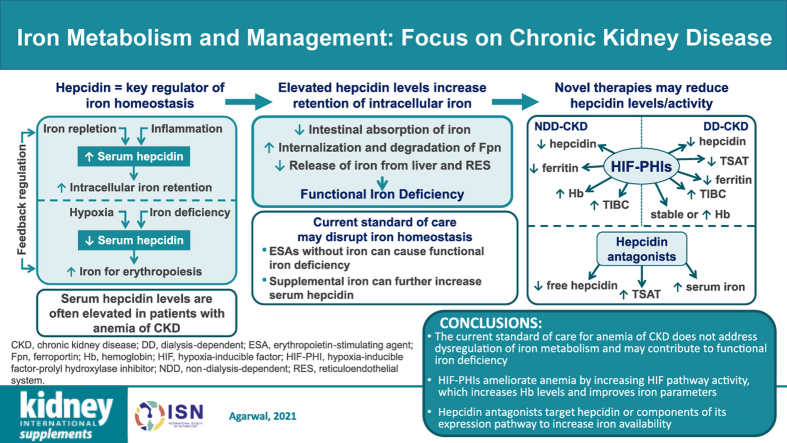
Graphical Abstract
Keywords: anemia, erythropoiesis, ferroportin, hepcidin, inflammation, iron
Abstract
Anemia is common in patients with chronic kidney disease (CKD) and results from the dysregulation of iron metabolism and erythropoiesis. Hepcidin is a key regulator of iron availability and leads to iron sequestration during the state of iron repletion. Decreases in the level of hepcidin in the presence of hypoxia and/or iron limitation allow for greater iron availability for erythropoiesis. However, kidney excretion of hepcidin decreases as the severity of CKD increases, whereas production of hepcidin is increased under inflammatory conditions often present in patients with CKD, both of which contribute to anemia. Assessment of iron status is, therefore, essential in the treatment of anemia. However, current laboratory tests for the determination of the adequate supply of iron have many limitations, including diurnal variation in the levels of biomarkers, lack of standardized reference methods across laboratories, and confounding by the presence of inflammation. In addition, the current treatment paradigm for anemia of CKD can further disrupt iron homeostasis; for example, treatment with erythropoiesis-stimulating agents in the absence of supplemental iron can induce functional iron deficiency. Moreover, supplemental iron can further increase levels of hepcidin. Several novel therapies, including hypoxia-inducible factor prolyl hydroxylase inhibitors and hepcidin inhibitors/antagonists, have shown promise in attenuating the levels and/or activity of hepcidin in anemia of CKD, thus ensuring the availability of iron for erythropoiesis.
Iron is essential for many biological processes because of its ability to engage in oxidation-reduction reactions, including, as a component of heme, the ability to transport oxygen throughout the body. However, an excess of iron can lead to the generation of reactive oxygen species, which can damage many cellular components. Regulation and sequestration of iron within the body is necessary to maintain this delicate balance.
Most of the iron in the body is derived from recycled red blood cells, with a lower amount contributed by dietary absorption. Anemia can result from low iron level and/or improper regulation of iron level in disorders such as chronic kidney disease (CKD), in addition to iron losses from blood draws, uremic bleeding, and hemodialysis. Anemia is defined as hemoglobin (Hb) level <13.0 g/dl for men and <12.0 g/dl for nonpregnant women.1,2 In CKD, comorbid anemia is due to inadequate erythropoietin (EPO) production by the kidneys and/or dysregulated iron homeostasis; decreased red blood cell survival also contributes to anemia development.3 In the United States, >38 million people (∼15% of the population) have CKD.4 Anemia is common among patients with advancing stages of CKD, with increasing prevalence from stage G3b CKD onward.5 The prevalence of anemia in US patients with CKD is 15% to 24% (about 5–9 million people), although treatment with erythropoiesis-stimulating agents (ESAs) is initiated only in a minority of those patients with Hb levels <10 g/dl.6,7
Herein, an overview is presented of iron homeostasis and the iron regulator hepcidin, with a focus on anemia of CKD. The limitations of both laboratory testing for determination of iron status and the current standard of care for managing iron deficiency in anemia of CKD are examined. Finally, novel therapeutics for anemia of CKD that target hepcidin are discussed.
Hepcidin Structure, Production, and Action
Hepcidin-25 is an important regulator of iron availability that leads to decreased iron transport and increased iron sequestration. It is a 25–amino acid peptide produced mainly in the liver, first discovered about 20 years ago.8,9 Hepcidin-25 contains 8 cysteine residues, all of which are disulfide bonded,8,9 resulting in a hairpin-like structure.10 Shorter lengths of hepcidin (hepcidin-20, hepcidin-22, hepcidin-23, and hepcidin-24) are produced by N-terminal degradation and may represent an additional mode of inactivation of hepcidin-25.9 These forms are not as well characterized, but appear to have antimicrobial and antifungal properties, as does hepcidin-25.9
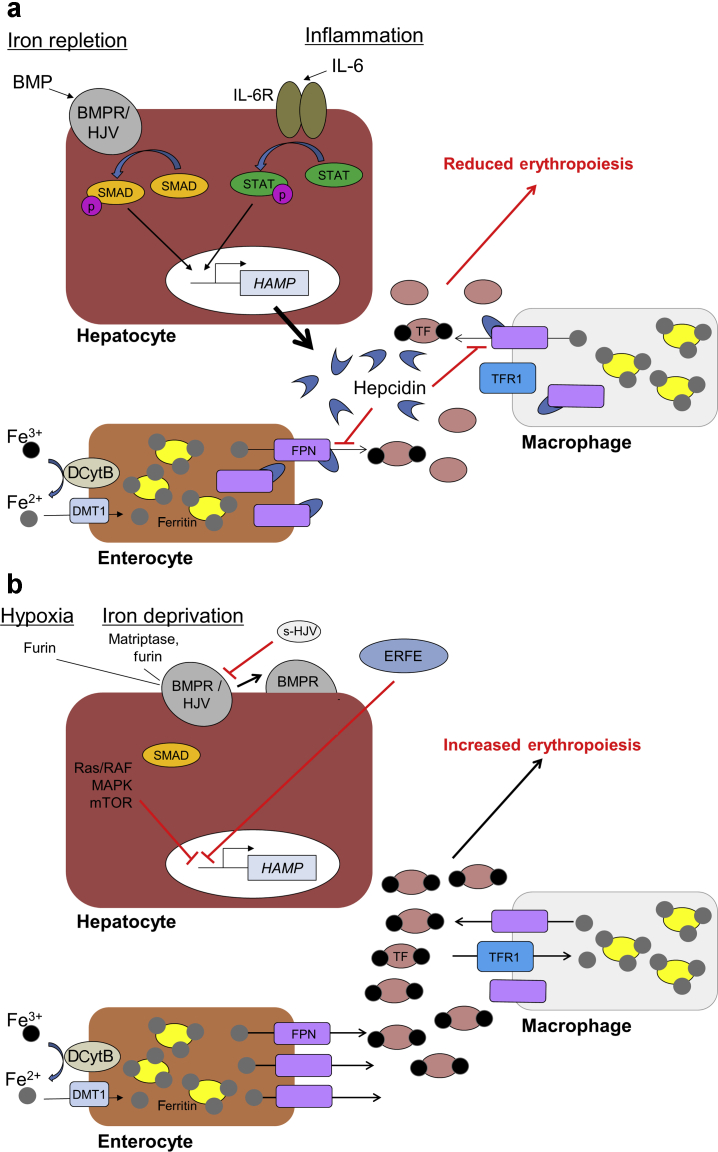
The expression of hepcidin is upregulated by inflammation (Figure 1a).11 Production of hepcidin-25 appears to be regulated primarily at the transcriptional level.12 The hepcidin antimicrobial peptide (HAMP) gene, which encodes hepcidin, is upregulated by bone morphogenetic protein (BMP) binding to type I and II BMP receptors and the coreceptor hemojuvelin. This complex phosphorylates Caenorhabditis elegans SMA/Drosophila mothers against decapentaplegic proteins, which then increase transcription of HAMP.13 The amount of BMP is regulated by hepatic iron stores so that hepcidin levels increase under iron-replete conditions.14 The inflammatory cytokine, interleukin-6, directly binds the signal transducer and activator of transcription-3 protein to the HAMP promoter, thus inducing the expression of hepcidin under inflammatory conditions.15 In addition to being a marker of inflammation, elevated hepcidin expression combined with reduced ferroportin expression and increased intracellular iron concentrations has been associated with the development and progression of cancer.16, 17, 18
Figure 1.
Regulation of hepcidin and iron homeostasis. (a) Under conditions of iron repletion, bone morphogenetic protein (BMP) is upregulated. Binding of BMP to the BMP receptor (BMPR)/hemojuvelin (HJV) complex activates phosphorylation of Caenorhabditis elegans SMA/Drosophila mothers against decapentaplegic (SMAD), which then activates transcription of hepcidin antimicrobial peptide (HAMP) gene. Under inflammatory conditions, interleukin-6 (IL-6) activates the Janus kinase/signal transducer and activator of transcription (STAT) pathway that also activates transcription of HAMP. Hepcidin is then secreted by the liver and is able to bind ferroportin (FPN), a key exporter of iron into the circulation, on the surface of iron exporting cells such as macrophages and enterocytes. The binding of hepcidin to FPN results in the internalization and subsequent degradation of FPN, reducing iron export into the circulation. This then reduces the amount of transferrin-bound iron within the circulation, limiting the availability of iron for key biological processes, including erythropoiesis. (b) Under conditions of hypoxia or iron deprivation, transcription of HAMP is inhibited in hepatocytes via multiple mechanisms that are regulated by hypoxia-inducible factor, including furin-mediated conversion of membrane-bound HJV to soluble HJV (s-HJV), which can interfere with BMP signaling; erythroferrone (ERFE) produced during erythropoiesis signaling; and activation of the Ras rapidly accelerated fibrosarcoma mitogen-activated protein kinase (Ras/RAF-MAPK)–mammalian target of rapamycin (mTOR) signaling pathway. In the absence of hepcidin, iron is able to mobilize from cells to be used in heme formation during erythropoiesis. DCytB, duodenal cytochrome B; DMT1, divalent metal transporter 1; IL-6R, IL-6 receptor; TF, transferrin; TFR1, TF receptor 1.
Several other regulators, such as hypoxia and iron deficiency, attenuate the expression of hepcidin (Figure 1b). Under iron deprivation conditions, the serine protease matriptase-2 (also called TMPRSS6) is increased and can cleave hemojuvelin, thus repressing hepcidin expression.19,20 The protease furin can also cleave hemojuvelin under conditions of hypoxia and iron deficiency, resulting in repression of hepcidin expression.21 Hepcidin is also downregulated via inhibition of BMP/Caenorhabditis elegans SMA/Drosophila mothers against decapentaplegic signaling by the hormone erythroferrone, which is upregulated by EPO.22 In addition, Ras/RAF mitogen-activated protein kinase and mammalian target of rapamycin signaling, which control liver nutrient homeostasis and boost hepatocyte proliferation, repress the expression of hepcidin.23
Iron homeostasis and hepcidin
Most of the iron in the body is stored in reticuloendothelial macrophages and the liver and is derived from recycled erythrocytes. Iron is gained through dietary absorption from the small intestine.3 After reduction to the ferrous form by duodenal cytochrome B, iron enters enterocytes via divalent metal transporter 1, a transmembrane protein that transports several metals, including ferrous iron (Figure 1).24 The protein transferrin binds iron to transport it to other types of cells, where it then binds to the transferrin receptor, is internalized, and the iron is extracted; the iron-free transferrin is released to the extracellular space, and the intracellular iron is stored within ferritin.12
Feedback regulation of hepcidin is mediated by iron levels in plasma and liver, the need for erythropoiesis, and the inflammatory state.12 Under normal, iron-replete conditions, hepcidin binds to ferroportin, an iron exporter that is required for the release of iron from enterocytes, macrophages, and hepatocytes into plasma or extracellular space, and causes its internalization and degradation, leading to intracellular iron retention.25
Absolute iron deficiency occurs when iron is depleted from the body, such as during blood loss. In contrast, functional iron deficiency is a state in which total body iron stores are sufficient but iron is not effectively mobilized from storage; this can occur under conditions of chronic inflammation or when erythropoiesis is stimulated to a large, supraphysiologic degree, such as during treatment with ESAs.26 A hallmark of functional iron deficiency in the presence of inflammation is an increased level of hepcidin.27 Patients with anemia of CKD may exhibit absolute iron deficiency (e.g., due to blood loss from hemodialysis), functional iron deficiency (e.g., due to high levels of inflammation), or both.
Tests for Clinical Measurement of Iron and their Limitations
A complicating factor in measuring iron levels and the response to treatment is that many of the commonly used tests for iron do not accurately reflect body iron load, especially in the presence of inflammation, which is common in CKD. Some of these parameters exhibit diurnal variation (including serum levels of iron and hepcidin),28,29 rendering sampling time important for comparisons. In addition, the wide variety of assay procedures, some of which show high intermethod variability,30 further complicates interpretation of these iron parameters. Despite their shortcomings, these iron parameters are commonly reported in clinical trials and in monitoring patients with anemia of CKD,1 in part because of the relative ease of obtaining blood samples compared with bone marrow examination (Table 131, 32, 33, 34).
Table 1.
Benefits and shortcomings of clinical iron measurements
| Iron parameter | Benefits | Shortcomings |
|---|---|---|
| Hepcidin | Ease of measurement | Diurnal variation28 High (∼49%) within-person variation31,32 |
| %Hypo | Sensitive, reliable marker of functional iron deficiency (when ≥6%)33 | Unreliable indicator of short-term changes in iron status |
| Reticulocyte Hb concentration | Ease of measurement (decreased in both absolute and functional iron deficiency)27 | Lack of standardized reference methods across laboratories31 Variations in results because of sample transport and storage31 |
| Serum ferritin | Reliably indicates iron status in noninflammatory conditions (decreased in absolute iron deficiency)27 May be used in combination with low TSAT (normal or elevated in functional iron deficiency)33 |
Unreliable indicator of iron status in the presence of inflammation12,27 |
| Serum iron | Ease of measurement | Diurnal variation29 High (∼30%) within-person variation31 Further decreased in inflammatory conditions31 |
| Soluble transferrin receptor | Ease of measurement (increased in absolute iron deficiency)27 | Lack of standardized reference methods across laboratories31 Affected by inflammation (decreased in functional iron deficiency)27 |
| TIBC | Ease of measurement | Diurnal variation12 |
| TSAT | Ease of measurement (decreased in both absolute and functional iron deficiency)27 May be used in combination with serum ferritin to diagnose functional iron deficiency33 |
Same as those of its components (serum iron and TIBC)34 |
| Bone marrow examination | Accuracy | Painful, cannot repeat often |
%Hypo, percentage of hypochromic red cells; Hb, hemoglobin; TIBC, total iron binding capacity; TSAT, transferrin saturation.
Serum ferritin
The primary role of ferritin is to function as the intracellular storage of iron, with a normal range of 15 to 300 μg/L.34,35 Serum ferritin is low (<100 ng/ml) in absolute iron deficiency in patients with CKD.36 However, although ferritin can reliably indicate iron status in the absence of inflammation, it is upregulated under inflammatory conditions. Thus, normal or high serum ferritin levels may not accurately indicate iron repletion in state of inflammation and functional iron deficiency.12,27 More than half of patients with CKD exhibit chronic inflammation with increased prevalence at higher stages of CKD; and in patients receiving dialysis,37 serum ferritin levels can be misleading. Thus, a transferrin saturation (TSAT) test is recommended to confirm iron deficiency in patients with inflammatory conditions who have serum ferritin values of 100 to 300 μg/L.27
Serum iron
Serum iron concentration reflects circulating iron primarily bound to transferrin; the normal range is typically between 65 and 175 μg/dl in men and between 50 and 170 μg/dl in women.38 Serum iron levels are low in iron deficiency34 and exhibit high intraindividual variation over time, and are further decreased under inflammatory conditions.31
Total iron binding capacity
Serum total iron binding capacity (TIBC) describes the amount of circulating transferrin available to bind iron, and is high when iron is deficient (>450 μg/dl).34,39 However, TIBC can be below normal in functional iron deficiency because iron stores are elevated. Transferrin levels are decreased under inflammatory conditions, further confounding TIBC interpretation.31 In addition, the amount of iron bound to transferrin is subject to diurnal variation and turnover occurs every few hours, complicating its measurement.12
Transferrin saturation
TSAT reflects the proportion of transferrin that is bound to iron and is low in absolute iron deficiency, with values of <20% indicative of iron deficiency.27,34,36 Because TSAT is calculated by dividing levels of serum iron by TIBC,34 TSAT mirrors any inaccuracies in the measurements of serum iron or TIBC as well as any effects of inflammation on either.
Soluble transferrin receptor
Iron deficiency decreases the amount of iron-laden transferrin and results in cleavage of the cell membrane–bound transferrin receptor to soluble transferrin receptor (sTfR).27 Because the majority of sTfR in serum is derived from erythroblasts and reticulocytes, increased levels of circulating sTfR indicate erythropoietic activity and the development of iron deficiency.40,41 The presence of inflammation can, however, inhibit erythropoiesis and thus reduce sTfR levels.27 The use of sTfR to assess iron status is also complicated by the lack of standardization across laboratories.31
Hypochromic red cells
The percentage of hypochromic red cells is often used to confirm a diagnosis of functional iron deficiency in patients with CKD.33 Red blood cells with intracellular Hb levels <28 g/dl are classified as hypochromic. A percentage of hypochromic red cells of ≥6% is considered more effective for the identification of functional iron deficiency than sTfR, zinc protoporphyrin, serum ferritin, and TIBC in patients with CKD receiving ESA therapy.42 However, percentage of hypochromic red cells assessment does not provide a reliable measure of short-term changes in iron status in response to iron administration.43
Reticulocyte Hb concentration
Reticulocyte Hb concentration is a sensitive measure of the amount of functional iron available for erythropoiesis31 and can detect short-term changes in iron status43 because it measures the amount of Hb in the reticulocytes (i.e., red blood cells that are only 1–2 days old, in contrast to total red blood cells, some of which may be up to 120 days old). Thus, reticulocyte Hb concentration mirrors the amount of iron available for incorporation into these new red blood cells.36 However, a drawback of this measurement also includes a lack of standardization of methods.
Hepcidin
Circulating hepcidin levels are high under iron-replete conditions but are also high in anemia of CKD because of the upregulation of hepcidin due to inflammation. Hepcidin can be measured via several different assays. Liquid chromatography–tandem mass spectrometry requires expensive, specialized equipment but can distinguish among the hepcidin isoforms. Enzyme-linked immunosorbent assay and other immunoassays, however, will detect all hepcidin isoforms, even the inactive forms, and can potentially overestimate levels of functional hepcidin. Recently, latex immunoassays have shown utility, being equivalent to liquid chromatography–tandem mass spectrometry for measurement of hepcidin-25 and can also be faster and easily performed at the clinical site.44
Notably, high interstudy and intraindividual variability has cast doubt on the utility of hepcidin levels as a biomarker of iron status.31,32 Serum hepcidin levels, measured via enzyme-linked immunosorbent assay, were significantly higher in adult patients with CKD compared with healthy adults. In addition, in both healthy volunteers and patients with CKD, ferritin and sTfR levels were significantly associated with hepcidin levels, whereas glomerular filtration rate was inversely correlated. The latter may indicate that, as glomerular filtration rate is impaired, hepcidin is no longer efficiently excreted in urine.28,45 In contrast, another study found that levels of serum hepcidin-25 (measured via matrix-assisted laser desorption ionization–time-of-flight mass spectrometry) were correlated with ferritin but not estimated glomerular filtration rate in patients with CKD.46 Similarly, serum hepcidin-25 levels, measured via liquid chromatography–tandem mass spectrometry, negatively correlated with Hb levels in patients with non–dialysis-dependent (NDD) CKD,47 but in another study, high levels of hepcidin and other inflammatory parameters were seen in patients with early CKD, but serum hepcidin did not correlate with Hb. Because hepcidin was measured via enzyme-linked immunosorbent assay, the discrepancy between the results may be due to the assay or a different patient population.48
Bone marrow examination
Iron stores may be determined by cytological assessment of bone marrow aspirate and use of Perls Prussian blue.33 Although this test may be considered “gold standard,” insufficient material often leads to inaccurate results, and the test provides no information on the amount of available iron for erythropoiesis.49 In addition, bone marrow examination is painful and often associated with postbiopsy complications, including bleeding, and therefore is generally not justifiable for assessing iron stores in patients with CKD.33
Combination of parameters to assess iron status
Several studies have examined the combination of >1 clinical measurement of iron to assess iron status. The most commonly used combination of iron parameters for the diagnosis of functional iron deficiency anemia in CKD is normal or high serum ferritin plus low TSAT.33,50 Although neither serum ferritin nor TSAT alone demonstrated high specificity and sensitivity, use of both did when bone marrow iron was used as a reference standard in patients with anemia of CKD.51 Ferritin and TSAT assay results poorly indicated body iron load in patients with dialysis-dependent (DD) CKD.52 A combined index of TSAT, TIBC, and ferritin showed the strongest association with Hb levels in patients with noninflammatory NDD CKD.53
Iron Deficiency in Patients with Anemia of CKD
Anemia of CKD occurs as a result of a relative deficiency of EPO, functional iron deficiency, impaired iron absorption, and/or blood loss due to dialysis.3,12 Iron-restricted erythropoiesis can also occur because of the elevated inflammatory state in patients with CKD as well as from ESAs used to treat anemia of CKD, as described in the next section. Levels of serum hepcidin-25 are elevated in patients with anemia of CKD, although there is high intrapatient variability and diurnal variation.28 Higher serum hepcidin levels, along with higher levels of inflammatory markers, such as C-reactive protein and interleukin-6, are frequently observed in patients on hemodialysis compared with healthy controls.54,55 In patients with NDD CKD, serum hepcidin levels were not associated with anemia in patients with earlier stages of CKD, but serum hepcidin was significantly associated with anemia in patients with later stages of CKD.56 Reduced renal excretion of hepcidin has been observed in patients with CKD, which may also contribute to the higher observed hepcidin levels.45
Iron supplementation in the current standard of care of anemia of CKD and its limitations
Anemia of CKD can be treated with iron supplementation to increase Hb levels with or without concomitant use of ESAs.1,57 Iron is frequently given along with ESA treatment because ESAs stimulate erythropoiesis that may lead to functional iron deficiency.1,58 Supplemental iron is generally administered either orally or i.v., and several considerations should guide the choice.
Oral iron is relatively inexpensive, and the route of administration avoids the need for i.v. access in patients who are not receiving hemodialysis.1 In addition, newer oral iron formulations, with improved tolerability and absorption, are in development (ferric maltol and Sucrosomial® iron) or have been approved (ferric citrate) for the treatment of anemia in patients with NDD CKD.59 However, oral iron is not readily absorbed and is thus not as effective as i.v. iron in raising Hb levels.1,60,61 In addition, the common gastrointestinal adverse effects of oral iron, although not serious, may limit adherence.1
The 2012 Kidney Disease: Improving Global Outcomes guideline recommends a trial of i.v. iron with or without concomitant ESA therapy in patients with CKD and anemia who require an increase in Hb levels or who have serum ferritin ≤500 ng/ml and TSAT ≤30%.1 In the recent Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) study, high-dose i.v. iron sucrose administration led to reduced ESA dose requirements in patients with DD CKD on maintenance hemodialysis compared with low-dose i.v. iron sucrose.62 Intravenous iron may be associated with more frequent serious adverse events than oral iron, including allergic/anaphylactoid reactions, cardiovascular events, and infections,63, 64, 65 as i.v. iron in CKD patients has been shown to promote oxidative damage to DNA, proteins, and lipids.66 However, in the PIVOTAL study, the risk of death or major cardiovascular event was lower with high-dose versus low-dose i.v. iron sucrose administration.62 This suggests that adverse events with i.v. iron may not be dose dependent.
Iron delivery via dialysate, in the form of ferric pyrophosphate citrate, is efficacious in patients with anemia of CKD who are receiving hemodialysis but cannot be administered to patients on peritoneal dialysis or patients not on dialysis.67
In addition to the increase in hepcidin levels seen in response to oral or i.v. iron in CKD patients,28,68 patients with DD CKD who received i.v. iron also showed higher levels of inflammatory markers compared with patients who did not receive i.v. iron.69 Iron overload must be avoided because high iron levels are associated with higher risks of hospitalization and mortality.70,71 However, the impact of iron in patients on dialysis remains a subject of debate. In the prospective, randomized, multicenter, open-label PIVOTAL trial in 2141 patients on hemodialysis, a high-dose, proactive i.v. iron regimen was associated with a decreased risk of the composite end point of death, myocardial infarction, stroke, or hospitalization for heart failure compared with a low-dose, reactive iron regimen.62 In addition, patients on the high-dose, proactive iron regimen had a nearly 20% lower monthly ESA dose than those on the low-dose, reactive iron regimen.62 Thus, the risks and benefits of i.v. iron must be carefully weighed.70,71
New therapies with the potential to restore iron homeostasis in patients with anemia of CKD
Several new strategies are being explored for the management of anemia of CKD (Table 272, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97). One strategy involves hypoxia-inducible factor (HIF), a heterodimeric transcription factor that is regulated by HIF–prolyl hydroxylase (PH), which targets it for degradation under oxygen-replete conditions98 (see the article by Haase99 in this supplement for detailed discussion). Under hypoxic conditions, HIF-α regulates genes encoding EPO and iron transporters, such as ferroportin, thereby facilitating erythropoiesis and iron utilization.100, 101, 102 Stabilization of HIF with inhibitors of HIF-PH is a strategy to increase the expression of EPO in the kidney103 to ameliorate anemia of CKD. In addition to stimulating EPO production104 and raising and/or maintaining Hb levels in patients with anemia of CKD, all reported HIF-PH inhibitors have shown hepcidin-lowering effects. Indeed, HIF indirectly decreases the expression of HAMP, the gene encoding hepcidin, through induction of erythropoiesis.105 HIF also modulates iron homeostasis via regulation of genes encoding divalent metal transporter 1, duodenal cytochrome B, transferrin, transferrin receptor, and ceruloplasmin.106
Table 2.
Randomized, controlled trials of newer therapies for anemia of CKD
| Therapy | Class | Patients | Trial phase, identifier | Treatment duration | Hb, g/dl | Hepcidin, μg/L | Serum iron, μg/dl | Ferritin, μg/L | Transferrin, g/L | TIBC, μmol/L | TSAT, % | sTfR, nmol/L | CHr, pg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Daprodustat | HIF-PH inhibitor | CKD stage 3–5, NDD | Phase 2, NCT0158789872 | 4 wk | Dose-dependent increase –0.12 to 0.95 |
Decreased –143.6 to –16.2 |
–1.7 to –0.4 μmol/L | Decreased –101.8 to –8.2 |
Increased 0.03 to 0.39 |
Increased 0.3 to 8.3 |
–3.4 to –2.6 | — | — |
| CKD stage 3–5, NDD | Phase 2, NCT0104739773 | 28 d | Increased | Decreased | Dose-dependent decrease | Decreased | — | Increased | Decreased | — | — | ||
| CKD stage 3–5, NDD | Phase 2, NCT0197757374 | 24 wk | Increased | Decreased –17.3 |
— | Decreased –37.9 |
— | Increased 3.1 mmol/L |
Decreased –4.5 |
— | — | ||
| HD, stable ESA | Phase 2, NCT0158792472 | 4 wk | Stable –1.06 to –0.08 |
–0.5 to 154.0 | Increased 2.2 to 5.0 μmol/L |
–80.8 to 74.2 | 0.1 to 0.2 | Increased 3.3 to 5.2 |
Increased 0.3 to 10.1 |
— | — | ||
| HD, stable ESA | Phase 2, NCT0201971975 | 4 wk | Dose-dependent increase –0.28 to 0.97 |
Decreased –60.4 to –76.2a |
Decreased –2.4 to –4.9 |
Decreased –50.6 to –113.3 |
0.4 to 0.8 | Increased 8.6 to 17.2 |
Decreased –31.3 to –50.4 |
— | — | ||
| CKD stage 5, HD, no ESA | Phase 2, NCT0104739773 | 28 d | Increased | Decreased | — | Decreased | — | Increased | Inconsistent | — | — | ||
| HD, chronic ESA hyporesponsiveness | Phase 2, NCT0207546376 | 16 wk | Increased | Fluctuated | — | Decreased | Increased | — | Increased | — | — | ||
| HD, stable ESA | Phase 2, NCT0197748277 | 24 wk | ∼0.1 | –20.6 | — | –59.9 | — | 5.5 | –4.4 | — | — | ||
| HD, no ESA | Phase 3, NCT0282932078 | 24 wk | Increased 0.79 |
Decreased –55.67% |
— | Decreased –107.03 |
— | Increased 9.34 |
Decreased –10.07 |
— | — | ||
| Desidustat | HIF-PH inhibitor | CKD stage 1–4, NDD | Phase 2, CTRI/2017/05/00853479 | 6 wk | Increased 1.57 to 2.92 |
Decreased –59.24 to –91.36 |
Stable –0.2 to 5.63 |
— | — | Increased 30.3 to 70.6 |
— | — | — |
| Enarodustat | HIF-PH inhibitor | NDD CKD | Phase 2, JapicCTI-15288180 | 6 wk | Increased | Decreased | — | Decreased | — | Increased | Decreased | — | — |
| HD, stable ESA | Phase 2, JapicCTI-15289281 | 6 wk | Dose-dependent increase –0.62 to 0.89 |
Decreased | — | Decreased | — | Increased | Decreased | — | — | ||
| Molidustat | HIF-PH inhibitor | NDD, ESA-naïve | Phase 2, NCT02021370 (DIALOGUE 1)82 | 16 wk | Increased 1.4 to 2.0 |
Decreased –18 |
Decreased –11 |
Decreased –99 |
— | Increased 3 |
Decreased –7 |
— | — |
| NDD, stable ESA | Phase 2, NCT02021409 (DIALOGUE 2)82 | 16 wk | Increased 0.4 to 0.9 |
Decreased –8 |
Decreased –8 |
Decreased –15 |
— | Stable –0.1 |
Stable –0.8 |
— | — | ||
| DD, stable ESA | Phase 2, NCT01975818 (DIALOGUE 4)82 | 16 wk | –2.4 to –0.1 | 7 | 7 | 54 | — | 3 | 2 | — | — | ||
| Roxadustat | HIF-PH inhibitor | CKD stage 3–4, NDD | Phase 2, NCT0076165783 | 4 wk | Increased 0.4 to 1.8 |
Decreased –225 to –70 |
Decreased –11.0 |
Decreased –68.8 |
— | Increased 41.8 μg/dl |
Decreased –8.1 |
— | — |
| CKD stage 3–5, NDD | Phase 3, NCT0265281984 | 8 wk | Increased 1.9 |
Decreased –56.14 |
Stable –0.24 μmol/L |
Decreased –93.3 |
Increased 0.73 |
Increased 18.20 |
Decreased –5.2 |
— | — | ||
| NDD CKD | Phase 2, NCT0159950785 | 8 wk | Increased 1.82 to 2.59 |
Decreased –37.8 to –37.2 | –8.1 to 0.2 μg/ml | Decreased –124 to –98 |
Increased 67.1 to 95.7 mg/dl |
Increased 65.1 to 102.0 μg/dl |
Decreased –8.66 to –3.85 |
Increased 2.71 to 3.68 mg/L | –1.13 to –0.87 | ||
| CKD stage 3–4, NDD, no ESA | Phase 2, NCT0124476386 | 16 or 24 wk | Increased 0.57 to 1.71 |
Decreased –27.7 |
1.1 μg/dl | Decreased –85.9 |
— | Increased 40.4 μg/dl |
–2.7 | — | Stable 0.2 |
||
| ESRD, incident HD or PD, ESA-naïve | Phase 2, NCT0141407587 | 12 wk | Increased 3.1 |
Decreased –63.4 to –12.6 |
–2.1 to 1.4 μmol/L | –120 to –25 | 5.5 to 9.5 μmol/L | 9.7 to 17.4 | –7.4 to 2.6 | Increased 1.8 to 4.3 ng/ml |
Decreased –2.2 to –1.0 |
||
| HD, ESA-naïve | Phase 3, NCT0278014188 | 24 wk | Increased 2.26 |
Decreased –23.199 |
Stable 0.4 μmol/L |
Decreased –74.34 |
Increased 0.648 |
Increased 13.5 |
Decreased –5.02 |
Increased 10.40 nmol/L |
Stable –0.85 |
||
| ESRD, HD, stable ESA | Phase 2, NCT0114766689 | 6 wk | Increased 0.3 |
Decreased –39.2 |
Increased 7.1 μg/dl |
Decreased –185.5 |
— | Increased 51.0 μg/dl |
Decreased –2.5 |
Increased 0.69 mg/L | Increased 0.3 |
||
| ESRD, HD, stable ESA | Phase 2, NCT01596855 | 9 wk | Increased 0.11 to 1.42 |
Decreased –102.7 to –25.7 |
–3.3 to 8.9 μg/ml | –162 to 21 | Increased 39.8 to 58.8 mg/dl |
Increased 41.5 to 59.1 μg/dl |
Decreased –8.98 to –3.77 |
Increased 0.51 to 2.05 mg/L |
–0.90 to 0.84 | ||
| ESRD, HD or PD, stable ESA | Phase 3, NCT0265280690 | 27 wk | Increased 0.7 ± 1.1 g/dl |
Decreased –30.2 ± 113.3 |
Stable 0.1 ± 8.3 μmol/L |
Increased –119 ± 208 |
Increased 0.40 ± 0.48 |
Increased 10.0 ± 11.9 |
Decreased –5.7% ± 15.4% |
||||
| HD, stable ESA | Phase 3, NCT0277976488 | 52 wk | Maintained 0.12 |
Decreased –6.159 |
Stable 0.4 μmol/L |
Decreased –23.99 |
Increased 0.495 |
Increased 10.0 |
Stable –3.93 |
Stable 3.72 nmol/L |
Stable –0.89 |
||
| Vadadustat | HIF-PH inhibitor | CKD stage 3–5, NDD | Phase 2, NCT0190648991 | 20 wk | Increased | Decreased | — | Decreased | — | Increased | — | ||
| CKD stage 3–4, NDD, no ESA | Phase 2, NCT0138109492 | 6 wk | Increased 0.70 to 1.39 |
Decreased –90 to –139 |
— | Decreased | — | Increased | |||||
| HD, ESA | Phase 2, NCT0226019393 | 16 wk | Maintained –0.03 to –0.14 |
Decreased –4.9 to –21.7 |
Increased 10.3 to 14.0 |
Decreased –39.0 to –115.4 |
— | Increased 24.9 to 27.7 μg/dl |
Increased 1.7 to 2.5 |
— | Stable | ||
| PRS-080#22 | Hepcidin antagonist | CKD stage 5, HD, stable ESA use | Phase 1, NCT0275416794 | Single dose | Not affected | Decreased | Increased | — | — | — | Increased | — | — |
| LY2928057 (monoclonal antibody against ferroportin) | Blocking interaction with hepcidin | ESRD, HD | Phase 1, NCT0199148395 | 6 wk | — | Increased | Increased | Decreased | — | — | Increased | — | — |
| LY3113593 (monoclonal antibody against BMP6) | Repression of hepcidin expression | ESRD, HD | Phase 1, NCT0214428595 | Single dose | Increased | Decreased | Increased 1.36-fold | Decreased | — | — | Increased | — | — |
| Vitamin D2 | Repression of hepcidin expression | HD | Phase 4, NCT0139582396 | 6 mo | — | — | — | 47 | — | — | –0.4 | — | — |
| Vitamin D3 | Repression of hepcidin expression | CKD, stage 3–4, NDD, no ESA use | Phase 1, NCT0198811697 | 6 wk | Stable | Stable | — | Stable | — | — | Stable | — | — |
CHr, reticulate Hb concentration; CKD, chronic kidney disease; DD, dialysis dependent; ESA, erythropoiesis-stimulating agent; ESRD, end-stage renal disease; Hb, hemoglobin; HD, hemodialysis; HIF-PH, hypoxia-inducible factor–prolyl hydroxylase; NDD, non–dialysis dependent; PD, peritoneal dialysis; sTfR, soluble transferrin receptor; TIBC, total iron binding capacity; TSAT, transferrin saturation.
Actual values are provided where available; however, some data were published only as graphs.
Median percentage change from baseline.
Several HIF-PH inhibitors are currently in development for the treatment of anemia of CKD, and 3 have been approved for use. The HIF-PH inhibitor roxadustat has received regulatory approval in China for treatment of anemia of CKD in patients with NDD CKD or DD CKD107,108 and in Japan for patients with DD CKD.109 Vadadustat110 and daprodustat111 have recently been approved for use in patients with DD and NDD CKD in Japan. In a randomized, double-blind, phase 3 trial of patients with NDD CKD, greater reductions in hepcidin levels were seen in those treated with roxadustat versus placebo. Serum iron levels were stable and similar in both groups, whereas transferrin and TIBC increased and ferritin decreased in the roxadustat group.84 Similarly, in a randomized, open-label, phase 3 trial in patients with DD CKD, greater reductions in hepcidin levels were seen in those treated with roxadustat compared with epoetin alfa. Serum iron levels were stable and transferrin levels increased, whereas TSAT decreased, in the roxadustat group.90
Trial data for other HIF-PH inhibitors in development, including daprodustat, molidustat, vadadustat, enarodustat, and desidustat, are consistent with these findings. In patients with NDD CKD, treatment with these HIF-PH inhibitors led to increases in Hb levels, TIBC, and/or reticulocyte concentration as well as decreases in hepcidin, ferritin, serum iron, and/or TSAT.74,79,80,112,113 Similarly, in patients on dialysis who were switched from an ESA to daprodustat, vadadustat, or enarodustat, Hb levels remained stable or increased, whereas hepcidin, ferritin, and/or TSAT decreased and TIBC increased.77,81,114 In a randomized, open-label, phase 2b trial in patients who received molidustat and iron supplementation, levels of hepcidin, TSAT, and TIBC remained stable, whereas ferritin decreased and iron concentration increased. In patients who received molidustat but no iron supplementation, hepcidin and ferritin decreased, whereas iron concentration and TIBC increased and TSAT remained stable.112
Another experimental therapy that appears to stabilize HIF is Angelica sinensis polysaccharide. Anemia and inflammation improved in a rat CKD model via stabilization of HIF-α by preventing its degradation, thus stimulating EPO expression.115 In addition, treatment of rats with CKD with Angelica sinensis polysaccharide reduced the expression of hepcidin and ferroportin and increased serum iron levels and expression of ferritin.
Several hepcidin antagonists are in development to treat anemia of CKD. A polyethylene glycolated anticalin protein (PRS-080#22) binds to and antagonizes hepcidin. In a phase 1 clinical trial in patients with anemia and DD CKD, increased serum iron and TSAT and decreased free hepcidin levels were seen following a single treatment with PRS-080#22.94 In addition, PRS-080#22 was well tolerated in both CKD patients and in healthy volunteers. Data are awaited for a recently completed phase 2 trial in patients with DD CKD and anemia (ClinicalTrials.gov, NCT03325621). Lexaptepid pegol (NOX-H94, a Spiegelmer product) is a pegylated mirror-image L-oligoribonucleotide that binds hepcidin with high affinity, thus blocking the function of hepcidin. Treatment with NOX-H94 was well tolerated in healthy volunteers and showed dose-dependent increases in serum iron and TSAT as well as hepcidin inhibition.116 In healthy volunteers with systemic inflammation induced by injection of Escherichia coli lipopolysaccharide, lexaptepid treatment resulted in a prolonged increase in serum iron levels compared with placebo, indicating that it may be useful for patients with chronic inflammation, including those with anemia of CKD117; whether lexaptepid moves to clinical trials remains to be determined.
Several other strategies for lowering hepcidin levels are currently in the preclinical or early clinical stages of development. Several therapies involve monoclonal antibodies to selectively inactivate components involved in iron metabolism. For example, an antihepcidin monoclonal antibody therapy treated anemia of inflammation in a mouse model.118 In addition, monoclonal antibodies against BMP6 and ferroportin, which respectively blocked interactions with its receptor and with hepcidin, led to increases in serum iron and decreases in ferritin in healthy volunteers and in patients with end-stage renal disease.95 In addition, nonanticoagulant heparins can inhibit the expression of hepcidin by inhibiting the BMP/Caenorhabditis elegans SMA/Drosophila mothers against decapentaplegic pathway, although this awaits testing in clinical trials.119
Finally, transcriptional repression of HAMP, the gene encoding hepcidin, is also the target of therapeutics but with mixed results to date. Vitamin D directly represses transcription of HAMP, and vitamin D deficiency is prevalent in patients with CKD.120 Hepcidin levels decreased by one-third in a study of healthy volunteers who received a single oral dose of vitamin D2 (100,000 IU).121 However, in a randomized trial in which patients on hemodialysis received vitamin D2 (50,000 IU) weekly, no significant changes were seen in serum ferritin levels, TSAT, or epoetin dose at 3 or 6 months, although hepcidin levels were not reported in this study.96 Vitamin D3 (calcitriol) supplementation for 6 weeks in patients with stage 3 or 4 CKD resulted in no changes in hepcidin, serum ferritin, TSAT, or Hb over time compared with placebo.97
Summary
Iron is highly regulated in the body, and a large part of this regulation is due to the peptide hepcidin. Dysregulation of iron homeostasis and high levels of hepcidin are observed in several chronic conditions, including anemia of CKD. Many commonly used diagnostic tests for assessing iron status may not accurately measure total body iron availability; therefore, more accurate and standardized methods of identifying iron deficiency in patients with CKD and inflammation should be pursued. The current standard of care for patients with anemia of CKD does not necessarily address this dysregulation of iron and in many cases can worsen the problem. Emerging therapies targeting other pathways (such as HIF-PH inhibitors, which allow for increased activity of the HIF pathway) ameliorate anemia by increasing Hb levels and improving iron homeostasis by decreasing hepcidin levels, increasing iron absorption by upregulating duodenal cytochrome B and divalent metal transporter 1, and increasing iron transport by upregulating transferrin and transferrin receptor 1.122 Hepcidin antagonists in development directly target hepcidin or components of its expression pathway to increase available iron. Great potential exists for newer therapies to address these challenges of the current treatment paradigm for anemia of CKD.
Disclosure
AKA has received research support from Akebia and FibroGen and consulting fees from AstraZeneca, Otsuka, and Janssen.
Acknowledgements
This article is published as part of a supplement supported by AstraZeneca. Jennifer L. Giel, PhD, and Meri Pozo, PhD, CMPP, of inScience Communications (New York, NY, USA), provided medical writing support, which was funded by AstraZeneca.
Roxadustat is being developed for clinical use by an alliance of FibroGen, Astellas, and AstraZeneca.
Author Contributions
AKA meets the International Committee of Medical Journal Editors criteria for authorship for this article and takes responsibility for the integrity of the work as a whole. AKA and medical writers from inScience Communications wrote the first draft of the article. AKA reviewed and edited subsequent drafts, approved the submission of the article, and is fully accountable for all aspects of the work.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 2.World Health Organization Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. https://www.who.int/vmnis/indicators/haemoglobin/en/ Available at:
- 3.Babitt J.L., Lin H.Y. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moranne O., Froissart M., Rossert J. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20:164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stauffer M.E., Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis J., Caspard H., Little D. Prevalence and risk factors of CKD anemia in the United States [abstract SA-PO240] J Am Soc Nephrol. 2019;30:826. [Google Scholar]
- 8.Krause A., Neitz S., Magert H.J. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 9.Park C.H., Valore E.V., Waring A.J. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 10.Hunter H.N., Fulton D.B., Ganz T. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277:37597–37603. doi: 10.1074/jbc.M205305200. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas G., Chauvet C., Viatte L. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz T., Nemeth E. Iron balance and the role of hepcidin in chronic kidney disease. Semin Nephrol. 2016;36:87–93. doi: 10.1016/j.semnephrol.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babitt J.L., Huang F.W., Wrighting D.M. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 14.Corradini E., Meynard D., Wu Q. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54:273–284. doi: 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrighting D.M., Andrews N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinnix Z.K., Miller L.D., Wang W. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010;2:43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S., Chen Y., Guo W. Disordered hepcidin-ferroportin signaling promotes breast cancer growth. Cell Signal. 2014;26:2539–2550. doi: 10.1016/j.cellsig.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Zhao B., Li R., Cheng G. Role of hepcidin and iron metabolism in the onset of prostate cancer. Oncol Lett. 2018;15:9953–9958. doi: 10.3892/ol.2018.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvestri L., Pagani A., Nai A. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang A.S., Anderson S.A., Wang J. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood. 2011;117:1687–1699. doi: 10.1182/blood-2010-06-287292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvestri L., Pagani A., Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111:924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 22.Arezes J., Foy N., McHugh K. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132:1473–1477. doi: 10.1182/blood-2018-06-857995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mleczko-Sanecka K., Roche F., da Silva A.R. Unbiased RNAi screen for hepcidin regulators links hepcidin suppression to proliferative Ras/RAF and nutrient-dependent mTOR signaling. Blood. 2014;123:1574–1585. doi: 10.1182/blood-2013-07-515957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanatori I., Kishi F. DMT1 and iron transport. Free Radic Biol Med. 2019;133:55–63. doi: 10.1016/j.freeradbiomed.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth E., Tuttle M.S., Powelson J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 26.Camaschella C. Iron deficiency. Blood. 2019;133:30–39. doi: 10.1182/blood-2018-05-815944. [DOI] [PubMed] [Google Scholar]
- 27.Dignass A., Farrag K., Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi: 10.1155/2018/9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashby D.R., Gale D.P., Busbridge M. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–981. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 29.Klausen T., Dela F., Hippe E. Diurnal variations of serum erythropoietin in trained and untrained subjects. Eur J Appl Physiol Occup Physiol. 1993;67:545–548. doi: 10.1007/BF00241652. [DOI] [PubMed] [Google Scholar]
- 30.Kamei D., Tsuchiya K., Miura H. Inter-method variability of ferritin and transferrin saturation measurement methods in patients on hemodialysis. Ther Apher Dial. 2017;21:43–51. doi: 10.1111/1744-9987.12479. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer C.M., Looker A.C. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am J Clin Nutr. 2017;106:1606S–1614S. doi: 10.3945/ajcn.117.155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy A.T., Witcher D.R., Luan P. Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometry. Blood. 2007;110:1048–1054. doi: 10.1182/blood-2006-11-057471. [DOI] [PubMed] [Google Scholar]
- 33.Thomas D.W., Hinchliffe R.F., Briggs C. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161:639–648. doi: 10.1111/bjh.12311. [DOI] [PubMed] [Google Scholar]
- 34.National Clinical Guideline Center (UK) Royal College of Physicians (UK); London, England: 2015. Anaemia Management in Chronic Kidney Disease: Partial Update 2015. [PubMed] [Google Scholar]
- 35.Worwood M. Indicators of the iron status of populations: ferritin. In: World Health Organization; Centers for Disease Control and Prevention, editor. Assessing the Iron Status of Populations. 2nd ed. WHO Press; Geneva, Switzerland: 2007. [Google Scholar]
- 36.Wish J.B. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1(suppl 1):S4–S8. doi: 10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 37.Cobo G., Lindholm B., Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33:iii35–iii40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers K., Ashraf M.A., Sharma S. StatPearls Publishing LLC; Island, FL: 2020. Physiology, hepcidin. StatPearls. Treasure. [PubMed] [Google Scholar]
- 39.Devkota B.P. Iron-binding capacity. https://emedicine.medscape.com/article/2085726-overview Available at:
- 40.Beard J. Indicators of the iron status of populations: free erythrocyte protoporphyrin and zinc protoporphyrin; serum and plasma iron, total iron binding capacity and transferrin saturation; and serum transferrin receptor. In: World Health Organization; Centers for Disease Control and Prevention, editor. Assessing the Iron Status of Populations. 2nd ed. WHO Press; Geneva, Switzerland: 2007. [Google Scholar]
- 41.Northrup-Clewes C.A. The interpretation of indicators of iron status during an acute phase response. In: World Health Organization; Centers for Disease Control and Prevention, editor. Assessing the Iron Status of Populations. 2nd ed. WHO Press; Geneva, Switzerland: 2007. [Google Scholar]
- 42.Tessitore N., Solero G.P., Lippi G. The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrol Dial Transplant. 2001;16:1416–1423. doi: 10.1093/ndt/16.7.1416. [DOI] [PubMed] [Google Scholar]
- 43.Hayes W. Measurement of iron status in chronic kidney disease. Pediatr Nephrol. 2019;34:605–613. doi: 10.1007/s00467-018-3955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamei D., Nagano M., Hanafusa N. Comparison between a novel latex immunoassay and LC-MS/MS for hepcidin-25 measurement. J Am Soc Nephrol. 2019;30:828. [Google Scholar]
- 45.Zaritsky J., Young B., Wang H.J. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–1056. doi: 10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters H.P., Laarakkers C.M., Swinkels D.W. Serum hepcidin-25 levels in patients with chronic kidney disease are independent of glomerular filtration rate. Nephrol Dial Transplant. 2010;25:848–853. doi: 10.1093/ndt/gfp546. [DOI] [PubMed] [Google Scholar]
- 47.Uehata T., Tomosugi N., Shoji T. Serum hepcidin-25 levels and anemia in non-dialysis chronic kidney disease patients: a cross-sectional study. Nephrol Dial Transplant. 2012;27:1076–1083. doi: 10.1093/ndt/gfr431. [DOI] [PubMed] [Google Scholar]
- 48.Sonkar S.K., Singh N.K., Sonkar G.K. Association of hepcidin and anemia in early chronic kidney disease. Saudi J Kidney Dis Transpl. 2019;30:315–324. doi: 10.4103/1319-2442.256838. [DOI] [PubMed] [Google Scholar]
- 49.Hughes D.A., Stuart-Smith S.E., Bain B.J. How should stainable iron in bone marrow films be assessed? J Clin Pathol. 2004;57:1038–1040. doi: 10.1136/jcp.2003.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodnough L.T., Nemeth E., Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 51.Kalantar-Zadeh K., Hoffken B., Wunsch H. Diagnosis of iron deficiency anemia in renal failure patients during the post-erythropoietin era. Am J Kidney Dis. 1995;26:292–299. doi: 10.1016/0272-6386(95)90649-5. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari P., Kulkarni H., Dheda S. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:77–83. doi: 10.2215/CJN.04190510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercadal L., Metzger M., Haymann J.P. A 3-marker index improves the identification of iron disorders in CKD anaemia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costa E., Swinkels D.W., Laarakkers C.M. Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in haemodialysis patients. Acta Haematol. 2009;122:226–229. doi: 10.1159/000253590. [DOI] [PubMed] [Google Scholar]
- 55.El Sewefy D.A., Farweez B.A., Behairy M.A. Impact of serum hepcidin and inflammatory markers on resistance to erythropoiesis-stimulating therapy in haemodialysis patients. Int Urol Nephrol. 2019;51:325–334. doi: 10.1007/s11255-018-2062-z. [DOI] [PubMed] [Google Scholar]
- 56.Lee S.W., Kim Y.H., Chung W. Serum hepcidin and iron indices affect anemia status differently according to the kidney function of non-dialysis chronic kidney disease patients: Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) Kidney Blood Press Res. 2017;42:1183–1192. doi: 10.1159/000485865. [DOI] [PubMed] [Google Scholar]
- 57.Mikhail A., Brown C., Williams J.A. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017;18:345. doi: 10.1186/s12882-017-0688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macdougall I.C., Hutton R.D., Cavill I. Poor response to treatment of renal anaemia with erythropoietin corrected by iron given intravenously. BMJ. 1989;299:157–158. doi: 10.1136/bmj.299.6692.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pergola P.E., Fishbane S., Ganz T. Novel oral iron therapies for iron deficiency anemia in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:272–291. doi: 10.1053/j.ackd.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Macdougall I.C., Bock A.H., Carrera F. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29:2075–2084. doi: 10.1093/ndt/gfu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Wyck D.B., Roppolo M., Martinez C.O. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68:2846–2856. doi: 10.1111/j.1523-1755.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 62.Macdougall I.C., White C., Anker S.D. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380:447–458. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 63.Agarwal R., Kusek J.W., Pappas M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015;88:905–914. doi: 10.1038/ki.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C., Graham D.J., Kane R.C. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314:2062–2068. doi: 10.1001/jama.2015.15572. [DOI] [PubMed] [Google Scholar]
- 65.Litton E., Xiao J., Ho K.M. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macdougall I.C., Bircher A.J., Eckardt K.U. Iron management in chronic kidney disease: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2016;89:28–39. doi: 10.1016/j.kint.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Fishbane S., Shah H.H. Ferric pyrophosphate citrate as an iron replacement agent for patients receiving hemodialysis. Hemodial Int. 2017;21(suppl 1):S104–S109. doi: 10.1111/hdi.12554. [DOI] [PubMed] [Google Scholar]
- 68.Gaillard C.A., Bock A.H., Carrera F. Hepcidin response to iron therapy in patients with non-dialysis dependent CKD: an analysis of the FIND-CKD Trial. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jairam A., Das R., Aggarwal P.K. Iron status, inflammation and hepcidin in ESRD patients: the confounding role of intravenous iron therapy. Indian J Nephrol. 2010;20:125–131. doi: 10.4103/0971-4065.70840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailie G.R., Larkina M., Goodkin D.A. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015;87:162–168. doi: 10.1038/ki.2014.275. [DOI] [PubMed] [Google Scholar]
- 71.Kalantar-Zadeh K., Regidor D.L., McAllister C.J. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- 72.Holdstock L., Meadowcroft A.M., Maier R. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27:1234–1244. doi: 10.1681/ASN.2014111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brigandi R.A., Johnson B., Oei C. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: a 28-day, phase 2A randomized trial. Am J Kidney Dis. 2016;67:861–871. doi: 10.1053/j.ajkd.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 74.Holdstock L., Cizman B., Meadowcroft A.M. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J. 2019;12:129–138. doi: 10.1093/ckj/sfy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akizawa T., Tsubakihara Y., Nangaku M. Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol. 2017;45:127–135. doi: 10.1159/000454818. [DOI] [PubMed] [Google Scholar]
- 76.Cizman B., Sykes A.P., Paul G. An exploratory study of daprodustat in erythropoietin-hyporesponsive subjects. Kidney Int Rep. 2018;3:841–850. doi: 10.1016/j.ekir.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meadowcroft A.M., Cizman B., Holdstock L. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J. 2019;12:139–148. doi: 10.1093/ckj/sfy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsubakihara Y., Akizawa T., Nangaku M. A 24-week anemia correction study of daprodustat in Japanese dialysis patients. Ther Apher Dial. 2020;24:108–114. doi: 10.1111/1744-9987.12962. [DOI] [PubMed] [Google Scholar]
- 79.Parmar D.V., Kansagra K.A., Patel J.C. Outcomes of desidustat treatment in people with anemia and chronic kidney disease: a phase 2 study. Am J Nephrol. 2019;49:470–478. doi: 10.1159/000500232. [DOI] [PubMed] [Google Scholar]
- 80.Akizawa T., Nangaku M., Yamaguchi T. A placebo-controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long-term trial. Am J Nephrol. 2019;49:165–174. doi: 10.1159/000496929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akizawa T., Nangaku M., Yamaguchi T. Enarodustat, conversion and maintenance therapy for anemia in hemodialysis patients: a randomized, placebo-controlled phase 2b trial followed by long-term trial. Nephron. 2019;143:77–85. doi: 10.1159/000500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Macdougall I.C., Akizawa T., Berns J.S. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14:28–39. doi: 10.2215/CJN.02510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Besarab A., Provenzano R., Hertel J. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30:1665–1673. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen N., Hao C., Peng X. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 85.Chen N., Qian J., Chen J. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373–1386. doi: 10.1093/ndt/gfx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Provenzano R., Besarab A., Sun C.H. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol. 2016;11:982–991. doi: 10.2215/CJN.06890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Besarab A., Chernyavskaya E., Motylev I. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akizawa T., Ueno M., Shiga T. Oral roxadustat three times weekly in ESA-naive and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: results from two phase 3 studies. Ther Apher Dial. 2020;24:628–641. doi: 10.1111/1744-9987.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Provenzano R., Besarab A., Wright S. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 90.Chen N., Hao C., Liu B.C. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 91.Pergola P.E., Spinowitz B.S., Hartman C.S. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90:1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 92.Martin E.R., Smith M.T., Maroni B.J. Clinical trial of vadadustat in patients with anemia secondary to stage 3 or 4 chronic kidney disease. Am J Nephrol. 2017;45:380–388. doi: 10.1159/000464476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haase V.H., Chertow G.M., Block G.A. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. 2019;34:90–99. doi: 10.1093/ndt/gfy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Renders L., Budde K., Rosenberger C. First-in-human phase I studies of PRS-080#22, a hepcidin antagonist, in healthy volunteers and patients with chronic kidney disease undergoing hemodialysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sheetz M., Barrington P., Callies S. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br J Clin Pharmacol. 2019;85:935–948. doi: 10.1111/bcp.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miskulin D.C., Majchrzak K., Tighiouart H. Ergocalciferol supplementation in hemodialysis patients with vitamin D deficiency: a randomized clinical trial. J Am Soc Nephrol. 2016;27:1801–1810. doi: 10.1681/ASN.2015040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panwar B., McCann D., Olbina G. Effect of calcitriol on serum hepcidin in individuals with chronic kidney disease: a randomized controlled trial. BMC Nephrol. 2018;19:35. doi: 10.1186/s12882-018-0823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang L.E., Gu J., Schau M. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haase V.H. Hypoxia-inducible factor–prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl. 2021;11:8–25. doi: 10.1016/j.kisu.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scortegagna M., Ding K., Zhang Q. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–3140. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 101.Taylor M., Qu A., Anderson E.R. Hypoxia-inducible factor-2alpha mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140:2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peyssonnaux C., Zinkernagel A.S., Schuepbach R.A. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paliege A., Rosenberger C., Bondke A. Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int. 2010;77:312–318. doi: 10.1038/ki.2009.460. [DOI] [PubMed] [Google Scholar]
- 104.Bernhardt W.M., Wiesener M.S., Scigalla P. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Q., Davidoff O., Niss K. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012;122:4635–4644. doi: 10.1172/JCI63924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haase V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dhillon S. Roxadustat: first global approval. Drugs. 2019;79:563–572. doi: 10.1007/s40265-019-01077-1. [DOI] [PubMed] [Google Scholar]
- 108.AstraZeneca. Roxadustat approved in China for the treatment of anaemia in non-dialysis-dependent patients with chronic kidney disease. https://www.astrazeneca.com/media-centre/press-releases/2019/roxadustat-approved-in-china-for-the-treatment-of-anaemia-in-non-dialysis-dependent-patients-with-chronic-kidney-disease-22082019.html Available at:
- 109.Astellas Pharma Inc Evrenzo® (roxadustat) tablets approved in Japan for the treatment of anemia associated with chronic kidney disease in dialysis patients. https://www.astellas.com/en/news/15096 Available at:
- 110.Akebia Akebia Therapeutics announces approval of vadadustat in Japan for the treatment of anemia due to chronic kidney disease in dialysis-dependent and non-dialysis dependent adult patients. https://ir.akebia.com/news-releases/news-release-details/akebia-therapeutics-announces-approval-vadadustat-japan Available at:
- 111.GlaxoSmithKline GSK receives first regulatory approval for Duvroq (daprodustat) in Japan for patients with anaemia due to chronic kidney disease. https://www.gsk.com/en-gb/media/press-releases/gsk-receives-first-regulatory-approval-for-duvroq-daprodustat-in-japan-for-patients-with-anaemia-due-to-chronic-kidney-disease/ Available at:
- 112.Akizawa T., Macdougall I.C., Berns J.S. Iron regulation by molidustat, a daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor, in patients with chronic kidney disease. Nephron. 2019;143:243–254. doi: 10.1159/000502012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nangaku M., Kondo K., Kokado Y. Randomized, open-label, active-controlled (darbepoetin alfa), phase 3 study of vadadustat for treating anemia in non-dialysis-dependent CKD patients in Japan. J Am Soc Nephrol. 2019;30:823. [Google Scholar]
- 114.Nangaku M., Kondo K., Ueta K. Anemia and iron metabolism: clinical research randomized, double-blinded, active-controlled (darbepoetin alfa), phase 3 study of vadadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2019;30:6. [Google Scholar]
- 115.Wang K., Wu J., Xu J. Correction of anemia in chronic kidney disease with Angelica sinensis polysaccharide via restoring EPO production and improving iron availability. Front Pharmacol. 2018;9:803. doi: 10.3389/fphar.2018.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boyce M., Warrington S., Cortezi B. Safety, pharmacokinetics and pharmacodynamics of the anti-hepcidin Spiegelmer lexaptepid pegol in healthy subjects. Br J Pharmacol. 2016;173:1580–1588. doi: 10.1111/bph.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Eijk L.T., John A.S., Schwoebel F. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood. 2014;124:2643–2646. doi: 10.1182/blood-2014-03-559484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sasu B.J., Cooke K.S., Arvedson T.L. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 119.Poli M., Asperti M., Ruzzenenti P. Non-anticoagulant heparins are hepcidin antagonists for the treatment of Anemia. Molecules. 2017;22:598. doi: 10.3390/molecules22040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim S.M., Choi H.J., Lee J.P. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J Ren Nutr. 2014;24:20–25. doi: 10.1053/j.jrn.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Bacchetta J., Zaritsky J.J., Sea J.L. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. 2014;25:564–572. doi: 10.1681/ASN.2013040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaplan J.M., Sharma N., Dikdan S. Hypoxia-inducible factor and its role in the management of anemia in chronic kidney disease. Int J Mol Sci. 2018;19:E389. doi: 10.3390/ijms19020389. [DOI] [PMC free article] [PubMed] [Google Scholar]