Graphical Abstract
Keywords: cardiovascular disease, chronic kidney disease, erythropoiesis-stimulating agents, heart failure, hypoxia-inducible factor–prolyl hydroxylase inhibitor, iron deficiency
Abstract
Cardiorenal syndrome includes a spectrum of disorders of the kidneys and heart in which loss of function in one organ contributes to reduced function in the other organ. Cardiorenal syndrome is frequently complicated by comorbid anemia, which leads to reciprocal and progressive cardiac and renal deterioration. The triad of heart failure, chronic kidney disease (CKD), and anemia is termed cardiorenal anemia syndrome (CRAS). There are currently no evidence-based recommendations for managing patients with CRAS; however, the treatment of these patients is multifactorial. Not only must the anemia be controlled, but heart failure and kidney injury must be addressed, in addition to other comorbidities. Intravenous iron and erythropoiesis-stimulating agents are the mainstays of treatment for anemia of CKD, addressing both iron and erythropoiesis deficiencies. Since erythropoiesis-stimulating agent therapy can be associated with adverse outcomes at higher doses in patients with CKD and is not used in routine practice in patients with heart failure, treatment options for managing anemia in patients with CRAS are limited. Several new therapies, particularly the hypoxia-inducible factor–prolyl hydroxylase inhibitors, are currently under clinical development. The hypoxia-inducible factor–prolyl hydroxylase inhibitors have shown promising results for treating anemia of CKD in clinical trials and may confer benefits in patients with CRAS, potentially addressing some of the limitations of erythropoiesis-stimulating agents. Updated clinical practice guidelines for the screening and management of anemia in cardiorenal syndrome, in light of potential new therapies and clinical evidence, would improve the clinical outcomes of patients with this complex syndrome.
Cardiorenal syndrome (CRS) includes a spectrum of disorders of the kidneys and heart in which loss of function in one organ leads to reduced function in the other.1 Cardiovascular disease (CVD), the leading cause of morbidity and mortality in chronic kidney disease (CKD),2 develops in 10% to 47% of patients with CKD, with a prevalence in those aged ≥66 years, double that of similar individuals without CKD.3,4 As kidney function declines, the risk of all-cause mortality increases, from a 20% increase in stage 3a CKD to a nearly 500% increase in stage 5 CKD.5 In patients with end-stage kidney disease, the risk of CVD-related mortality is 20 to 30 times higher than in the general population.6
Conversely, CKD has been reported in 25% to 50% of patients with heart failure (HF),7,8 which affects an estimated 5.7 million people in the United States.9 The risk of mortality in patients with HF is increased by 56% with CKD of any stage and by 131% in those with moderate or severe kidney impairment.10 The risk of CVD in patients with CKD is further increased by concomitant diabetes11,12 or hypertension,12 both of which are more prevalent in patients with CKD than without.13
Patients with CKD and HF have an increased risk of anemia, an independent risk factor for the development and progression of both CVD and CKD.14 The prevalence of anemia increased from 21% to 70% in patients with CKD as kidney function declined and from 9% to 79% in patients with HF as disease severity worsened from New York Heart Association (NYHA) functional class I to IV.15 In patients with newly diagnosed HF, the incidence of anemia was 17%, with 21% of these patients having iron deficiency anemia,16 whereas in patients with congestive HF, the overall incidence of anemia was 56%.15 In a small set of patients, bone marrow iron deficiency was detected in 40% and anemia in 17% of 42 patients with HF17 and in 73% of 37 patients with both end-stage HF and anemia.18
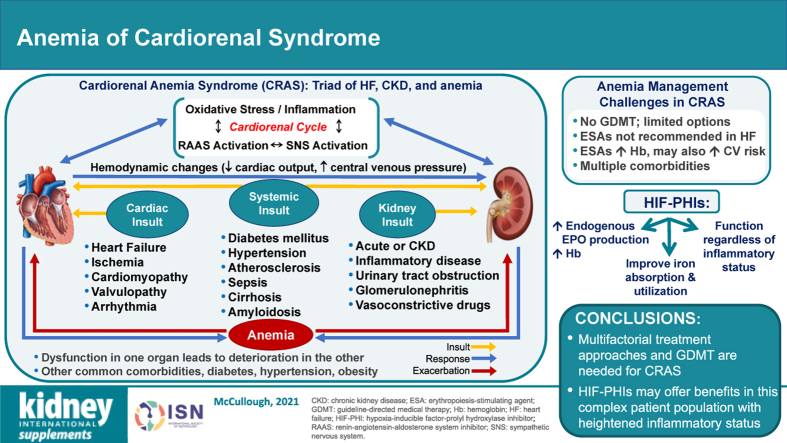
Anemia often develops in patients with CRS, and leads to reciprocal and progressive cardiac and renal deterioration.19 This triad of pathophysiological conditions is termed cardiorenal anemia syndrome (CRAS), a complex disease that is associated with adverse clinical outcomes, increased risk of hospitalization and mortality,7,8,20 and decreased quality of life (QOL).21 This review will highlight the unique challenges and integrative treatment strategies associated with the management of patients with CRAS.
Characteristics of CRS
Pathophysiology of CRS
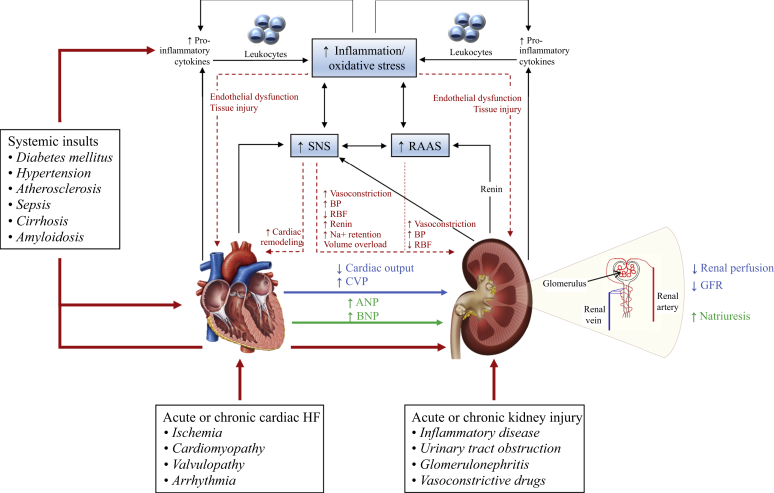
CRS arises when shifts in blood flow and organ perfusion lead to activation of corrective mechanisms that become maladaptive and negatively impact kidney and heart function, with progressive degeneration in each system.22, 23, 24 The syndrome is classified into 5 different types based on the primary organ of dysfunction (heart or kidney) and the nature of the dysfunction (acute or chronic)23 (Table 11,23,25). Key pathophysiological pathways involved in CRS are summarized in Figure 11,24,26, 27, 28 and have recently been reviewed in detail.1,24
Table 1.
| Type | Definition | Major mechanisms |
|---|---|---|
| Type I |
Acute CRS Abrupt worsening of heart function (e.g., ACS or acute HF) resulting in AKI |
Neurohormonal, abnormal cell signaling, hemodynamic abnormalities |
| Type II |
Chronic CRS Chronic abnormalities in heart function (e.g., chronic HF) resulting in progressive and permanent CKD |
Chronic neurohormonal hyperactivation |
| Type III |
Acute renocardiac syndrome Abrupt worsening of kidney function (e.g., AKI caused by volume overload, inflammatory surge, or metabolic disturbances in uremia; glomerulonephritis) resulting in acute heart disorder (e.g., acute HF, arrhythmia, or ischemia) |
Acute volume overload, hyperkalemia, acidosis |
| Type IV |
Chronic renocardiac syndrome CKD (e.g., chronic glomerular disease) resulting in decreased heart function (e.g., HF, cardiac hypertrophy, or increased risk of adverse CV events) |
Chronic pressure and volume overload, uremic cardiomyopathy, metabolic/micronutrient abnormalities |
| Type V |
Secondary CRS Systemic condition (e.g., amyloidosis, sepsis, cirrhosis, or diabetes mellitus) resulting in loss of both heart and kidney function |
Cytokine storm, danger-associated molecular abnormalities |
ACS, acute coronary syndrome; AKI, acute kidney injury; CKD, chronic kidney disease; CRS, cardiorenal syndrome; CV, cardiovascular; HF, heart failure.
Figure 1.
A schematic diagram of the mechanisms underlying cardiorenal syndrome.1,24,26, 27, 28 Dysfunction in either the heart or kidneys, caused by systemic insults, triggers hemodynamic and nonhemodynamic changes, culminating in cardiorenal syndrome (CRS) and propagating a vicious cycle (double-headed black arrows). Black arrows indicate pathophysiological interactions in CRS; blue arrow, hemodynamic changes; dotted red arrows, consequences of nonhemodynamic changes; and green arrow, mechanism to correct renal function (decompensated). ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; BP, blood pressure; CVP, central venous pressure; GFR, glomerular filtration rate; HF, heart failure; Na+, sodium; RAAS, renin-angiotensin-aldosterone system; RBF, renal blood flow; SNS, sympathetic nervous system.
CRS originating from either heart or kidney defects ultimately leads to activation of neurohormonal networks, such as the sympathetic nervous system, renin-angiotensin-aldosterone system (RAAS), and vasopressin pathways, and triggers a cycle of maladaptive processes leading to dysfunction of both organs.22,29, 30, 31 Oxidative injury is a common link between heart and kidney damage because both primary heart and kidney failure activate the RAAS, and neurohormonal activation (especially angiotensin II) in one organ leads to production of reactive oxygen species (ROS), causing deterioration in the other organ.22 Oxidative stress amplifies cardiac remodeling, leading to hypertrophy,32 and disrupts the endothelial barrier, causing tissue injury.24 Maladaptive cell signaling, which manifests as changes in interleukins, cytokines, and micro-RNAs, in CRS due to common comorbidities (e.g., diabetes or hypertension) causes structural and functional changes of the heart or kidney microvasculature, leading to endothelial barrier dysfunction and amplified inflammation, ROS production, cardiac remodeling, and abnormalities in glomerular and tubular function.33,34
Cardiorenal anemia syndrome
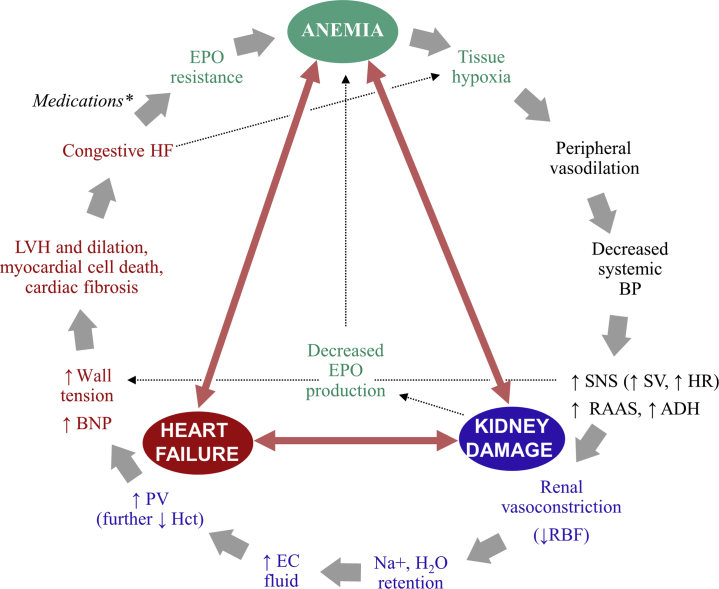
Chronic kidney disease and its associated pressure overload, volume overload, and cardiomyopathy can contribute to congestive HF and development of anemia in CRS through multifactorial mechanisms acting simultaneously.19 Conversely, anemia of CRS exacerbates both CKD and CVD by blunting erythropoietin (EPO) production, impairing oxygen delivery, and generating tissue hypoxia that leads to peripheral vasodilation and further stimulation of neurohormonal activity that propagates the CRS cycle (i.e., decreased renal perfusion and increased cardiac burden) (Figure 217,19,35).19,36 In CRAS, each of the 3 conditions activates each other and is associated with an increase in sympathetic activity, RAAS activity, oxidative stress, and inflammation that results in tissue damage,19 creating a vicious cycle of damage.
Figure 2.
The cycle of damage in cardiorenal anemia syndrome.17,19,35 ∗Medications used in heart failure (HF) associated with anemia include angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and β-blockers. ADH, antidiuretic hormone (vasopressin); BNP, brain natriuretic peptide; BP, blood pressure; EC, extracellular; EPO, erythropoietin; Hct, hematocrit; HR, heart rate; LVH, left ventricular hypertrophy; Na+, sodium; PV, plasma volume; RAAS, renin-angiotensin-aldosterone system; RBF, renal blood flow; SNS, sympathetic nervous system; SV, stroke volume.
Patients with both CKD and CVD may have low EPO levels because of kidney damage, which causes erythropoiesis deficiency.35,37 In addition, CKD- and CVD-induced proinflammatory cytokines suppress renal EPO secretion and exert a negative effect on EPO receptors in the bone marrow, leading to endogenous EPO resistance.38, 39, 40 Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), often used to manage CVD and CKD, have also been associated with decreased hemoglobin (Hb) levels, likely due to decreased EPO expression and/or EPO resistance from inhibition of early erythroid progenitor cell proliferation in the bone marrow.35,41 The release of proinflammatory cytokines, as occurs in both CKD and CVD, increases hepcidin-25 concentrations (see Haase42 and Agarwal43 in this supplement), which cause functional iron deficiency by decreasing intestinal iron absorption and inhibiting iron release from internal stores, leading to impaired Hb synthesis and iron-restricted erythropoiesis.37,40 Moreover, iron deficiency can be caused or exacerbated by blood loss associated with hemodialysis or frequent phlebotomy,44,45 underlying nutritional defects, or reduced intestinal iron absorption due to certain drugs, malignancies, or inflammatory bowel diseases.35
Dysregulation of phosphate homeostasis can prompt pathophysiological changes in bone mineral metabolism and may cause or worsen anemia of CRS. Impaired renal phosphate clearance in CKD may lead to decreased vitamin D activation and consequent hyperphosphatemia, reduced intestinal calcium absorption, and hypocalcemia.46 This shift in calcium homeostasis causes excessive parathyroid hormone secretion, which may inhibit EPO synthesis and shorten red blood cell (RBC) survival.47 Hyperphosphatemia is linked to increased blood pressure and left ventricular (LV) hypertrophy.48 In addition, elevated levels of fibroblast growth factor 23, expressed during hyperphosphatemia, are associated with bone disorders and can both directly and indirectly affect anemia as well as directly stimulate myocardial hypertrophy and fibrosis.49,50 Fibroblast growth factor 23 directly inhibits EPO production in the kidneys, which leads to reduced RBC production, and stimulates release of proinflammatory cytokines.49,50 Because systemic inflammation stimulates fibroblast growth factor 23 production, a positive feedback loop is created that indirectly promotes acceleration of anemia of CKD and inflammatory-cytokine mediated functional iron deficiency via hepcidin upregulation.50
Oxidative stress is an important factor in the development and progression of CRAS. Elevated expression of renal NAD phosphate oxidase during kidney dysfunction and increased activation by uremic toxins, which accumulate in CKD, lead to excessive production of ROS.51 Inflammation also leads to generation of ROS.24 Increased ROS and reduced antioxidant defense mechanisms, with resultant oxidative stress, contribute to anemia52 and alter the membrane properties of RBCs to enhance their removal by the spleen.51 Furthermore, reductions in Hb caused by iron deficiency exacerbate oxidative stress.51 Treatment-related iron overload, (e.g., i.v. iron) also results in increased labile iron levels, stimulating ROS production and oxidative stress.51
Several comorbidities are thought to contribute to the development of anemia in patients with CKD or CVD. The risk of anemia is significantly associated with diabetes, irrespective of kidney function.53 Patients with diabetes and all levels of kidney function have a higher prevalence of anemia than the general population, when matched for glomerular filtration rate, and many have iron deficiency (50% with Hb <11 g/dl; 43% with World Health Organization–defined anemia [<12 g/dl, women; <13 g/dl, men]).54 Although more common in patients with kidney dysfunction,53,54 development of anemia is also thought to be related to diabetes, perhaps resulting from EPO deficiency as a consequence of diabetic nephropathy, interstitial fibrosis, vascular lesions or cytokine-induced inhibition of synthesis, or glycation of the EPO receptor secondary to hyperglycemia.55 Testosterone deficiency is also implicated as a cause of anemia in men and women with and without kidney impairment, with bioavailable testosterone levels being linearly correlated with Hb levels and prevalence of anemia.56,57
Epidemiology and clinical consequences of CRAS
Although the definition of CRAS varies, several studies have defined the syndrome as HF with an estimated glomerular filtration rate (eGFR) of <60 ml/min per 1.73 m2 (i.e., stage 3–5 CKD) and anemia with Hb levels of <13 g/dl for males and <12 g/dl for females.7,8,58, 59, 60 Using this definition, the prevalence of CRAS among patients with HF ranges from 19% to 44%,7,8,58, 59, 60 and the prevalence of anemia in patients with CRS ranges from 39% to 45%.8 However, anemia may be underdiagnosed in patients with HF, depending on the definitions and methods used.61,62 Predictors for CRAS in patients with HF include diabetes, high natriuretic peptide levels, and the use of i.v. diuretics.8 Similarly, predictors of anemia in patients with HF include high natriuretic peptide levels, reduced kidney function (<60 ml/min per 1.73 m2), diabetes mellitus, LV ejection fraction ≥30%, and male sex.62
Patients with CRAS have a considerably increased risk of adverse clinical outcomes when compared with patients with HF alone.8,58,59,63 In a prospective study in outpatients with HF, CRAS was significantly associated with all-cause mortality (hazard ratio 2.0; 95% confidence interval, 1.4–2.8; P < 0.001) and was a stronger predictor of this outcome than LV dysfunction, absence of β-blocker treatment, and age.58 Similarly, the mortality rate of patients with HF was significantly higher among patients with CRAS versus those without CRAS (74% vs. 46% over a mean follow-up of ∼3–4 months; P < 0.001).59 In hospitalized patients with mild to severe HF, CRAS significantly increased the risk for the composite of cardiac death, nonfatal myocardial infarction, or rehospitalization for HF (33.4% vs. 11.2%; P < 0.001) or for renal replacement therapy (6.5% vs. 0.8%; P < 0.001).8
In a retrospective study, hospitalized patients with CVD at risk of HF, but with no prior history of HF, had a significantly higher risk of major adverse cardiovascular (CV) events when both CKD and anemia were also present (32%; P < 0.001) compared with comorbid CKD alone (19%), anemia alone (14%), or no CKD and no anemia (12%).63 Among patients with well-controlled hypertension on ARB therapy and no evidence of moderate or severe (NYHA class III or IV) HF, those with anemia had a significantly increased risk of adverse CV and renal events compared with patients without anemia (hazard ratio 1.82; 95% confidence interval, 1.12–2.96; P = 0.0163),14 indicating that anemia correction is essential in patients with CRS or at risk of CRS.
Although QOL in patients with CRAS was not evaluated, significant alterations in psychological state due to depression, anxiety, and decreased vitality and daily activities were reported in patients with type II CRS compared with patients with HF and no kidney impairment.64
The Management of Patients with CRS
Guideline-directed medical therapy (GDMT), such as β-blockers, ACEIs, and ARBs, has improved the management and clinical outcomes of patients with HF and those with CKD over the past few decades.65,66 However, the management of CRAS is complicated and lacks specific recommendations that take into account progressively deteriorating kidney function, different stages of HF, and the presence/absence of diabetes, hypertension, and other comorbidities. The clinical manifestation of CRAS is a combination of these factors and may require different approaches in individual patients as well as frequent medication changes that may deviate from GDMT. For example, initiation of ACEIs or ARBs can cause a decrease in kidney function in a subset of patients, and the risk of hyperkalemia from prolonged use of these medications is increased in patients with CKD or diabetes.67,68 Acute kidney injury is also common in patients with indications for RAAS inhibitors, and can result in hypotension, necessitating a dose reduction or withdrawal of the RAAS inhibitor.67 Mineralocorticoid receptor antagonists (such as spironolactone and eplerenone), neprolysin inhibition with sacubitril in combination with valsartan, ivabradine, and the combination of long-acting nitrates and hydralazine can therefore be appropriate oral therapy and part of treatment to achieve the most effective and best tolerated personalized regimen to reduce the risks of HF hospitalization and CV death in specific patients.69, 70, 71, 72, 73 Recently, sodium-glucose transporter 2 inhibitors have been demonstrated to reduce HF hospitalization, progression of kidney disease, and CV death in large outcomes trials in patients with type 2 diabetes mellitus.74 One sodium-glucose transporter 2 inhibitor, dapagliflozin, has been shown to reduce HF hospitalization and CV death in both diabetics and nondiabetics.75 In addition, correction and prevention of anemia with sodium-glucose transporter 2 inhibitors have been demonstrated in type 2 diabetes mellitus patients, including in patients with anemia of CKD.76,77
Most patients who develop chronic CRS have a history of hypertension, diabetes mellitus, or both. A typical patient would have at least a 20-year history of type 2 diabetes mellitus, hypertension, obesity, and coronary heart disease, with LV ejection fraction <45% and effort intolerance on moderate or greater exertion. Their laboratory profile would be an eGFR <45 ml/min per 1.73 m2, urine albumin-to-creatinine ratio 30 to 300 mg/g, blood-brain natriuretic peptide >100 pg/ml, galectin-3 >17 pg/ml, and suppression of tumorigenesis 2 >35 ng/ml,78 with a large fraction also having a baseline troponin concentration above the upper limit of normal. Therefore, management of CRS generally aims to reduce blood pressure and improve glycemic control.25 Based on GDMT for patients with HF (Table 279, 80, 81), a typical patient with CRS will most likely receive therapy with an ACEI or ARB, β-blocker, and/or mineralocorticoid receptor antagonists,66 and may also receive glucose-lowering medications, featuring the sodium-glucose transporter 2 inhibitor class.25,82 Angiotensin-converting enzyme inhibitors and ARBs are also recommended for the management of patients with CKD (Table 279, 80, 81), including those with acute decompensated HF.83,84 Selected inpatients with acute decompensated HF and evidence of poor renal perfusion may also benefit from infusions of positive inotropic agents (e.g., dopamine, dobutamine, and milrinone) or loop diuretics (e.g., furosemide and torsemide) for relief of congestion.85 Patients with advanced CKD or end-stage kidney disease may require dialysis, but there is a lack of evidence to support use of pharmacotherapy in patients with comorbid HF and end-stage kidney disease.85,86 Both i.v. and oral loop diuretics reduce fluid retention, helping to correct volume overload, in patients with congestive HF and can improve renal perfusion in those with impaired kidney function. However, aggressive treatment with high doses of these agents can result in increased sympathetic nervous system and RAAS activity, and increased renal sodium reabsorption, causing further impairment of kidney function.67,85
Table 2.
| ACCF/AHA stage | NYHA functional classification | Recommendation | Treatment options |
|---|---|---|---|
| HF | |||
| A: At high risk for HF but without structural heart disease or symptoms of HF | None | Control hypertension and lipid disorders Control or avoid conditions that could contribute/lead to HF: obesity, diabetes mellitus, tobacco use, known cardiotoxic agents |
Diuretic-based therapy ACEIs ARBs β-Blockers Statins Glucose-lowering agents |
| B: Structural heart disease but without signs or symptoms of HF | I | Treatment based on clinical history Control hypertension and lipid disorders Control or avoid conditions that could contribute/lead to HF: obesity, diabetes mellitus, tobacco use, known cardiotoxic agents |
Diuretic-based therapy ACEIsa ARBs β-Blockers History of MI or ACS and reduced EF: ACEIs,a ARBs, β-blockers History of MI or ACS: statins Reduced EF, no history of MI: ACEIs,a β-blockers Structural cardiac abnormality, no history of MI or ACS: BP-controlling agents |
| C: Structural heart disease with prior or current symptoms of HF | I–IV | HFrEF: Specific education to facilitate self-care required (monitor symptoms, weight, and activity; control sodium intake; and take medications) Treatment based on clinical history Control hypertension and lipid disorders Control or avoid conditions that could contribute/lead to HF: obesity, diabetes mellitus, tobacco use, known cardiotoxic agents HFpEF: Control hypertension and relieve symptoms of volume overload Coronary revascularization for patients with CAD and angina/myocardial ischemia despite GDMT Manage AF |
HFrEF: ACEIa or ARB plus β-blocker; diuretic as needed (patients with fluid retention) Additional therapies based on patient and disease characteristicsb: aldosterone antagonists, angiotensin receptor–neprilysin inhibitor, hydral-nitrates, ivabradine, SGLT2i HFpEF: ACEIs, ARBs, or β-blockers In selected patientsc: aldosterone receptor antagonists |
| D: Refractory HF, requiring specialized interventions | IV | Thorough evaluation to ascertain diagnosis is correct and there are no remediable etiologies or alternative explanations for advanced symptoms Restrict fluids Provide inotropic support if in cardiogenic shock, or refractory to GDMT and device therapy, or hospitalized with severe systolic dysfunction, low BP and depressed CO, or requiring palliative therapy |
Dopamine, dobutamine, milrinone |
| CKD | |||
| All grades | Individualize BP targets and agents according to age, coexistent CVD and other comorbidities, risk of progression of CKD, presence or absence of retinopathy (in patients with diabetes), and treatment tolerance Reduce protein and salt intake Glycemic control in patients with concomitant diabetes mellitus |
ACEIs or ARBs Patients with CKD and diabetes: ACEIs or ARBs, SGLT2i statins, antiplatelet therapy, glucose-controlling therapy, as indicated |
|
ACCF, American College of Cardiology Foundation; ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; AF, atrial fibrillation; AHA, American Heart Association; ARB, angiotensin receptor blocker; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CO, cardiac output; CVD, cardiovascular disease; EF, ejection fraction; GDMT, guideline-directed medical therapy; HF, heart failure; HFpEF, HF with preserved EF; HFrEF, HF with reduced EF; hydral, hydralazine; LVEF, left ventricular EF; MI, myocardial infarction; NYHA, New York Heart Association; SGLT2i, sodium-glucose transporter 2 inhibitor.
The preferred treatment option if not contraindicated.
Aldosterone antagonist for patients with NYHA class II to IV HF, creatinine clearance >30 ml/min, and potassium <5.0 mEq/L; discontinue ACEI or ARB and initiate angiotensin receptor–neprilysin inhibitor for patients with NYHA class II to III HF, adequate BP on ACEI or ARB, and no contraindication to ARB or sacubitril; initiate hydral-nitrates in Black patients with NYHA class III to IV HF; insert implantable cardioverter-defibrillator in patients with NYHA class II to III HF and LVEF ≤35% (also >1-year survival and >40 days post-MI); cardiac resynchronization therapy or device for patients with NYHA class II to IV HF, LVEF ≤35%, normal sinus rhythm QRS ≥150 seconds, and left bundle-branch block; ivabradine for patients with NYHA class II to III HF, normal sinus rhythm, and heart rate ≥70 beats/minute on maximally tolerated dose of β-blocker.
EF ≥45%, increased B-type natriuretic peptide levels or HF admission within 1 year, estimated glomerular filtration rate >30 ml/min, creatinine <2.5 mg/dl, and potassium <5.0 mEq/L.
The management of patients with CRAS
Management of CRAS requires a multidisciplinary approach that considers functional and absolute iron status, the classification of heart and kidney disease, and prognostic indicators for worsening clinical condition (e.g., eGFR, urine albumin-to-creatinine ratio, natriuretic peptide concentrations, galectin-3, suppression of tumorigenesis 2, troponin, LV ejection fraction, and Hb levels).87, 88, 89 However, GDMT in patients with CRAS is limited. Kidney Disease: Improving Global Outcomes convened an international, multidisciplinary conference to discuss the treatment of anemia in patients with CKD and HF and concluded that ESAs have no effect on the prevention or the treatment of HF in patients with CKD.70 Parenteral iron was deemed suitable for patients with concomitant HF and CKD, improving functional capacity, symptoms, and eGFR, whereas hypoxia-inducible factor–prolyl hydroxylase (HIF-PH) inhibitors were highlighted as promising for these patients.70
When considered separately, additional guidance for treating anemia in HF and CKD is available. However, the respective requirements can differ and must be reconciled in patients who present with anemia of CRS. The American College of Cardiology Foundation, American Heart Association Task Force on Clinical Practice Guidelines, and Heart Failure Society of America recommend evaluation for anemia in routine baseline assessment of all patients with HF.79 Similarly, the 2012 Kidney Disease: Improving Global Outcomes guideline recommends regular assessment of Hb levels in patients with CKD, with increasing frequency as kidney function declines.90 Evaluation should include a complete blood cell count, absolute reticulocyte count, serum ferritin levels, serum transferrin saturation, and serum vitamin B12 and folate levels to assess the potential cause of anemia and rule out absolute iron deficiency.90 As discussed by others in this issue of the supplement, i.v. iron and ESAs are the mainstays of treatment for anemia of CKD, addressing both iron and EPO deficiencies.90 However, higher ESA doses needed to achieve anemia correction have been associated with adverse outcomes in patients with CKD, and ESAs are not recommended in patients with HF and anemia; only i.v. iron, in cases of iron deficiency, is recommended for patients with HF and anemia.79 Patients with CRAS therefore pose a treatment dilemma for clinicians as many need greater improvements in symptoms despite maximized GDMT.
Intravenous iron
Intravenous iron therapy improves iron parameters in patients with HF and iron deficiency (serum ferritin <100 μg/L or 100–299 μg/L when transferrin saturation <20%), with or without anemia, including those with or without CKD or CRS.91, 92, 93, 94 In the Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial in patients with chronic HF in which the mean eGFR was ∼64 ml/min per 1.73 m2, i.v. ferric carboxymaltose was associated with significant increases in Hb, serum ferritin, and transferrin saturation, compared with placebo (P < 0.001), in patients with anemia (Hb ≤12 g/dl) and in the overall study population.91 These improvements in iron parameters were accompanied by improvements in NYHA functional class, 6-minute walk tests, and QOL at 24 weeks.91 In the Ferric Iron in Heart Failure (FERRIC-HF) trial, i.v. iron sucrose, compared with no treatment, significantly increased serum ferritin (P < 0.01), transferrin saturation (P < 0.05), NYHA functional class (P < 0.01), and fatigue score (P < 0.01) QOL over 18 weeks and improved QOL relative to baseline.93 There were no changes in Hb, irrespective of the presence of anemia (Hb ≤12.5 g/dL).93 Improvements in iron parameters were maintained at 12 months in patients with stable HF NYHA class II or III (mean eGFR of ∼65 ml/min per 1.73 m2) receiving i.v. ferric carboxymaltose in the Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure (CONFIRM-HF) study.94 Notably, patients receiving i.v. iron continued to have significantly better 6-minute walk test results, fatigue scores, and QOL, along with reduced risk of hospitalization for worsening HF or all-cause death at 12 months (P < 0.05 for all).94 The beneficial effect of i.v. iron therapy on the 6-minute walk test was also seen in patients with HF and an eGFR of <60 ml/min per 1.73 m2 at week 24.94
A retrospective study of patients with CRS and anemia (Hb <12 g/dl) showed that i.v. iron sucrose used alone at 200 mg/wk for 6 weeks significantly increased mean Hb levels from baseline (from 10.6 to 11.9 g/dl; P < 0.001), similar to combined treatment with weekly subcutaneous epoetin-β (from 10.2 to 12.4 g/dl; P < 0.001).92 Notably, baseline iron status did not predict increases in Hb in patients who received iron alone or with ESAs. Treatment with appropriately dosed i.v. iron without ESAs, consistent with recommended treatment guidelines, may be sufficient for anemia management in patients with CRS and anemia.92 Similar findings among patients on hemodialysis indicate that optimal i.v. iron usage may allow for a reduction in ESA dosing, thereby reducing ESA hyporesponsiveness and drug toxicity.44
Therefore, i.v. iron appears to have a key role in the management of anemia in patients with CRAS, and is a recommended therapy for patients with both CKD and anemia90 and HF and iron deficiency to improve clinical functioning and QOL.79,95 In aggregate, data suggest that i.v. iron not only treats iron deficiency anemia, but also influences the natural history of HF and is associated with improvements in symptoms and functional capacity, reductions in hospitalization for worsening HF, and the composite end point of hospitalization for worsening HF or all-cause death.
Erythropoiesis-stimulating agents
Although ESA therapy is beneficial in many patients, a substantial proportion of patients with CKD or congestive HF have ESA resistance, likely as a result of antagonism of EPO by proinflammatory cytokines, and do not achieve improvements in Hb levels at usual therapeutic ESA doses.40
In addition to improving Hb and iron parameters, ESA therapy has been associated with reductions in LV mass and wall thickness and long-term reductions in renal composite outcomes in Japanese patients with CKD.96 Combined ESA therapy and i.v. iron also increase Hb levels and stabilize falling creatinine clearance in patients with HF.97 However, in iron-replete patients with systolic HF and mild-to-moderate anemia, ESA therapy did not significantly reduce the risk of the composite outcome of death from any cause or first hospitalization for worsening HF, despite significant increases in Hb levels.98 Based on the results of this and other studies, ESAs are not recommended by the American College of Cardiology Foundation, the American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Failure Society of America as therapy to improve morbidity and mortality, or by the European Society of Cardiology in patients with HF and anemia.79,95
For patients with CKD, higher ESA doses to achieve a target Hb >11 g/dl are not recommended because of increased risk of CV-related adverse events or mortality,80,99 most likely related to CV toxicity of high ESA doses and not due to the normalization of Hb. In the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) study, low exposure to epoetin alfa and achievement of Hb >11.5 g/dl or >12.7 g/dl reduced the risk of the composite outcome (death, HF, stroke, and myocardial infarction) in patients with CKD, whereas patients with the highest exposure to the ESA and lowest achieved Hb levels (≤11.5 g/dl) had an increased risk of the composite end point.100
In the Mechanisms of Erythropoietin Action in the Cardiorenal Syndrome (EPOCARES) study in patients with CRAS, epoetin-β for 50 weeks led to significant increases in Hb and hematocrit levels compared with standard care.101 However, significant increases in C-terminal fibroblast growth factor 23 were also observed with epoetin-β therapy, which were positively associated with an increased risk of mortality, providing a possible mechanism for the increased CV risk.101
Consistent with the limited data regarding the use of ESAs in patients with CRAS, and lack of recommendation for their use in HF,79 real-world data suggest that a relatively low proportion of patients with CRAS (10%) are prescribed ESA therapy, with previous iron therapy and more severe CKD being predictors of initiating ESA therapy.102
RBC transfusions
RBC transfusion (RBCT) is indicated when ESA therapy is ineffective, when the associated risks of ESA therapy outweigh its potential benefits, or when rapid correction of acute anemia is necessary.90 In general, RBCTs should be avoided in patients with chronic CKD anemia or in those eligible for organ transplant to minimize the risk of adverse outcomes (e.g., CV events and allosensitization).90 The risks associated with RBCTs likely also apply to patients with CRAS, but data regarding the use of RBCTs in these patients are limited.
According to the American Association of Blood Banks’ clinical guidelines, Hb <8 g/dl is recommended as the threshold for considering RBCT in hospitalized patients with preexisting CVD; however, data are insufficient to make any such recommendation for patients with acute coronary syndrome.103 A systematic review indicated an Hb threshold of 7 to 8 g/dl for RBCT was safe in patients undergoing heart surgery, but data in patients with coronary artery disease, acute coronary syndrome, or congestive HF remain limited.104 RBCT in patients with acute coronary syndrome has been associated with increased mortality risk.105 The results of meta-analyses of randomized trials support a restricted rather than a liberal transfusion approach that would likely apply to most patients with CRAS.106 Given the lack of data regarding the use of RBCT specifically in patients with CRAS, a careful risk-benefit assessment is needed.
New treatment options for anemia management
Given the shortcomings and challenges with the current treatment modalities, patients with CRAS would greatly benefit from additional treatment options for anemia. As discussed by others in this supplement, HIF-PH inhibitors promote physiological EPO production by inhibiting the enzyme responsible for downregulation of the transcription factor that controls EPO expression and corresponding erythropoiesis.107 Several oral HIF-PH inhibitors (e.g., daprodustat, desidustat, enarodustat, molidustat, roxadustat, and vadadustat) have advanced to global phase 3 trials following promising phase 2 trials in which these agents maintained or increased Hb levels, without clinically relevant adverse events, in patients with CKD and anemia.108, 109, 110 HIF-PH inhibitors have direct effects on iron metabolism through regulation of several genes involved in iron transport and absorption, independent of EPO stimulation.108 In addition, these agents reduced serum hepcidin108,110 and cholesterol levels110 and may have additional beneficial effects independent of EPO production or iron metabolism regulation, such as the ability to alleviate ischemic injury and improve heart function, vascular pathology, and diabetic nephropathy.111, 112, 113 The increases in Hb levels observed with HIF-PH inhibitors occur with much lower circulating EPO levels than seen with ESAs and, according to studies to date, do not seem to be influenced by baseline inflammation status, perhaps as a result of their effects on hepcidin levels.111 However, HIF has several gene targets affecting multiple organs and cellular functions, and the full range of effects of HIF-PH inhibitors, including angiogenesis and glucose metabolism, has yet to be elucidated.111
The hepcidin antagonist PRS-080#22 improved functional iron deficiency and was well tolerated in a phase 1 study in patients on hemodialysis; phase 2 trials are currently underway (NCT03325621 and NCT02754167), but exclude patients with congestive HF NYHA class III or IV.114 Further studies, particularly in patients with CRAS, are needed to assess the effects of hepcidin inhibition on cardiac and kidney function and to appraise its utility in the treatment of anemia of CRAS.
Conclusions
The management of anemia in patients with CRAS is challenging as multiple guideline-recommended medications are required to effectively control the loss of heart and kidney function in these patients, and data regarding the optimal strategy for patients with CRAS are limited. Updated clinical practice guidelines for the screening and management of anemia in CRS, particularly in light of potential new therapies and clinical evidence, would improve the clinical outcomes of patients with this complex syndrome.
Disclosure
PAM has acted as a consultant to Akebia and AstraZeneca.
Acknowledgements
This article is published as part of a supplement supported by AstraZeneca. Medical writing assistance was provided by Rohan Keshwara, PhD and Caroline Spencer, PharmB of inScience Communications (Philadelphia, PA, USA), funded by AstraZeneca.
Roxadustat is being developed for clinical use by an alliance of FibroGen, Astellas, and AstraZeneca.
Author Contributions
PAM meets the International Committee of Medical Journal Editors criteria for authorship for this article and takes responsibility for the integrity of the work as a whole. PAM and medical writers from inScience Communications wrote the first draft of the article. PAM reviewed and edited subsequent drafts, approved the submission of the article, and is fully accountable for all aspects of the work.
References
- 1.Rangaswami J., Bhalla V., Blair J.E.A. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 2.Cozzolino M., Mangano M., Stucchi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33:iii28–iii34. doi: 10.1093/ndt/gfy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2018 USRDS Annual Data Report: Executive Summary. Am J Kidney Dis. 2019;73:A9–A22. [Google Scholar]
- 4.Yuan J., Zou X.R., Han S.P. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: results from the Chinese cohort study of chronic kidney disease (C-STRIDE) BMC Nephrol. 2017;18:23. doi: 10.1186/s12882-017-0441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Subbiah A.K., Chhabra Y.K., Mahajan S. Cardiovascular disease in patients with chronic kidney disease: a neglected subgroup. Heart Asia. 2016;8:56–61. doi: 10.1136/heartasia-2016-010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Jarallah M., Rajan R., Al-Zakwani I. Incidence and impact of cardiorenal anaemia syndrome on all-cause mortality in acute heart failure patients stratified by left ventricular ejection fraction in the Middle East. ESC Heart Fail. 2019;6:103–110. doi: 10.1002/ehf2.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C.J., Choi I.J., Park H.J. Impact of cardiorenal anemia syndrome on short- and long-term clinical outcomes in patients hospitalized with heart failure. Cardiorenal Med. 2016;6:269–278. doi: 10.1159/000443339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Writing Group Members. Mozaffarian D., Benjamin E.J. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 10.Smith G.L., Lichtman J.H., Bracken M.B. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 11.Foley R.N., Murray A.M., Li S. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 12.He J., Shlipak M., Anderson A. Risk factors for heart failure in patients with chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh N.I., Hwang S.-J., Larson M.G. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
- 14.Kim-Mitsuyama S., Soejima H., Yasuda O. Anemia is an independent risk factor for cardiovascular and renal events in hypertensive outpatients with well-controlled blood pressure: a subgroup analysis of the ATTEMPT-CVD randomized trial. Hypertens Res. 2019;42:883–891. doi: 10.1038/s41440-019-0210-1. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg D.S., Wexler D., Blum M. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 16.Ezekowitz J.A., McAlister F.A., Armstrong P.W. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 17.Grote Beverborg N., Klip I.T., Meijers W.C. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004519. [DOI] [PubMed] [Google Scholar]
- 18.Nanas J.N., Matsouka C., Karageorgopoulos D. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48:2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg D.S., Wexler D., Iaina A. Anemia, chronic renal disease and congestive heart failure - the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. 2006;38:295–310. doi: 10.1007/s11255-006-0064-8. [DOI] [PubMed] [Google Scholar]
- 20.Kosiborod M., Smith G.L., Radford M.J. The prognostic importance of anemia in patients with heart failure. Am J Med. 2003;114:112–119. doi: 10.1016/s0002-9343(02)01498-5. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg D.S., Wexler D., Iaina A. The role of anemia in the progression of congestive heart failure: is there a place for erythropoietin and intravenous iron? Transfus Altern Transfus Med. 2008;6:26–37. [PubMed] [Google Scholar]
- 22.Bock J.S., Gottlieb S.S. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 23.Ronco C. Cardiorenal syndromes: definition and classification. In: Ronco C., Costanzo M.R., Bellomo R., Maisel A.S., editors. Vol 164. KARGER; Basel, Switzerland: 2010. pp. 33–38. (Contrib Nephrol). [DOI] [PubMed] [Google Scholar]
- 24.Yogasundaram H., Chappell M.C., Braam B. Cardiorenal syndrome and heart failure-challenges and opportunities. Can J Cardiol. 2019;35:1208–1219. doi: 10.1016/j.cjca.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough P.A., Tumlin J.A., Szerlip H. Cardiorenal syndromes: advances in determining diagnosis, prognosis and therapy. J Cardiovasc Dis Diagn. 2015;3:1–28. [Google Scholar]
- 26.Bonventre J.V., Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J., Wang Y., Geng X. Metabolic acidosis as a risk factor for the development of acute kidney injury and hospital mortality. Exp Ther Med. 2017;13:2362–2374. doi: 10.3892/etm.2017.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharfuddin A.A., Molitoris B.A. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 29.Hartupee J., Mann D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. doi: 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lymperopoulos A., Rengo G., Koch W.J. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarlane S.I., Winer N., Sowers J.R. Role of the natriuretic peptide system in cardiorenal protection. Arch Intern Med. 2003;163:2696–2704. doi: 10.1001/archinte.163.22.2696. [DOI] [PubMed] [Google Scholar]
- 32.Takimoto E., Kass D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 33.Mihai S., Codrici E., Popescu I.D. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:2180373. doi: 10.1155/2018/2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 35.Palazzuoli A., Antonelli G., Nuti R. Anemia in cardio-renal syndrome: clinical impact and pathophysiologic mechanisms. Heart Fail Rev. 2011;16:603–607. doi: 10.1007/s10741-011-9230-x. [DOI] [PubMed] [Google Scholar]
- 36.Westenbrink B.D., Visser F.W., Voors A.A. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. Eur Heart J. 2006;28:166–171. doi: 10.1093/eurheartj/ehl419. [DOI] [PubMed] [Google Scholar]
- 37.Grote Beverborg N., van Veldhuisen D.J., van der Meer P. Anemia in heart failure: still relevant? JACC Heart Fail. 2018;6:201–208. doi: 10.1016/j.jchf.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555–559. doi: 10.1089/jir.1998.18.555. [DOI] [PubMed] [Google Scholar]
- 39.Morceau F., Dicato M., Diederich M. Pro-inflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediat Inflamm. 2009;2009:405016. doi: 10.1155/2009/405016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Putten K., Braam B., Jie K.E. Mechanisms of disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Rev. 2008;4:47–57. doi: 10.1038/ncpneph0655. [DOI] [PubMed] [Google Scholar]
- 41.Ajmal A., Gessert C.E., Johnson B.P. Effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on hemoglobin levels. BMC Res Notes. 2013;6:443. doi: 10.1186/1756-0500-6-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haase V.K. Hypoxia-inducible factor–prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl. 2021;11:8–25. doi: 10.1016/j.kisu.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal A.K. Iron metabolism and management: focus on chronic kidney disease. Kidney Int Suppl. 2021;11:46–57. doi: 10.1016/j.kisu.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roger S.D., Tio M., Park H.C. Intravenous iron and erythropoiesis-stimulating agents in haemodialysis: a systematic review and meta-analysis. Nephrology (Carlton) 2017;22:969–976. doi: 10.1111/nep.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zumbrennen-Bullough K., Babitt J.L. The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients. Nephrol Dial Transplant. 2014;29:263–273. doi: 10.1093/ndt/gft443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charytan D.M., Fishbane S., Malyszko J. Cardiorenal syndrome and the role of the bone-mineral axis and anemia. Am J Kidney Dis. 2015;66:196–205. doi: 10.1053/j.ajkd.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka M., Komaba H., Fukagawa M. Emerging association between parathyroid hormone and anemia in hemodialysis patients. Ther Apher Dial. 2018;22:242–245. doi: 10.1111/1744-9987.12685. [DOI] [PubMed] [Google Scholar]
- 48.Segall L., Nistor I., Covic A. Heart failure in patients with chronic kidney disease: a systematic integrative review. Biomed Res Int. 2014;2014:937398. doi: 10.1155/2014/937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coe L.M., Madathil S.V., Casu C. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. 2014;289:9795–9810. doi: 10.1074/jbc.M113.527150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Czaya B., Faul C. The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci. 2019;20:4195. doi: 10.3390/ijms20174195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuhu F., Bhandari S. Oxidative stress and cardiovascular complications in chronic kidney disease, the impact of anaemia. Pharmaceuticals (Basel) 2018;11:103. doi: 10.3390/ph11040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bissinger R., Bhuyan A.A.M., Qadri S.M. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J. 2019;286:826–854. doi: 10.1111/febs.14606. [DOI] [PubMed] [Google Scholar]
- 53.El-Achkar T.M., Ohmit S.E., McCullough P.A. Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: the Kidney Early Evaluation Program. Kidney Int. 2005;67:1483–1488. doi: 10.1111/j.1523-1755.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- 54.Thomas M.C., MacIsaac R.J., Tsalamandris C. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care. 2003;26:1164–1169. doi: 10.2337/diacare.26.4.1164. [DOI] [PubMed] [Google Scholar]
- 55.Deray G., Heurtier A., Grimaldi A. Anemia and diabetes. Am J Nephrol. 2004;24:522–526. doi: 10.1159/000081058. [DOI] [PubMed] [Google Scholar]
- 56.Carrero J.J., Barany P., Yilmaz M.I. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrol Dial Transplant. 2012;27:709–715. doi: 10.1093/ndt/gfr288. [DOI] [PubMed] [Google Scholar]
- 57.Ferrucci L., Maggio M., Bandinelli S. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166:1380–1388. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu K.J., Kearney L.G., Hare D.L. Cardiorenal anemia syndrome as a prognosticator for death in heart failure. Am J Cardiol. 2013;111:1187–1191. doi: 10.1016/j.amjcard.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Pallangyo P., Fredrick F., Bhalia S. Cardiorenal anemia syndrome and survival among heart failure patients in Tanzania: a prospective cohort study. BMC Cardiovasc Disor. 2017;17:59. doi: 10.1186/s12872-017-0497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scrutinio D., Passantino A., Santoro D. The cardiorenal anaemia syndrome in systolic heart failure: prevalence, clinical correlates, and long-term survival. Eur J Heart Fail. 2011;13:61–67. doi: 10.1093/eurjhf/hfq167. [DOI] [PubMed] [Google Scholar]
- 61.Belmar Vega L., de Francisco A., Albines Fiestas Z. Iron deficiency in patients with congestive heart failure: a medical practice that requires greater attention. Nefrología. 2016;36:249–254. doi: 10.1016/j.nefro.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Tang W.H., Tong W., Jain A. Evaluation and long-term prognosis of new-onset, transient, and persistent anemia in ambulatory patients with chronic heart failure. J Am Coll Cardiol. 2008;51:569–576. doi: 10.1016/j.jacc.2007.07.094. [DOI] [PubMed] [Google Scholar]
- 63.Minamisawa M., Miura T., Motoki H. Prognostic impact of cardio-renal-anemia syndrome in patients at risk for heart failure. Circulation. 2017;136 :A14876. [Google Scholar]
- 64.Bivol E., Grib L. Psychosocial stress and quality of life in patients with type 2 cardiorenal syndrome. Arch Balkan Medical Union. 2019;54:147–154. [Google Scholar]
- 65.Balakumaran K., Patil A., Marsh S. Evaluation of a guideline directed medical therapy titration program in patients with heart failure with reduced ejection fraction. Int J Cardiol Heart Vasc. 2019;22:1–5. doi: 10.1016/j.ijcha.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhagat A.A., Greene S.J., Vaduganathan M. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail. 2019;7:1–12. doi: 10.1016/j.jchf.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark A., Kalra P., Petrie M. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. Heart. 2019;105:904–910. [Google Scholar]
- 68.Raebel M.A. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: hyperkalemia with ACEI and ARB. Cardiovasc Ther. 2012;30:e156–e166. doi: 10.1111/j.1755-5922.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 69.Cole R.T., Kalogeropoulos A.P., Georgiopoulou V.V. Hydralazine and isosorbide dinitrate in heart failure: historical perspective, mechanisms, and future directions. Circulation. 2011;123:2414–2422. doi: 10.1161/CIRCULATIONAHA.110.012781. [DOI] [PubMed] [Google Scholar]
- 70.House A.A., Wanner C., Sarnak M.J. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:1304–1317. doi: 10.1016/j.kint.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Jhund P.S., McMurray J.J. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart. 2016;102:1342–1347. doi: 10.1136/heartjnl-2014-306775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koruth J.S., Lala A., Pinney S. The clinical use of ivabradine. J Am Coll Cardiol. 2017;70:1777–1784. doi: 10.1016/j.jacc.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 73.Sica D.A. Mineralocorticoid receptor antagonists for treatment of hypertension and heart failure. Methodist Debakey Cardiovasc J. 2015;11:235–239. doi: 10.14797/mdcj-11-4-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kluger A.Y., Tecson K.M., Lee A.Y. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol. 2019;18:99. doi: 10.1186/s12933-019-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McMurray J.J.V., Solomon S.D., Inzucchi S.E. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 76.Maruyama T., Takashima H., Oguma H. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol Ther. 2019;21:713–720. doi: 10.1089/dia.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefansson B.V., Heerspink H.J.L., Wheeler D.C. Correction of anemia by dapagliflozin in patients with type 2 diabetes. J Diabetes Complications. 2020;34:107729. doi: 10.1016/j.jdiacomp.2020.107729. [DOI] [PubMed] [Google Scholar]
- 78.Parikh R.H., Seliger S.L., Christenson R. Soluble ST2 for prediction of heart failure and cardiovascular death in an elderly, community-dwelling population. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yancy C.W., Jessup M., Bozkurt B. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 80.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 81.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 82.Lo K.B., Gul F., Ram P. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2020;10:1–10. doi: 10.1159/000503919. [DOI] [PubMed] [Google Scholar]
- 83.Oliveros E., Oni E.T., Shahzad A. Benefits and risks of continuing angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, and mineralocorticoid receptor antagonists during hospitalizations for acute heart failure. Cardiorenal Med. 2020;10:69–84. doi: 10.1159/000504167. [DOI] [PubMed] [Google Scholar]
- 84.Singhania G., Ejaz A.A., McCullough P.A. Continuation of chronic heart failure therapies during heart failure hospitalization - a review. Rev Cardiovasc Med. 2019;20:111–120. doi: 10.31083/j.rcm.2019.03.562. [DOI] [PubMed] [Google Scholar]
- 85.Obi Y., Kim T., Kovesdy C.P. Current and potential therapeutic strategies for hemodynamic cardiorenal syndrome. Cardiorenal Med. 2016;6:83–98. doi: 10.1159/000441283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rangaswami J., McCullough P.A. Heart failure in end-stage kidney disease: pathophysiology, diagnosis, and therapeutic strategies. Semin Nephrol. 2018;38:600–617. doi: 10.1016/j.semnephrol.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 87.Ibrahim N.E., Januzzi J.L., Jr. Established and emerging roles of biomarkers in heart failure. Circ Res. 2018;123:614–629. doi: 10.1161/CIRCRESAHA.118.312706. [DOI] [PubMed] [Google Scholar]
- 88.McCullough P.A. Practical experience using galectin-3 in heart failure. Clin Chem Lab Med. 2014;52:1425–1431. doi: 10.1515/cclm-2014-0278. [DOI] [PubMed] [Google Scholar]
- 89.McCullough P.A., Olobatoke A., Vanhecke T.E. Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011;12:200–210. doi: 10.3909/ricm0624. [DOI] [PubMed] [Google Scholar]
- 90.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 91.Anker S.D., Comin Colet J., Filippatos G. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 92.Ben-Assa E., Shacham Y., Shashar M. Target hemoglobin may be achieved with intravenous iron alone in anemic patients with cardiorenal syndrome: an observational study. Cardiorenal Med. 2015;5:246–253. doi: 10.1159/000433564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okonko D.O., Grzeslo A., Witkowski T. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 94.Ponikowski P., van Veldhuisen D.J., Comin-Colet J. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC): developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 96.Akaishi M., Hiroe M., Hada Y. Effect of anemia correction on left ventricular hypertrophy in patients with modestly high hemoglobin level and chronic kidney disease. J Cardiol. 2013;62:249–256. doi: 10.1016/j.jjcc.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Silverberg D. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transpl. 2003;18:viii7–viii12. doi: 10.1093/ndt/gfg1084. [DOI] [PubMed] [Google Scholar]
- 98.Swedberg K., Young J.B., Anand I.S. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 99.US Food and Drug Administration FDA drug safety communication: modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-modified-dosing-recommendations-improve-safe-use-erythropoiesis Available at:
- 100.McCullough P.A., Barnhart H.X., Inrig J.K. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol. 2013;37:549–558. doi: 10.1159/000351175. [DOI] [PubMed] [Google Scholar]
- 101.Eisenga M.F., Emans M.E., van der Putten K. Epoetin beta and C-terminal fibroblast growth factor 23 in patients with chronic heart failure and chronic kidney disease. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jackevicius C.A., Co M.J., Warner A.L. Predictors of erythropoietin use in patients with cardiorenal anaemia syndrome. Int J Pharm Pract. 2015;23:199–204. doi: 10.1111/ijpp.12133. [DOI] [PubMed] [Google Scholar]
- 103.Carson J.L., Guyatt G., Heddle N.M. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 104.Carson J.L., Stanworth S.J., Alexander J.H. Clinical trials evaluating red blood cell transfusion thresholds: an updated systematic review and with additional focus on patients with cardiovascular disease. Am Heart J. 2018;200:96–101. doi: 10.1016/j.ahj.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 105.Rao S.V., Jollis J.G., Harrington R.A. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 106.Holst L.B., Petersen M.W., Haase N. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Semenza G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 108.Haase V.H., Chertow G.M., Block G.A. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. 2019;34:90–99. doi: 10.1093/ndt/gfy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaplan J.M., Sharma N., Dikdan S. Hypoxia-inducible factor and its role in the management of anemia in chronic kidney disease. Int J Mol Sci. 2018;19:389. doi: 10.3390/ijms19020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Provenzano R., Besarab A., Wright S. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 111.Coyne D.W., Goldsmith D., Macdougall I.C. New options for the anemia of chronic kidney disease. Kidney Int Suppl (2011) 2017;7:157–163. doi: 10.1016/j.kisu.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ghadge S.K., Messner M., Van Pham T. Prolyl-hydroxylase inhibition induces SDF-1 associated with increased CXCR4+/CD11b+ subpopulations and cardiac repair. J Mol Med (Berl) 2017;95:825–837. doi: 10.1007/s00109-017-1543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Young J.M., Williams D.R., Thompson A.A.R. Thin air, thick vessels: historical and current perspectives on hypoxic pulmonary hypertension. Front Med (Lausanne) 2019;6:93. doi: 10.3389/fmed.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Renders L., Budde K., Rosenberger C. First-in-human phase I studies of PRS-080#22, a hepcidin antagonist, in healthy volunteers and patients with chronic kidney disease undergoing hemodialysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212023. [DOI] [PMC free article] [PubMed] [Google Scholar]