury.12 In the context of ischaemia/reperfusion injury (acute myocardial infarction), remote ischaemic conditioning has been consistently associated with cardioprotection.13 A central mechanism underlying the benefits of remote ischaemic conditioning is mitochondrial protection.12 Conditioning before the index injury [remote ischemic PREconditioning (RIPC)]14 has been shown to be very effective; however, initiation of conditioning during the index injury (remote ischaemic PERconditioning) confers a weaker protection.15 Nevertheless, anthracycline administration is a fully controlled and programmed intervention, and the cardiac injury event is thus predictable. Therefore, given the central role of mitochondrial damage in AIC, RIPC seems a good candidate therapy for testing in this context. In this study, we tested RIPC as a cardioprotective strategy in a validated large-animal model of AIC induced by serial intracoronary administrations of doxorubicin.16 The intracoronary approach offers the advantage of avoiding the myelosuppressive adverse effects of systemic administration of higher doses of anthracyclines that severely compromises long-term large-animal studies. Monitoring by cardiac magnetic resonance (CMR) revealed that the severe cardiac dysfunction associated with serial intracoronary doxorubicin administration is significantly attenuated by RIPC applied immediately before each anthracycline administration. RIPC prevented severe anthracycline-induced mitochondrial fragmentation and dysregulated autophagy.
2. Methods
2.1 Study design
All animal studies were conducted at the CNIC and approved by the local CNIC Institutional and regional animal research committees. All animal procedures conformed to EU Directive 2010/63EU and Recommendation 2007/526/EC regarding the protection of animals used for experimental and other scientific purposes.
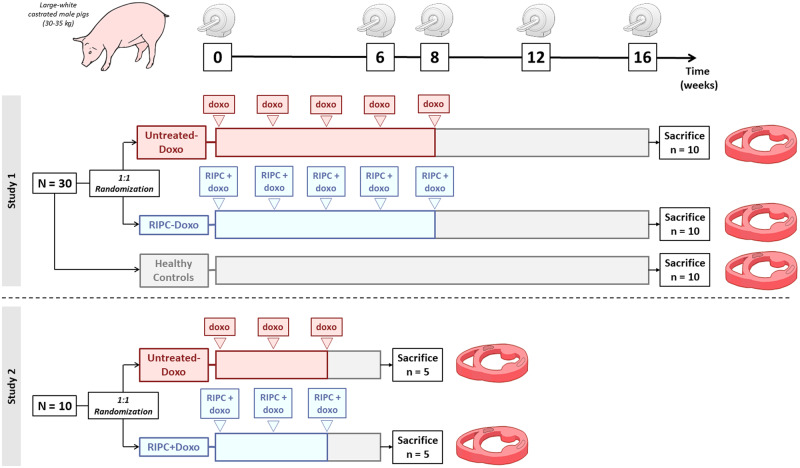
The study design is summarized in Figure 1. In Study 1, we tested the efficacy of RIPC in a model of overt AIC with long-term evaluation: 20 Large-White male pigs (3 months old, weighting ∼30 kg) received a series of 5 intracoronary doxorubicin injections in order to induce severe AIC.16 The 0.45 mg/kg injections were spaced 2 weeks apart (Weeks 0, 2, 4, 6, and 8). Before commencing injections, animals were randomized 1:1 to RIPC or no pretreatment. There were thus two AIC treatment groups: those with preconditioning (RIPC-Doxo) and those with no pretreatment (Untreated-Doxo). RIPC consisted on 3 cycles of 5 min lower limb ischaemia followed by 5 min reperfusion before each doxorubicin injection. All pigs underwent serial CMR exams at Weeks 0, 6, 8, 12, and 16. After the 16-week CMR exam, animals were sacrificed, and cardiac tissue was harvested for histology, transmission electron microscopy (TEM), and protein expression analysis. Another group of 10 pigs with no exposure to doxorubicin served as healthy controls.
Figure 1.
Study design. Study 1 consisted of 30 castrated Large-White male pigs; 20 pigs were randomized the AIC protocol with no pretreatment (Untreated-Doxo) or preceded at each cycle by RIPC (RIPC-Doxo). An additional 10 animals were monitored as healthy controls. CMR scans were performed at baseline (Week 0) and at 6, 8, 12, and 16 weeks. Animals were sacrificed, and hearts were excised for ex vivo analysis. Study 2 consisted of 10 animals randomized to Untreated-Doxo or RIPC-Doxo and sacrificed 2 weeks after the third doxorubicin dose. CMR scans were performed at baseline (Week 0) and at 6 weeks.
In Study 2, 10 new pigs (randomized to RIPC or no treatment) were administrated with only 3 doxorubicin intracoronary injections, a ‘subclinical’ protocol associated with preserved cardiac function.16 The RIPC-Doxo and Untreated-Doxo pigs in Study 2 underwent CMR exams at Weeks 0 and 6. After the 6-week CMR exam, pigs were sacrificed and cardiac tissue harvested for histology, TEM, and protein expression analysis.
2.2 Remote ischaemic preconditioning
RIPC was induced under general anaesthesia (20 mg/kg of ketamine, 2 mg/kg of xylazine, and 0.5 mg/kg of midazolam by intramuscularily injection) by securing a tourniquet around the right hind leg to produce transient ischaemia. Total ischaemia was assured by checking visually for the congestive reaction in the lower extremity and by Doppler echography in the inner side of the thigh, pressing the tourniquet until artery flow ceased. Each of the three RIPC cycles consisted of 5 min of total ischaemia followed by 5 min of reperfusion. The initiation of doxorubicin intracoronary injection was approximately 10 min after the end of the third conditioning cycle.
2.3 Intracoronary doxorubicin injection
Doxorubicin was administered according to a previously detailed protocol.16 In brief, animals were anaesthetized (20 mg/kg of ketamine, 2 mg/kg of xylazine, and 0.5 mg/kg of midazolam by intramuscularily injection) and maintained with intravenously perfusion of the same anaesthetics cocktail (per hour) they were also endotracheally intubated. Intramuscular buprenorphine (0.01 mg/kg) was also intramuscular administrated before the catheterism. The right femoral artery was then repeatedly accessed in each catheterization by the Seldinger technique, and a 5-Fr sheath was inserted. Pigs were anticoagulated with 150 IU/kg of intravenous heparin, and a 4-Fr coronary diagnostic catheter was inserted via a femoral sheath and placed at the origin of the left coronary artery. Under angiography guidance, a 0.014 mm coronary guide wire was positioned distally in the left anterior descending (LAD) coronary artery. The catheter was docked selectively in the proximal LAD, and a 0.45 mg/kg dose of doxorubicin (Farmiblastina®, Pfizer) diluted in 30 mL saline was given as a slow bolus injection over 3 min. Electrocardiographic and haemodynamic parameters were monitored during doxorubicin administration. Once infusion was complete, normal coronary flow was documented by coronary angiography, the material was removed, and the animal was allowed to recover.
2.4 CMR imaging
All studies were performed with a Philips 3-T Achieva Tx whole body scanner (Philips Healthcare, Best, The Netherlands) equipped with a 32-element phased-array cardiac coil. The CMR protocol included a standard segmented cine steady-state free-precession (SSFP) sequence to provide high-quality anatomical references, T1-mapping, and T1W late gadolinium enhancement (LGE) sequences. The imaging parameters for the cine SSFP sequence were as follows: field of view (FOV), 280 mm × 280 mm; slice thickness, 6 mm with no gaps; repetition time (TR), 2.8 ms, echo time (TE), 1.4 ms; flip angle, 45°; cardiac phases, 30; voxel size, 1.8 mm × 1.8 mm; and number of excitations, 3. The T1-mapping sequence (Modified Look-Locker Inversion recovery) was based on a 5(3)3 scheme using a single-shot SSFP readout sequence (TR/TE/flip angle = 2.1 ms/1.05 ms/350) with an in-plane acquisition resolution of 1.5 × 1.8 mm2 and an 8 mm slice thickness. The T1 mapping sequence was triggered at mid-diastole and acquired from a single short-axis mid-apical slice. LGE imaging was performed 15 min after intravenous administration of 0.2 mM/kg gadopentetate dimeglumine contrast agent using an 3D inversion recovery spoiled turbo field echo sequence (TR/TE/flip angle = 2.4 ms/1.13 ms/100) with an isotropic resolution of 1.5 × 1.5 × 1.5 mm3 on a FOV of 340 × 340 × 320 mm3 in the foot-head (FH), left-right (LR), and anterior-posterior (AP) directions. Data were acquired in mid-diastole with a 151.2 ms acquisition window. Acquisition was accelerated using a net SENSE factor of 2.25 (1.5 × 1.5 in the AP and LR directions) with a bandwidth of 853 Hz per pixel. Inversion time was adjusted before acquisition using a look-locker scout sequence with different inversion times to ensure proper nulling of the healthy myocardium signal. For the analysis, 3D volume was reconstructed in short axis, two chamber, and four chamber views with a slice thickness of 6 mm.
2.5 Image analysis
CMR studies were analysed by an experienced observer and reviewed by a blinded experienced independent observer using dedicated software (IntelliSpace Portal, Philips Healthcare, Best, the Netherlands). LV cardiac borders were traced in each cine image to obtain LV end-diastolic mass, LV end-diastolic volume (LVEDV), end-systolic volume (LVESV), and LVEF. Wall thickening values for the assessment of infused (LV region supplied by the LAD) and remote contractile function were also obtained by tracing cardiac borders in systole and diastole. T1 maps were generated by using a maximum-likelihood expectation–maximization algorithm and fitting the MR signal to a T1 inversion recovery with three independent model parameters. T1 relaxation maps were quantitatively analysed by placing a wide transmural region of interest at the infused myocardial area (irrigated by the LAD) of the corresponding slice in all studies.
2.6 Ex vivo analysis
After sacrifice by intravenous injection of pentobarbital in overdose (50 mg/kg intravenously), samples of the doxorubicin-infused region (anterior wall) and the remote area (posterior wall) were collected for histology, TEM, and protein expression analysis. For histology, samples were fixed in 4% formalin and embedded in paraffin, and 4-μm sections were cut and stained with haematoxylin and eosin, Masson trichrome, and Sirius Red. Sirius Red sections were scanned, and 20 × 20 magnification images were captured for collagen quantification using a modified macro.17
2.7 Transmission electron microscopy
Samples from the infused area were maintained in 4% glutaraldehyde in 10% paraformaldehyde for 24–48 h. Tissues were then post-fixed in 1% osmium tetroxide in water for 1 h at room temperature. Samples were washed with water and block-stained with 0.5% uracil acetate in water for 10 min. Samples were then dehydrated through a series of aqueous alcohol solutions (30%, 50%, 70%, 95%, and 100%) and a final passage through acetone. After this, samples were included in Durcupan epoxy resin through increasing resin:acetone mixtures (1:3 then 3:1) and, finally, pure resin. Resin-included samples were polymerized in an oven at 60°C for 48 h. Ultrathin (60 nm) slices were cut with a Leica Ultracut S ultramicrotome and were deposited on 200 mesh copper grids. Grids were counterstained with uranyl acetate and lead citrate. Images were obtained with a Jeol Jem1010 (100 kV) transmission electron microscope linked to a Gatan camera (Orius 200 SC model); acquired images were processed with Digital Micrograph software. Individual images were acquired using ImageJ software (1.52p Wayne Rasband from the US National Institutes of Health) at the following magnifications: 6000× for tissue evaluation, 10 000× for mitochondrial quantification (10 images with a total of ∼7440 μm2 of myocardium extension and a mean of ∼250 mitochondria per animal), and 40 000× for the assessment of mitochondrial cristae.
2.8 Western blotting
Heart tissue from infused area was lysed in Radioimmunoprecipitation assay (RIPA) buffer supplemented with a protease and phosphatase inhibitor cocktail. Protein content was quantified with the Bio-Rad Bicinchoninic acid (BCA) protein assay. Protein samples were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis, and proteins were transferred to nitrocellulose membranes. After blocking, membranes were incubated with the following antibodies: BECLIN1 (Cell Signaling, 3738S), p62 (Cell Signaling, 5114S), DRP1 (Cell Signaling, 8570S), and GAPDH (Abcam, ab8245) at 4°C overnight. Bound antibodies were detected after staining with a corresponding secondary antibody. Quantitative densitometric analysis was performed using ImageJ Fiji software.
2.9 mtDNA quantification
mtDNA was quantified by quantitative real-time polymerase chain reaction (PCR) using SYBR Green, and mtDNA content was presented as the ratio of mitochondria-encoded 16S to nuclear-encoded HPRT. 16S primers: forward 5´-CGATGTTGGATCAGGACACC-3´, reverse 5´-CTGAGACGCGTTTGTGAAGTT-3´. HPRT primers: forward 5´-GGCCAGTTCGGGAATGATCT-3´, reverse 5´-CCCCCAGTCCCCCAAATCTA-3´.
2.10 Statistical analysis
According to the data distribution, continuous variables were calculated as mean ± standard deviation (SD) or median ± interquartile range (IQR). In text, data are reported as mean ± SD, while in the main figure, data are reported as mean ± standard error (SE) for better visual interpretation. Data normality was assessed with the Shapiro–Wilk test. Cardiac mass and volume data were indexed by body weight using the modified Brody formula. Differences were considered statistically significant at P < 0.05. All data were analysed with RStudio [RStudio Team (2015): Integrated Development for RStudio, Inc., Boston, MA, USA], and graphics were created with ggplot2.
Sample size in each treatment group (RIPC-Doxo and Untreated-Doxo) was calculated in order to detect an 8% between-group difference in LVEF at 16-week CMR (study primary endpoint) with an SD of 6.5%, no casualties (previously defined in the animal model16) a power of 0.8, and 0.05 of bilateral significance. The primary endpoint is similar to that in other cardioprotection studies, and the sample size calculation resulted in 10 animals per group. Two-group comparisons were by t-test, taking account of RIPC exposure at the end of the study (16 weeks). Comparisons over time within each group for all the CMR data were made by repeated one-way analysis of variance (ANOVA) measurements with Bonferroni correction.
3. Results
During doxorubicin injections, none of the pigs showed adverse events (changes in electrocardiogram -ECG- or systolic arterial pressure). One pig randomized to RIPC died immediately after the third anthracycline injection due to a complication related to the invasive procedure and was replaced to maintain the pre-specified sample size. Another pig from Untreated-Doxo group died after the final CMR exam at 16 weeks, we decided not to use ex vivo samples for water quantification and TEM/WB from this animal.
3.1 RIPC prevents anthracycline-induced cardiac dysfunction
The series of five doxorubicin injections in Study 1 caused a progressive deterioration of LV systolic function in pigs allocated to the non-RIPC AIC group (Untreated-Doxo). LVEF remained unchanged until the fourth doxorubicin injection. From there on, LVEF progressively deteriorated to the end of the study. LV systolic function deterioration was significantly blunted in pigs receiving RIPC before each doxorubicin injection (RIPC-Doxo group) (Figure 2). LVEF at Week 16 (primary study endpoint) was significantly higher in the RIPC-Doxo group (41.5% ± 9.1% and 32.5% ± 8.7% for RIPC-Doxo vs. Untreated-Doxo; P = 0.04). Cardiac dysfunction was already overt at Week 12 CMR (LVEF = 43.2% ± 8.1% and 33.4% ± 9.1% in RIPC-Doxo vs. Untreated-Doxo; P = 0.02) (Figure 2A). There was no between-group difference in 16-week LVEDV (156.0 ± 38.4 mL and 168.0 ± 69.7 mL in RIPC-Doxo vs. Untreated-Doxo; p = 0.63), whereas LVESV was non-significantly lower in the RIPC group (93.1 ± 35.2 mL and 118.0 ± 64.3 mL in RIPC-Doxo vs. Untreated-Doxo; P = 0.30) (Supplementary material online, Figure S1). All CMR data from Study 1 are detailed in Supplementary material online, Table S1.
Figure 2.
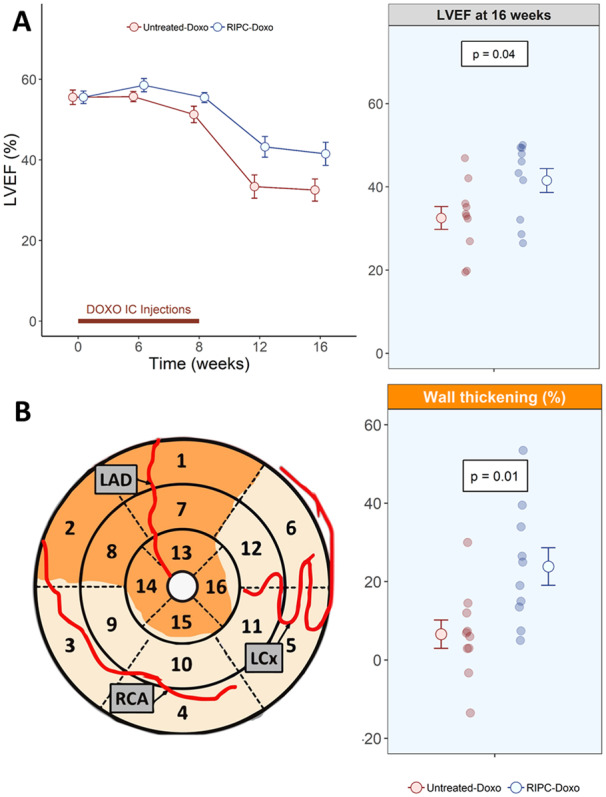
Functional CMR in Study 1. (A) LVEF over time (left) and at 16 weeks (right). Grouped data are presented as mean ± SE and individual data at the end of the study as a dotplot. N = 10 animals per group. The significance of differences between Untreated-Doxo and RIPC-Doxo groups at 16 weeks was assessed by the Student’s t-test. (B) Wall thickening at 16 weeks in the doxorubicin-infused area (mean of the AHA segments 7 and 8). Grouped data are presented as mean ± SE and individual data at the end of the study as a dotplot. N = 10 animals per group. The significance of differences between Untreated-Doxo and RIPC-Doxo at 16 weeks was assessed by the Student’s t-test.
More preserved LVEF after RIPC could be due to preserved contractile function in the doxorubicin-infused region or to a compensatory contractile function effect in the remote area. To test these possibilities, we measured wall thickening as an index of contractility in the infused and remote LV regions (Figure 2B). RIPC was associated with a significant contractile improvement only in the infused region (23.8% ± 15.2% and 6.59% ± 11.4% in RIPC-Doxo vs. Untreated-Doxo; P = 0.01, Figure 2B). Remote myocardium contractility did not differ significantly between groups (58.4% ± 28.1% vs. 45.4% ± 21.0% in RIPC-Doxo vs. Untreated-Doxo; P = 0.26) (Supplementary material online, Figure S2).
3.2 RIPC reduces myocardial fibrosis associated with anthracycline-induced cardiotoxicity
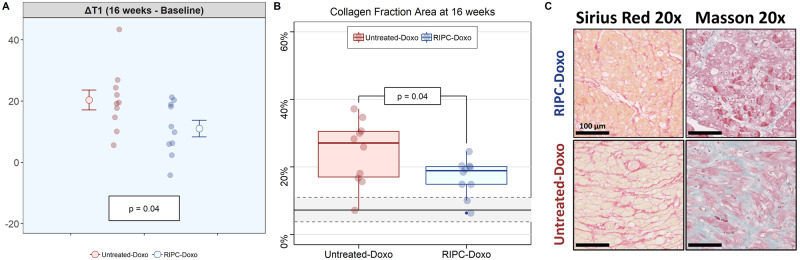
Myocardial tissue changes were evaluated in vivo by serial T1 mapping evaluations and ex vivo in samples harvested at the end of the 16-week protocol. T1 relaxation times increased from baseline to Week 16 in both treatment groups. In the RIPC-Doxo group, baseline T1 was 1150 ± 58 ms and increased to 1300 ± 109 ms at Week 16 (P = 0.03). In the Untreated-Doxo group, T1 increased from 1110 ± 63 ms at baseline to 1410 ± 108 ms at 16 weeks (P = 0.01). The increase in T1 relaxation time was significantly smaller in the RIPC group (11.0 ± 8.58% and 20.3 ± 10.3% in RIPC-Doxo vs. Untreated-Doxo; P = 0.04) (Figure 3A). Correlated with the CMR data, Sirius Red staining at the end of the study revealed a significantly smaller collagen area in the RIPC group (16.9% ± 5.4% and 24.4% ± 9.6% in RIPC-Doxo vs. Untreated-Doxo; P = 0.04) (Figure 3B).
Figure 3.
CMR and histology analysis of cardiac tissue. (A) Native T1 differential at the end of the study (16 weeks – baseline) (right). Grouped data are presented as mean ± SE and individual data at the end of study as a dotplot. N = 10 animals per group. The significance of differences between Untreated-Doxo and RIPC-Doxo groups at 16 weeks was assessed by the Student’s t-test. (B) Collagen fraction area at 16 weeks. Samples were obtained from the medium antero-septal region. Data are presented grouped in a boxplot and ungrouped (individual data) as a dotplot. N = 10 animals per group. Shaded grey lines represented mean and standard deviation of the collagen data from Healthy Control group. The significance of differences between Untreated-Doxo and RIPC-Doxo at 16 weeks was assessed by the Student’s t-test. (C) Representative histology images illustrating myocardial interstitial fibrosis (SR and Masson’s trichrome) at 16 weeks.
3.3 RIPC attenuates mitochondrial fragmentation in end-stage anthracycline-induced cardiotoxicity
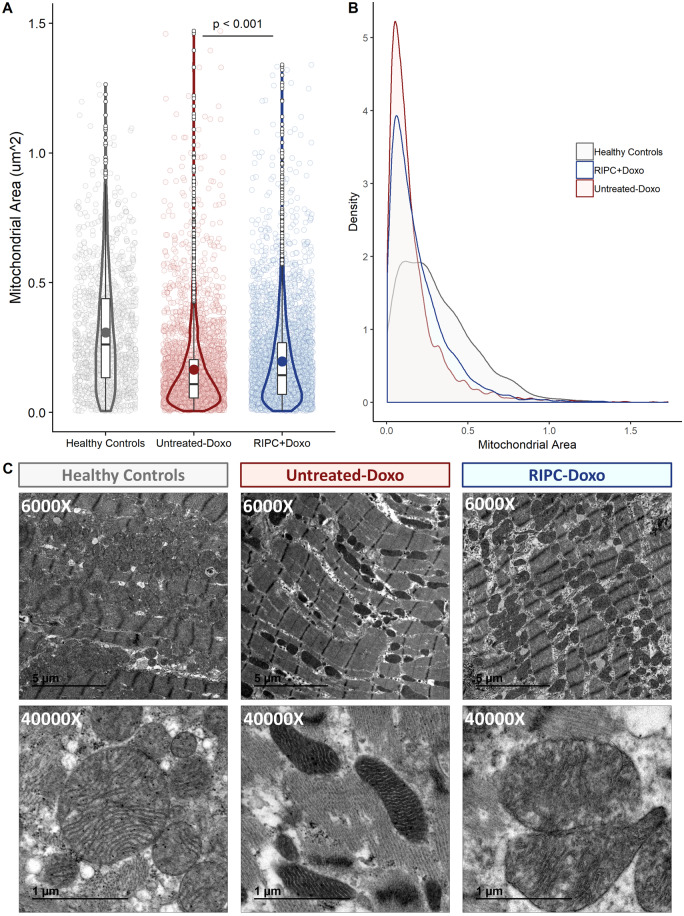
TEM evaluation of myocardial samples obtained at Week 16 revealed massive mitochondrial fragmentation in non-RIPC pigs (median ± IQR mitochondrial size 0.31 ± 0.30 μm2 and 0.17 ± 0.15 μm2 for healthy controls vs. Untreated-Doxo pigs; P < 0.001). Fragmentation was less severe in pigs undergoing RIPC before each doxorubicin injection (0.20 ± 0.2 μm2 and 0.17 ± 0.15 μm2 for RIPC-Doxo vs. Untreated-Doxo; P < 0.001). Area distribution analysis confirmed that the size distribution of mitochondria in RIPC-Doxo pigs was between that of controls and animals (Figure 4A,B). In addition to the differences in mitochondria size, the Untreated-Doxo animals had elongated and aberrantly shape mitochondria, likely as a result of fragmentation; these, with massive loss of cristae and electrodense aggregates due to the subsequent cristae rupture (Figure 4C).
Figure 4.
TEM analysis of myocardial mitochondria after 16 weeks of AIC (Study 1). (A) Individual mitochondrial area in the infused area in each animal group (dotplot) with its boxplot and violin plot related distribution. (B) Distribution of mitochondrial density vs. mitochondrial area in each animal group. (C) Representative TEM images at 6000× and 40 000× showing cardiac mitochondria replicates for each animal group. N = 5 animals per group. Data were analysed by linear regression nested according to treatment group and animal ID (Untreated-Doxo and RIPC-Doxo).
3.4 RIPC prevents disruption of mitochondrial dynamics and dysregulated autophagy occurring early in the course of AIC
We next examined mitochondrial dynamics and other processes associated with cell damage during the subclinical stages of AIC, when cardiac function was still unaffected. For this, we included a new group of 10 pigs (Study 2) that received only three doxorubicin injections; as before, the pigs were first randomized to RIPC or no pretreatment (N = 5 per arm). Pigs were sacrificed at Week 6 (2 weeks after the third doxorubicin injection). CMR performed immediately before sacrifice revealed normal LVEF in both groups (57.7% ± 2.08% and 55.3% ± 5.16% in the RIPC-Doxo and Untreated-Doxo groups vs. 64.1 ± 6.24% in healthy controls). LV volumes and LV mass were also unaffected (Supplementary material online, Table S2).
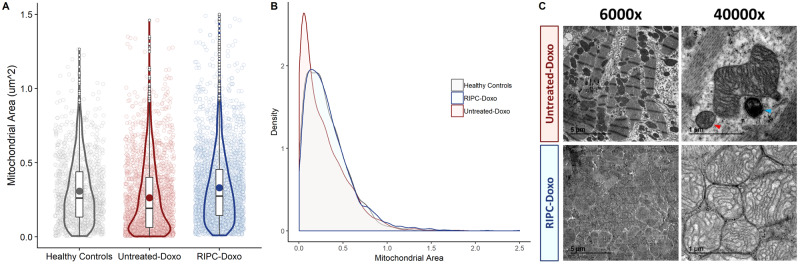
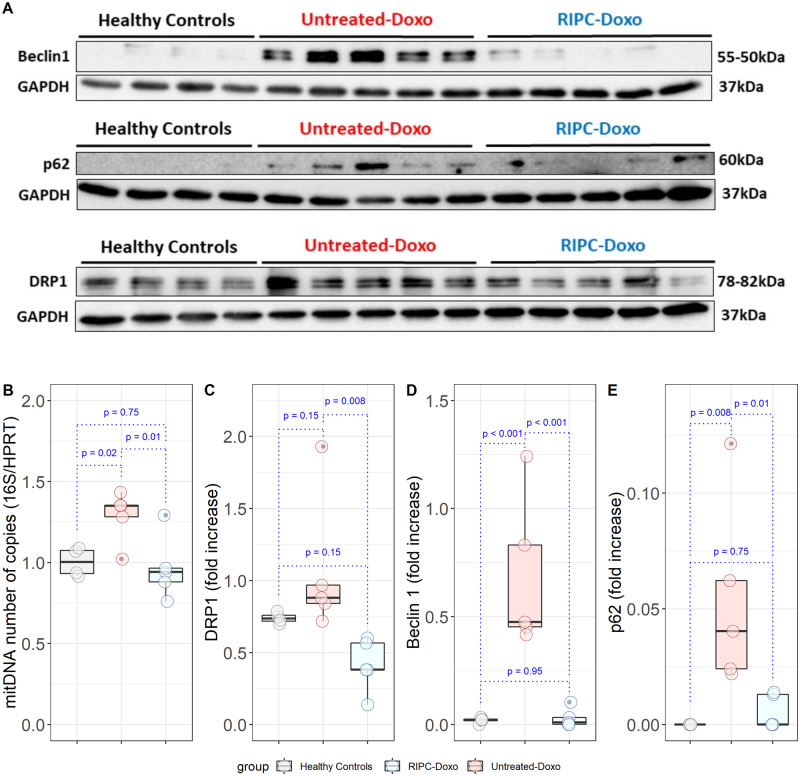
Despite normal heart function, Untreated-Doxo pigs at this early stage of doxorubicin exposure had severe alterations in mitochondrial morphology, with a clear fragmented phenotype (median ± IQR mitochondrial size = 0.27 ± 0.34 μm2 in Untreated-Doxo pigs vs. 0.308 ± 0.30 μm2 in healthy controls; P < 0.001). This fragmented phenotype was not present in myocardial mitochondria from RIPC-Doxo pigs, and mitochondria in this group were significantly larger than in Untreated-Doxo pigs (0.34 ± 0.31 μm2 vs. 0.27 ± 0.34 μm2 for RIPC-Doxo vs. Untreated-Doxo; P < 0.001) (Figure 5A). The area distribution curve for RIPC-Doxo pigs superimposed that of healthy controls, whereas Untreated-Doxo pigs showed a more fragmented distribution (Figure 5B). Irregularly shaped, small, and fragmented mitochondria surrounded by ribosomes were found in Untreated-Doxo pigs receiving only three doxorubicin injections (Study 2). The morphology of mitochondria from RIPC-Doxo pigs in Study 2 presented some disruption of cristae but conservation of shape and size (Figure 5C). Western blot analysis revealed that the mitochondrial fragmentation in Untreated-Doxo pigs was associated with a significant upregulation of the fission master regulator dynamin-related protein-1 (DRP1) (Figure 6A,C). Doxorubicin-induced DRP-1 upregulation was blocked RIPC (Figure 6A,C). mtDNA quantification confirmed the fragmented mitochondrial phenotype of Untreated-Doxo pigs, which had more mtDNA copies than RIPC-Doxo pigs and healthy controls (Figure 6B). Given the role of mitochondrial dysfunction in autophagy dysregulation and the impact on the latter on cardiac function, we next explored the impact of disrupted mitochondrial dynamics on the autophagy machinery. Expression of the autophagy-related proteins Beclin 1 and p62 was significantly higher in Untreated-Doxo pigs than in RIPC-Doxo pigs receiving doxorubicin and healthy controls (Figure 6D,E). Original WB unedited gels are detailed in Supplementary material online, Figure S3.
Figure 5.
TEM analysis of myocardial mitochondria after 6 weeks of AIC (Study 2). (A) Individual mitochondrial area in the infused area in each animal group (dotplot) with its boxplot and violin plot related distribution. (B) Distribution of mitochondrial density vs. mitochondrial area in each animal group. (C) Representative TEM images at 6000× and 40 000× showing all cardiac mitochondria replicates for each animal group. N = 5 animals per group. Data were analysed by linear regression nested according to treatment group and animal ID (Untreated-Doxo and RIPC-Doxo). The red arrowhead indicates a fragmented mitochondria and the blue arrowhead a secondary lysosome.
Figure 6.
Myocardial mitochondria protein expression in the infused area after 6 weeks of AIC (Study 2). (A) Western blot analysis of the myocardial expression of Beclin1, p62, and DRP1 in all animals. (B) Myocardial mitochondrial DNA content. (C–E) Quantification of protein expression (fold-increased, normalized to GAPDH) for (C) DRP1, (D) Beclin 1, and (E) p62. (n = 4 in Healthy controls, 5 in Untreated-Doxo, and 5 in RIPC-Doxo groups). Significance differences between groups were calculating using one-way ANOVA.
4. Discussion
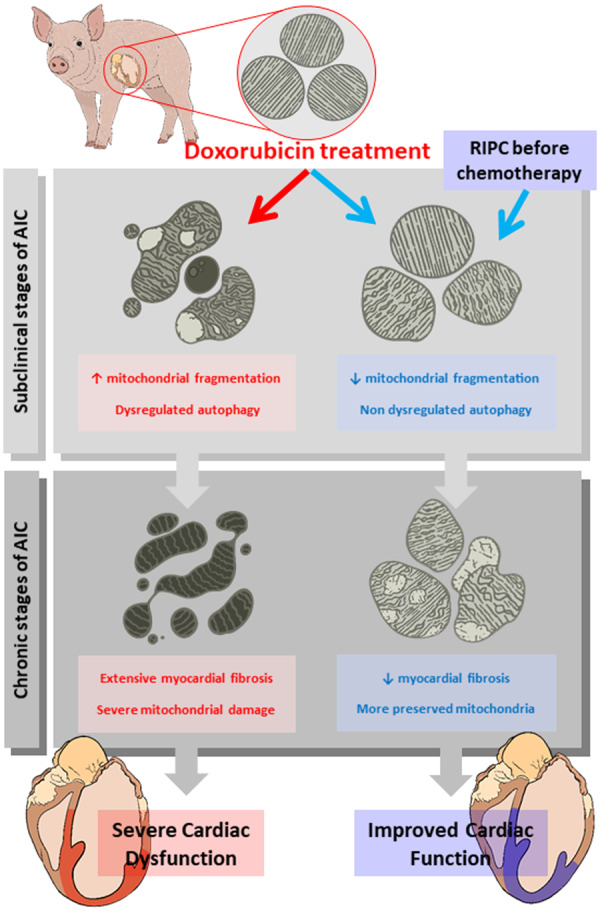
In this study, we tested the cardioprotective effect of RIPC before doxorubicin administration in a large-animal model of AIC. Long-term serial CMR evaluation showed that RIPC significantly attenuates cardiac dysfunction associated with AIC. Hearts from RIPC-treated animals had significantly less myocardial fibrosis. Doxorubicin caused significant mitochondrial damage from the early stages after administration: at subclinical AIC stages (before overt cardiac dysfunction), doxorubicin induced a severe fragmented mitochondria phenotype and upregulated autophagy markers. RIPC applied before doxorubicin injections prevented the mitochondrial morphological abnormalities and the disruption of mitochondrial dynamics. These data identify RIPC as a potential cardioprotective strategy to prevent AIC and support the translation of this therapeutic strategy to the clinic.
Anthracyclines have been in clinical use for more than 50 years and, alone or in combination with other agents, remain the first line therapy for many cancer types. AIC is one of the most feared side effects of these efficacious chemotherapy agents. Most recent data show that up to 30% of patients treated with anthracyclines develop some degree of cardiotoxicity, and AIC manifests as significant cardiac dysfunction in up to 10% of patients.3,6 Severe AIC is more frequent among especially vulnerable populations, such as the elderly. The unavoidable trade-off between cancer and chronic HF places a major burden on individuals and healthcare systems.
The current approach to AIC includes early detection by serial imaging and the use of non-specific HF therapies such as beta-blockers or ACE-inhibitors.4,18 However, even when these are initiated early after AIC diagnosis, recovery cardiac function is usually incomplete.7 Currently, the only FDA-approved drug for the prevention of AIC is dexrazoxane, an iron chelator that has been shown to reduce myocardial injury in a paediatric population.8,19 However, dexrazoxane is not used in daily practice, and its efficacy for AIC prevention in adults is uncertain; more clinical trials are needed to clearly define its cardioprotective effect.20 Moreover, most recent clinical trials with ACE-inhibitors or beta-blockers showed inconsistent reductions in AIC.21–23 There is therefore a clinical need to identify effective preventive therapies for AIC.
Remote ischaemic conditioning has been extensively validated in experimental studies12,13 and early clinical trials24 as an intervention able to ameliorate cardiac damage associated with acute myocardial infarction (AMI). The protective effect of remote conditioning is stronger when it is applied before the index episode (PREconditioning); unfortunately, ischaemia onset in AMI is an unpredictable event, and remote conditioning in this context is therefore only feasible during ongoing infarction (PERconditioning).12 Despite promising results from RIC-STEMI trial,25 in the largest clinical trial in AMI patients, the CONDI-2/ERIC-PPCI trial, remote ischaemic perconditioning did not improve clinical outcomes.15 Unlike AMI, anthracycline administration is a fully programmed event, and it is thus possible to schedule RIPC. The positive results reported here and the ease of implementing this strategy in the clinic identify RIPC as a strong candidate for testing in future clinical trials. The safety of RIPC demonstrated in many trials in AMI add to the attraction of this strategy.
Ischaemic conditioning has scarcely been tested in the context of AIC. Schjøtt et al.26 tested ischaemic preconditioning in isolated rat hearts before epirubicin administration; local ischaemic preconditioning of explanted hearts was associated with an attenuation of epirubicin-induced cardiac dysfunction. More recently, Maulik et al.27 tested ischaemic preconditioning in cultured cardiomyocytes exposed to doxorubicin. Supporting the ex vivo data,26 their results showed that cardiomyocytes subjected to ischaemic preconditioning were protected against doxorubicin-induced cell death.27
Importantly, ischaemic preconditioning in these studies did not impact a competitive advantage to cancer cells exposed to doxorubicin, providing an additional safety benefit to this strategy. Very recently, Gertz et al.28 published the first in vivo evidence of the cardioprotection afforded by RIPC in a mouse model of AIC. RIPC was associated with improved survival and attenuated cardiac fibrosis and autophagy. However, the anthracycline regime in that study did impact cardiac function, and thus the cardioprotective effect of RIPC was less clear.28 In line with the Gertz et al. study,28 our results in pigs show that RIPC triggers a significant reduction in the cardiac expression of key autophagy factors.
Despite of the lack of experimental evidence from animal studies to support the ability of RIPC to prevent AIC, two small clinical trials are ongoing. Chung et al.29 (NCT02471885) are testing a series of four RIPC cycles (5 min each) in the arm before each chemotherapy cycle in a population of 128 patients receiving anthracyclines for various types of cancer. In another trial (NCT03166813), Li et al. are testing a different RIPC protocol (three cycles of 5 min) applied either in the arm or in the leg before each chemotherapy cycle in a paediatric population (4–18 years old) receiving anthracyclines. Both trials have high-sensitivity cardiac troponin T (hs-cTnT) after the end of chemotherapy as the primary endpoint.
Our study provides the first demonstration that RIPC protects the heart against AIC in a large-animal model. Unlike the results from mice,28 in our model, anthracyclines had a clear cardiocytotoxic effect, and we were able to detect differences in cardiac function and cardiac contractile function between animals receiving RIPC or no pretreatment. We also found differences in T1 mapping values implicated in AIC.30 In agreement with the results from mice,28 at the end of the study protocol we found that RIPC-treated animals had significantly less fibrosis (collagen fraction) that animals receiving no pretreatment.
The mechanism by which RIPC exerts its cardioprotective effect against AIC is incompletely understood. There is evidence identifying neuronal and humoral mediators that transfer the protective signal from the periphery (e.g. the leg undergoing brief intermittent ischaemia episodes) to the heart.31 At the heart’s level, there is firm evidence that mitochondrial damage plays a central role in AIC2,.32,33 To investigate the mechanism of protection, we therefore explored mitochondrial dynamics (fusion/fission) and authophagy.34 Our TEM analysis revealed that mitochondria from doxorubicin-injected pigs not undergoing RIPC were abnormally small, had irregular and aberrant morphology, and contained a hyper-dense matrix, confirming previous reports.32,35 Morphological abnormalities were present very early in course of AIC (before overt cardiac dysfunction) and progressed to an extreme phenotype by the end of the 16-week protocol. RIPC prevented most of these morphological abnormalities from early in the course of AIC. Confirming these findings, protein expression of the mitochondrial fission master regulator DRP-1 was upregulated by doxorubicin treatment and normalized by RIPC, as was autophagy, evaluated early in the course of AIC by the expression of Beclin-1 and p62. Although autophagy is essentially protective and not a deleterious process (at least in the context of myocardial infarction),36 when dysregulated it can lead to cell death.37 Mitochondrial morphology abnormalities have emerged as essential trigger of autophagy. We propose that the severe mitochondrial dysfunction triggered by doxorubicin exposure induces a dysregulated autophagy in cardiomyocytes that leads to subsequent heart dysfunction. RIPC, by preserving mitochondrial integrity during AIC, halts this process and results in a long-term cardiac protection (Graphical Abstract).
In conclusion, this study provides evidence of the efficacy of RIPC in preventing AIC in a translational large-animal model. RIPC-mediated cardioprotection starts early in the course of AIC, in the subclinical phase, and has important downstream benefits on cardiac function. Our study supports the execution of adequately powered well-designed clinical trials to confirm this benefit in the clinical setting.
4.1 Study limitations
The intracoronary route for doxorubicin injection used here does not mimic the clinical scenario and is a highly aggressive model. We16 and others38,39 have used this administration route in the past as a means of avoiding the myelosuppression associated with systemic doxorubicin injection, which is pronounced in pigs.40 Nevertheless, the protective effect of RIPC in this extreme model strongly supports its translational potential. A further limitation in this study is the use of healthy juvenile pigs, which are free of the comorbidities frequent in cancer patients developing AIC, many of whom are elderly. In this study, we have not evaluated the expression of some pro-survival signals, such as STAT-3, known to be upregulated by after RIPC in the context of myocardial infarction.41,42
Translational perspective
Serial cardiac magnetic resonance (CMR) evaluation of a highly translatable large-animal model of anthracycline-induced cardiotoxicity (AIC) shows that cumulative exposure to doxorubicin results in significantly reduced left ventricular ejection fraction (LVEF) and extensive mitochondrial fragmentation. Remote ischaemic preconditioning (RIPC) applied before each doxorubicin cycle preserved cardiac contractility and LVEF in long-term CMR exams. RIPC prevented doxorubicin-induced irreversible mitochondrial fragmentation and dysregulated autophagy. RIPC is as an attractive strategy for testing in clinical trials in AIC.
Data availability
The grouped CMR data underlying this article are available in the article and in its online supplementary material. The individual data will be shared on reasonable request to the corresponding author.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
Carlos Galán-Arriola has designed and performed all the experiments, data analysis and drafted the manuscript. Rocio Villena-Gutiérrez and Eduardo Oliver are responsible for the western blot and mitochondrial DNA analyses. María I Higuero-Verdejo participated in the in vivo experiments and TEM analysis. Gonzalo J. López and Gonzalo Pizarro have been responsible for all the CMR acquisition and blinded analysis of the studies. Antonio de Molina-Iracheta and Claudia Pérez-Martínez have been responsible for the histopathological processing and analysis. Iván A. Díaz-Rengifo, Rodrigo D. García, David González-Calle, and Manuel Lobo have been contributed to the data acquisition and supported the in vivo experiments. Pedro L Sánchez, Raúl Córdoba, and Valentin Fuster, have been provided critical revision to the manuscript. Javier Sánchez-González has been responsible for the CMR protocol development and provided critical revision to the manuscript. Borja Ibanez is responsible for the study conception and design, obtaining funding for the study, made critical revision to the paper and made final approval of it. All the authors read and approved the manuscript.
Conflict of interest: Javier Sánchez-González is employed by Philips Healthcare. The rest of the authors have nothing to declare.
Supplementary Material
Acknowledgements
We thank Eugenio Fernández, Tamara Córdoba, Inés Sanz, Lorena Domínguez, Nuria Valladares, Antonio Benítez, Santiago Rodriguez-Colilla, and Rubén Mota for technical and veterinary support at the CNIC animal facility and farm. We also thank Marta Gavilán, Ángel Macías, and Braulio Pérez for technical support in CMR studies. Simon Bartlett (CNIC) provided English editing.
Funding
This study is part of a project that has received funding from the European Research Council (ERC) under the European Union Horizon 2020 Research and Innovation Programme (ERC-Consolidator Grant agreement No. 819775 to B.I). The study was also partially funded by an ERA-CVD Joint Translational Call 2016 [funded through the Instituto de Salud Carlos III (ISCIII) and the European Regional Development Fund (ERDF), # AC16/00021] and by a Health Research Project from the ISCIII-FIS (# PI16/02110). Carlos Galán-Arriola and Rocío Villena-Gutiérrez are P-FIS fellows (Instituto de Salud Carlos III). This study forms part of a research agreement between the CNIC and Philips Healthcare. The CNIC is supported by the ISCIII, the Ministerio de Ciencia e Innovación, and the Pro-CNIC Foundation and is a Severo Ochoa Center of Excellence (MEIC award SEV-2015-0505).
References
- 1. Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh E.. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 2. J Am Coll Cardiol 2017;70:2552–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitry MA, Edwards JG.. Doxorubicin induced heart failure: phenotype and molecular mechanisms. Int J Cardiol Heart Vasc 2016;10:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Achenbach S, Agewall S, Badimon L, Barón-Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol Ç, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 4. López-Fernández T, López de Sá Areses E, Valbuena López SC, Dalmau González-Gallarza R, López Sendón Henchel JL, Martín García A, Santaballa Beltrán A, Montero Luis Á, García Sanz R, González Ferrer JJ, Mitroi C, Arenas M, Virizuela Echaburu JA, Marco Vera P, Barreiro-Pérez M, Mazón Ramos P, Velasco del Castillo S, Hinojar Baydes R, Zamorano JL, Pérez de Isla L, Calvo-Iglesias F, Íñiguez Romo A, Castro Fernández A, González-Caballero E, Plana Gómez JC.. Cardio-onco-hematology in clinical practice. Position paper and recommendations. Rev Esp Cardiol 2017;70:474–486. [DOI] [PubMed] [Google Scholar]
- 5. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, Decara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P.. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27560:911–939. [DOI] [PubMed] [Google Scholar]
- 6. López-Sendón J, Álvarez-Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, Cardinale D, Canales Albendea M, Feliu Batlle J, Rodríguez Rodríguez I, Rodríguez Fraga O, Albaladejo A, Mediavilla G, González-Juanatey JR, Martínez Monzonis A, Gómez Prieto P, González-Costello J, Serrano Antolín JM, Cadenas Chamorro R, López Fernández T.. Classification, prevalence, and outcomes ofanticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020;41:1720–1729. [DOI] [PubMed] [Google Scholar]
- 7. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM.. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 8. Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE.. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 2004;351:145–153. [DOI] [PubMed] [Google Scholar]
- 9. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T.. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 2019;280:163–175. [DOI] [PubMed] [Google Scholar]
- 10. Anker MS, Hadzibegovic S, Lena A, Belenkov Y, Bergler‐Klein J, Boer RA, Farmakis D, Haehling S, Iakobishvili Z, Maack C, Pudil R, Skouri H, Cohen‐Solal A, Tocchetti CG, Coats AJS, Seferović PM, Lyon AR; for the Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology. Recent advances in cardio-oncology: a report from the ‘Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019’. ESC Heart Fail 2019;6:1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh ETH, Chang HM.. Oncocardiology - past, present, and future: a review. JAMA Cardiol 2016;1:1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon DM.. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bromage DI, Pickard JMJ, Rossello X, Ziff OJ, Burke N, Yellon DM, Davidson SM.. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: a systematic review and meta-analysis. Cardiovasc Res 2017;113:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P.. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 15. Hausenloy DJ, Kharbanda RK, Møller UK, Ramlall M, Aarøe J, Butler R, Bulluck H, Clayton T, Dana A, Dodd M, Engstrom T, Evans R, Lassen JF, Christensen EF, Garcia-Ruiz JM, Gorog DA, Hjort J, Houghton RF, Ibanez B, Knight R, Lippert FK, Lønborg JT, Maeng M, Milasinovic D, More R, Nicholas JM, Jensen LO, Perkins A, Radovanovic N, Rakhit RD.. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet 2019;6736:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, López GJ, Molina-Iracheta A, de Pérez-Martínez C, Agüero J, Fernández-Jiménez R, Martín-García A, Oliver E, Villena-Gutierrez R, Pizarro G, Sánchez PL, Fuster V, Sánchez-González J, Ibanez B.. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol 2019;73:779–791. [DOI] [PubMed] [Google Scholar]
- 17. Hadi AM, Mouchaers KTB, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A, Laarse WJ, van der, Beliën J.. Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol 2011;34:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Zamorano JL, Aboyans V, Achenbach S, Agewall S, Badimon L, Barón-Esquivias G, Baumgartner H, Bax JJ, Bueno H, Carerj S, Dean V, Erol Ç, Fitzsimons D, Gaemperli O, Kirchhof P, Kolh P, Lancellotti P, Lip GYH, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Roffi M, Torbicki A, Vaz Carneiro A, Windecker S; Authors/Task Force Members. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology ESC. Eur J Heart Fail 2017;19:9–139. [DOI] [PubMed] [Google Scholar]
- 19. Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, Barry EV, Asselin BL, Athale U, Clavell LA, Larsen E, Moghrabi A, Samson Y, Michon B, Schorin MA, Cohen HJ, Neuberg DS, Orav EJ, Colan SD.. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 2010;11:950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macedo AVS, Hajjar LA, Lyon AR, Nascimento BR, Putzu A, Rossi L, Costa RB, Landoni G, Nogueira-Rodrigues A, Ribeiro ALP.. Efficacy of dexrazoxane in preventing anthracycline cardiotoxicity in breast cancer. JACC CardioOncology 2019;1:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heck SL, Gulati G, Hoffmann P, Knobelsdorff-Brenkenhoff F, Von Storås TH, Ree AH, Gravdehaug B, Røsjø H, Steine K, Geisler J, Schulz-Menger J, Omland T.. Effect of candesartan and metoprolol on myocardial tissue composition during anthracycline treatment: the PRADA trial. Eur Heart J Cardiovasc Imaging 2018;19:544–552. [DOI] [PubMed] [Google Scholar]
- 22. Avila MS, Ayub-Ferreira SM, Barros Wanderley MR, de Dores Cruz F, das Gonçalves Brandão SM, Rigaud VOC, Higuchi-dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, Paula Costa RL, de Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA.. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 2018;71:2281–2290. [DOI] [PubMed] [Google Scholar]
- 23. Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W, Munster PN.. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol 2019;73:2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT.. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010;375:727–734. [DOI] [PubMed] [Google Scholar]
- 25. Gaspar A, Lourenço AP, Pereira MÁ, Azevedo P, Roncon-Albuquerque R, Marques J, Leite-Moreira AF.. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res Cardiol 2018;113:14. [DOI] [PubMed] [Google Scholar]
- 26. Schjøtt J, Olsen H, Berg K, Jynge P.. Pretreatment with ischaemia attenuates acute epirubicin-induced cardiotoxicity in isolated rat hearts. Pharmacol Toxicol 1996;78:381–386. [DOI] [PubMed] [Google Scholar]
- 27. Maulik A, Davidson SM, Piotrowska I, Walker M, Yellon DM.. Ischaemic preconditioning protects cardiomyocytes from anthracycline-induced toxicity via the PI3K pathway. Cardiovasc Drugs Ther Cardiovascular Ther 2018;32:245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gertz ZM, Cain C, Kraskauskas D, Devarakonda T, Mauro AG, Thompson J, Samidurai A, Chen Q, Gordon SW, Lesnefsky EJ, Das A, Salloum FN.. Remote ischemic pre-conditioning attenuates adverse cardiac remodeling and mortality following doxorubicin administration in mice. JACC CardioOncology 2019;1:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung R, Maulik A, Hamarneh A, Hochhauser D, Hausenloy DJ, Walker JM, Yellon DM.. Effect of remote ischaemic conditioning in oncology patients undergoing chemotherapy: rationale and design of the ERIC-ONC study - a single-center, blinded, randomized controlled trial. Clin Cardiol 2016;39:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jordan JH, Todd RM, Vasu S, Hundley WG.. Cardiovascular magnetic resonance in the oncology patient. JACC Cardiovasc Imaging 2018;11:1150–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kleinbongard P, Skyschally A, Heusch G.. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch - Eur J Physiol 2017;469:159–181. [DOI] [PubMed] [Google Scholar]
- 32. Zhang S, Liu X, Bawa-Khalfe T, Lu L-S, Lyu YL, Liu LF, Yeh E.. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 33. Takemura G, Fujiwara H.. Doxorubicin-induced cardiomyopathy. From the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 2007;49:330–352. [DOI] [PubMed] [Google Scholar]
- 34. Koleini N, Kardami E.. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017;8:46663–46680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farhad H, Staziaki PV, Addison D, Coelho-Filho OR, Shah RV, Mitchell RN, Szilveszter B, Abbasi SA, Kwong RY, Scherrer-Crosbie M, Hoffmann U, Jerosch-Herold M, Neilan TG.. Characterization of the changes in cardiac structure and function in mice treated with anthracyclines using serial cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 2016;9:e003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Przyklenk K, Dong Y, Undyala VV, Whittaker P.. Autophagy as a therapeutic target for ischaemia/reperfusion injury? Concepts, controversies, and challenges. Cardiovasc Res 2012;94:197–205. [DOI] [PubMed] [Google Scholar]
- 37. Roca-Agujetas V, Dios C, De Lestón L, Marí M, Morales A, Colell A.. Recent insights into the mitochondrial role in autophagy and its regulation by oxidative stress. Oxid Med Cell Longev 2019;2019:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christiansen S, Perez-Bouza A, Schälte G, Hilgers RD, Autschbach R.. Selective left ventricular adriamycin-induced cardiomyopathy in the pig. J Heart Lung Transplant 2008;27:86–92. [DOI] [PubMed] [Google Scholar]
- 39. Goetzenich A, Hatam N, Zernecke A, Weber C, Czarnotta T, Autschbach R, Christiansen S.. Alteration of matrix metalloproteinases in selective left ventricular adriamycin-induced cardiomyopathy in the pig. J Heart Lung Transpl 2009;28:1087–1093. [DOI] [PubMed] [Google Scholar]
- 40. Vleet J, Van LA G, Vj F.. Pathologic features of adriamycin toxicosis in young pigs: nonskeletal lesions. Am J Vet Res 1979;40:1537–1552. [PubMed] [Google Scholar]
- 41. Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G.. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 2015;117:279–288. [DOI] [PubMed] [Google Scholar]
- 42. Kleinbongard P, Amanakis G, Skyschally A, Heusch G.. Reflection of cardioprotection by remote ischemic perconditioning in attenuated ST-segment elevation during ongoing coronary occlusion in pigs evidence for cardioprotection from ischemic injury. Circ Res 2018;122:1102–1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The grouped CMR data underlying this article are available in the article and in its online supplementary material. The individual data will be shared on reasonable request to the corresponding author.