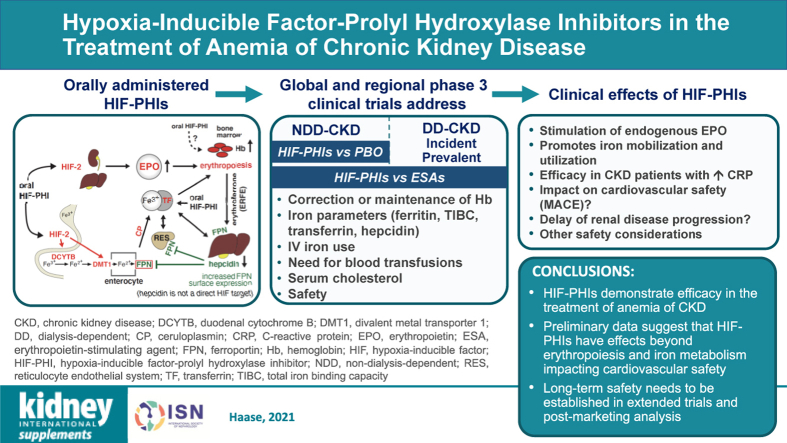
Graphical Abstract
Keywords: anemia, chronic kidney disease, erythropoietin, hepcidin, hypoxia-inducible factor, iron, prolyl hydroxylase domain dioxygenase
Abstract
Hypoxia-inducible factor–prolyl hydroxylase domain inhibitors (HIF-PHIs) are a promising new class of orally administered drugs currently in late-stage global clinical development for the treatment of anemia of chronic kidney disease (CKD). HIF-PHIs activate the HIF oxygen-sensing pathway and are efficacious in correcting and maintaining hemoglobin levels in patients with non–dialysis- and dialysis-dependent CKD. In addition to promoting erythropoiesis through the increase in endogenous erythropoietin production, HIF-PHIs reduce hepcidin levels and modulate iron metabolism, providing increases in total iron binding capacity and transferrin levels, and potentially reducing the need for i.v. iron supplementation. Furthermore, HIF-activating drugs are predicted to have effects that extend beyond erythropoiesis. This review summarizes clinical data from current HIF-PHI trials in patients with anemia of CKD, discusses mechanisms of action and pharmacologic properties of HIF-PHIs, and deliberates over safety concerns and potential impact on anemia management in patients with CKD.
Erythropoiesis-stimulating agents (ESAs) are recombinant versions of human erythropoietin (EPO) and the current mainstay of treatment for anemia of chronic kidney disease (CKD), typically in conjunction with iron supplementation.1,2 Although ESAs have decreased blood transfusion needs, reduced cardiovascular morbidity and mortality, and improved symptoms associated with severe anemia of CKD with hemoglobin (Hb) target levels of 10 to 11 g/dl,3, 4, 5, 6 higher Hb target levels (i.e., ≥13 g/dl) increase the risk for cardiovascular and cerebrovascular events, vascular access thrombosis, progression to end-stage renal disease, and overall mortality.3,7, 8, 9
The hypoxia-inducible factor (HIF)–prolyl hydroxylase domain (PHD) pathway regulates cellular responses to hypoxia and is involved in multiple diseases, including anemia, polycythemia, ischemic diseases, pulmonary arterial hypertension, and cancer.10 HIF-PHD inhibitors (HIF-PHIs) are a new class of drugs that activate HIF transcription factors and have broad therapeutic potential in clinical medicine.11 As anemia therapy, HIF-PHIs promote erythropoiesis primarily through increased endogenous EPO production and modulation of iron metabolism.
HIF-PHIs reversibly inhibit HIF-PHD dioxygenases, which belong to a larger family of enzymes that utilize molecular oxygen and 2-oxoglutarate for hydroxylation.12 HIF-PHIs are currently in advanced global clinical development for anemia management in patients with CKD. Four compounds are approved for marketing in Japan: daprodustat (Duvroq, Kyowa Kirin Co., Ltd.),13, 14, 15 roxadustat (Evrenzo, Astellas Pharma; for dialysis-dependent CKD),16, 17, 18 vadadustat (Vafseo, Akebia Therapeutics),19,20 and enarodustat (Enaroy, Japan Tobacco Inc.).21 Roxadustat is also approved for marketing in China.22,23 Licensing of HIF-PHIs is expected soon in North America and Europe.
This review discusses the mechanisms of action and pharmacologic properties of HIF-PHIs, summarizes clinical data from HIF-PHI trials in patients with anemia of CKD, and considers their clinical efficacy, safety, and potential advantages compared with the current standard of care.
Discovery of HIF and Mechanism of Action
A classic response to hypoxia is the increase in red blood cell mass. This response is mediated by the glycoprotein hormone EPO, which was purified from the urine of patients with aplastic anemia in 197724; the EPO gene was cloned in 1985.25,26 The molecular mechanisms that regulate the hypoxic induction of EPO, however, were not understood until the discovery that human hepatoma cells were capable of producing EPO under hypoxic conditions.27 This discovery facilitated the identification and characterization of a regulatory DNA sequence located within the 3′ enhancer region of the EPO gene,28 which was shown to bind a nuclear factor under hypoxia, named HIF-1.29 HIF-1 was subsequently purified from large-scale HeLa and Hep3B cell cultures, sequenced, and found to consist of 2 protein subunits, HIF-1α and HIF-1β (described below).30,31 Further studies demonstrated that in addition to the hypoxic induction of EPO in the kidney and liver, HIF-1 played a more general role, regulating hypoxia-sensitive genes, including those encoding glycolytic enzymes (e.g., phosphoglycerate kinase 1 and lactate dehydrogenase A) and angiogenic factors, such as vascular endothelial growth factor (VEGF).32, 33, 34, 35 Subsequently, HIF transcriptional activity was found to be controlled by hydroxylation of specific proline residues within the oxygen-regulated HIF-1α subunit, leading to its ubiquitylation and proteasomal degradation.36, 37, 38 HIF-α hydroxylation is mediated by a family of prolyl-4-hydroxylases, PHD enzymes, which function as the primary oxygen sensors of the HIF pathway.39, 40, 41, 42 In 2019, the Nobel Prize in Physiology or Medicine was awarded to Professor William Kaelin, Jr., Sir Peter Ratcliffe, and Gregg Semenza for their contributions to delineating the molecular mechanisms underlying HIF oxygen sensing.43
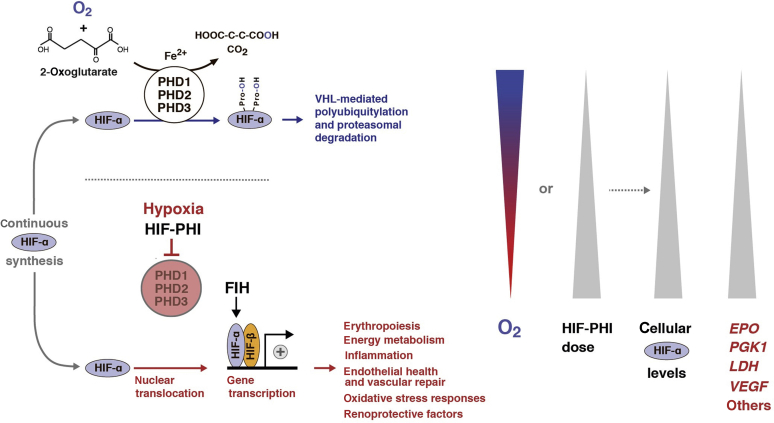
The HIF/PHD oxygen-sensing pathway plays a central role in cellular adaptation to hypoxia, regulating biologic processes essential for cell survival. These include glycolysis, mitochondrial metabolism, angiogenesis, immune responses, and erythropoiesis (Figure 1).10 HIF transcription factors, of which HIF-1 and HIF-2 are extensively studied, belong to a larger family of proteins that regulate responses to environmental stresses and are composed of 2 subunits: an oxygen-sensitive α-subunit (HIF-1α, HIF-2α, or HIF-3α) and a constitutively expressed β-subunit.44 HIF-1α and HIF-2α heterodimerize with HIF-β to form HIF-1 and HIF-2 transcription factors, respectively.
Figure 1.
Schematic diagram of the hypoxia-inducible factor (HIF) pathway. HIF-α is constitutively produced and rapidly degraded under normoxic conditions. Degradation of HIF-α is mediated by prolyl hydroxylase domain (PHD) 1, PHD2, and PHD3 enzymes, which hydroxylate specific proline residues within HIF-α. Hydroxylated HIF-α is ubiquitylated by the von Hippel–Lindau (VHL)–E3 ubiquitin ligase complex, leading to its proteasomal degradation. PHDs utilize O2 and 2-oxoglutarate as substrates in an iron-dependent reaction, resulting in the formation of hydroxylated HIF-α, succinate, and CO2. Hypoxia or HIF–PHD inhibitors (PHIs) reduce PHD catalytic activity, which leads to cellular accumulation of HIF-α, its nuclear translocation, heterodimerization with HIF-β, and increased transcription of HIF-regulated genes, which are involved in multiple biological processes. Factor-inhibiting HIF (FIH) modulates HIF transcriptional activity via hydroxylation of a C-terminal asparagine residue within HIF-α. EPO, erythropoietin; LDH, lactate dehydrogenase; PGK1, phosphoglycerate kinase 1; VEGF, vascular endothelial growth factor.
During normoxia, continuously synthesized HIF-α subunits are hydroxylated by PHD enzymes, of which there are 3 isoforms (PHD1, PHD2, and PHD3).39, 40, 41, 42 PHD enzymes are dioxygenases that utilize molecular oxygen and 2-oxoglutarate as substrates for HIF-α hydroxylation in an iron-dependent manner. This reaction initiates degradation of HIF-α via von Hippel–Lindau–mediated ubiquitylation and prevents the formation of HIF transcription factors.45 In contrast, hypoxia and pharmacologic HIF-PHD inhibition impairs HIF-α degradation, increasing intracellular HIF-α levels and resulting in the formation of transcriptionally active HIF heterodimers.45 An independent second hypoxic switch operates at the C-terminal end of HIF-α, with the hydroxylation of an asparagine residue by factor-inhibiting HIF. This asparagine modification modulates the recruitment of coactivators to the HIF transcriptional complex.46,47
HIF in Erythropoiesis
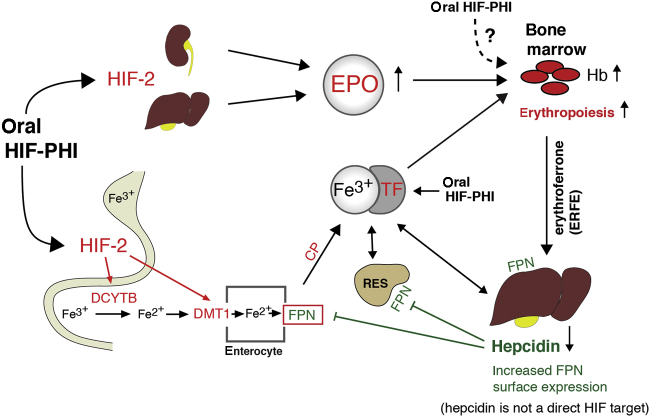
Although HIF-1 was first identified as the transcription factor that regulates EPO in human hepatoma cells, in vivo hypoxic stimulation of EPO and erythropoiesis is primarily mediated by HIF-2.48, 49, 50 Its α-subunit was initially described as vascular HIF-1α–like factor (referred to as endothelial PAS domain protein-1),44 but it was soon realized that HIF-2α was not restricted to endothelial cells. HIF-2 induces hepatic and renal EPO expression and promotes the transcription of several iron metabolism and transport genes, including duodenal cytochrome b (DCYTB), divalent metal transporter 1 (DMT1), and ferroportin (FPN1) (Figure 2).50,51 HIF-1 and HIF-2 have been shown to regulate transferrin (TF), TF receptor 1, and ceruloplasmin (CP).48,49,52 Therefore, pharmacologic HIF activation is predicted to enhance enteral iron uptake and transport through increased expression of iron metabolism and transport genes. Indeed, increased DCYTB and DMT1 expression was observed after administration of an HIF-activating compound in normal and inflamed rodents.53
Figure 2.
Overview of hypoxia-inducible factor (HIF) regulation of erythropoiesis. Reprinted from Advances in Chronic Kidney Disease, volume 26, issue 4, Sanghani NS, Haase VH, Hypoxia-inducible factor activators in renal anemia: current clinical experience, pages 253–266, Copyright © 2019, with permission from the National Kidney Foundation, Inc.51 In response to hypoxia or HIF– prolyl-hydroxylase inhibitors (PHIs), HIF-2 stimulates erythropoietin (EPO) production in the kidneys and liver. This promotes erythropoiesis and leads to increased iron demand in the bone marrow. HIF coordinates erythropoisis with iron metabolism, as it regulates genes involved in iron uptake, release from internal stores, and transport (highlighted in red). Absorbed and stored iron is released into the circulation via ferroportin (FPN) and complexed with transferrin (TF) for transport to liver, bone marrow, reticulocyte endothelial system (RES), and other organs. FPN surface expression is regulated by hepcidin, whereas HIF-2 participates in the transcriptional regulation of FPN. Erythroferrone (ERFE) mediates suppression of hepcidin production in the liver under conditions of accelerated erythropoiesis. Ceruloplasmin (CP) is an HIF-regulated copper-carrying ferroxidase that catalyzes the oxidation of ferrous (Fe2+) to ferric (Fe3+) iron. DCYTB, duodenal cytochrome B (cytochrome b reductase 1); DMT1, divalent metal transporter 1; Hb, hemoglobin.
A key regulator of iron metabolism is hepcidin, a small hepatic peptide that facilitates degradation of the cell surface iron exporter ferroportin and controls iron availability for erythropoiesis.54 Hepcidin plays a central role in the pathogenesis of anemia of inflammation and is elevated in patients with CKD due to reduced renal clearance and the presence of uremic inflammation.55, 56, 57 Elevated serum hepcidin levels in CKD are associated with atherosclerosis, cardiovascular disease, and increased mortality.57
Increased serum hepcidin impairs the release of iron from internal stores and reduces intestinal iron uptake, whereas low serum hepcidin has the inverse effect.58 Hepcidin synthesis is suppressed when iron stores are low or when iron demands increase as a result of accelerated erythropoiesis (e.g., under hypoxic or anemic conditions or following ESA administration).59, 60, 61, 62 Conversely, hepcidin production increases in response to iron loading or increased systemic iron. Serum hepcidin levels also increase with inflammation, mediated by inflammatory cytokine signaling in hepatocytes.59 Erythropoietic drive during ineffective erythropoiesis generates an overriding signal that results in the suppression of hepcidin transcription, even with excessive systemic iron.63
Erythroferrone, which is produced by bone marrow erythroid progenitor cells, is the main mediator of hepcidin suppression during increased erythropoietic activity.63 This appears to involve blockade of bone morphogenetic protein signaling pathways that normally increase hepcidin transcription.64 Similar to ESA administration, stimulation of erythropoiesis with HIF-PHIs lowers serum hepcidin levels.51 Cell culture– and animal-based studies have established that both liver-specific and systemic HIF activation suppress hepcidin transcription in an EPO-dependent manner that requires erythropoiesis.65, 66, 67 However, HIF-PHI effects that are independent of erythropoiesis cannot be completely excluded.
Pharmacology of HIF-PHIs
HIF-PHIs in current clinical development are potent reversible inhibitors of all 3 PHD isoforms, with in vitro half-maximal inhibitory concentrations in the submicromolar to low micromolar range (Table 168, 69, 70, 71, 72, 73, 74, 75, 76).68,70,71 HIF-PHIs chelate at the catalytic-site iron, stabilizing both HIF-1α and HIF-2α and resulting in dose-dependent increases in HIF-regulated gene expression.68 However, differences between daprodustat, molidustat, roxadustat, and vadadustat were found in the kinetics of HIF-α stabilization and relative expression levels of HIF-regulated genes in cells exposed to equimolar amounts of compound.68 Significant activity against factor-inhibiting HIF and other 2-oxoglutarate–dependent dioxygenases was not detected.68 Because of differences in pharmacokinetics, the effective dosing schedules for HIF-PHIs vary, with roxadustat being administered 3 times weekly, compared with once-daily administration for daprodustat, enarodustat, molidustat, and vadadustat.51,77,78 Based on phase 1 and 2 data, higher once-daily doses of daprodustat and molidustat may be required in patients on dialysis to achieve target Hb levels.79,80
Table 1.
Summary of the pharmacologic properties of hypoxia-inducible factor–prolyl hydroxylase inhibitors
| Variable | Daprodustat68,69 (GSK1278863) | Desidustat70 (ZYAN1) | Enarodustat71,72 (JTZ-951) | Molidustat68,73,74 (BAY 85-3934) | Roxadustat68,75 (FG-4592; ASP1517; AZD9941) | Vadadustat68,76 (AKB-6548; MT-6548) | |
|---|---|---|---|---|---|---|---|
| Pharmacodynamics | |||||||
| IC50, μmol/L | |||||||
| MALDI-TOF binding assay | PHD1: 1.50 PHD2: 2.87 PHD3: 0.61 |
PHD1: 1.45 PHD2: 1.85 PHD3: 0.72 |
PHD1: 1.40 PHD2: 1.26 PHD3: 1.32 |
PHD1: 0.84 PHD2: 2.30 PHD3: 0.26 |
|||
| In vitro assay in HepG2 cells | 11.2a | ||||||
| Fluorescent enzyme assay | PHD1: 0.016 PHD2: 0.061 PHD3: 0.101 |
||||||
| Pharmacokinetics | |||||||
| Participants | Japan, n = 13b | Cauc, n = 12b | Cauc, n = 56b | Mixed,c n = 6d | Japan, n = 9b | Japan, n = 15b | Cauc, n = 8b |
| Dose, mg | 100 | 100 | 10–300 | 10 | 50 | 100 | 450 |
| AUC, μg·h/mle | 5.20f | 3.55f | 3.7–116.2 | 7.33f | 1.11 | 88.7f | 397f |
| Cmax, μg/mle | 2.32 | 1.60 | 0.6–17.9 | 0.986 | 0.56 | 10.6 | 52.6 |
| Tmax, hg | 1.50 | 1.50 | 1.25–3.00 | 0.5 | 0.75 | 2.0 | 2.0 |
| t½, he | 2.25 | 1.86 | 7.0–11.4 | 25.9h; 8.96i | 10.4 | 13.1 | 5.8j |
| CL/F, L/he | 21.70 | 31.4 | 2.1–2.9 | 1.52 | 45.1 | 1.18 | NR |
| CLR, L/he | NR | NR | NR | NR | 0.693 | 0.0261 | NR |
| Metabolizing enzymes | CYP2C8, major CYP3A4, minor |
NR | CYP2C8, CYP2C9, CYP3A4 | UGTs | CYP2C8, major | UGT1A9, major | |
AUC, area under the concentration-time curve; Cauc, Caucasian; CL/F, apparent oral clearance; CLR, renal clearance; Cmax, peak plasma concentration; CYP, cytochrome P450; IC50, 50% PHD inhibitory concentration; Japan, Japanese; MALDI-TOF, matrix-assisted laser desorption ionization–time of flight; NR, not reported; PHD, prolyl hydroxylase domain; t½, elimination half-life; Tmax, time to Cmax; UGT, uridine 5'-diphospho-glucuronosyltransferase.
PHD enzyme isoform not specified.
Single-dose oral administration in fasted, healthy volunteers.
Participants were either Caucasian (n = 1), Black (n = 4), or American Indian/Alaskan Native (n = 1).
Single-dose oral administration in patients with end-stage renal disease on maintenance hemodialysis.
Mean.
Reported as AUC from time 0 to infinity.
Median.
Terminal t½.
Effective t½.
Value of 7.8 in patients with hepatic impairment.
Pharmacokinetic profiles
The pharmacokinetic parameters for single-dose HIF-PHIs, which are rapidly absorbed after oral administration, are summarized in Table 1.69, 70, 71, 72, 73,75,76 Roxadustat and daprodustat are primarily oxidized by cytochrome P450 (CYP) 2C8, with a minor contribution of CYP3A4 to daprodustat metabolism.81,82 In addition to CYP2C8-mediated oxidation, roxadustat undergoes phase 2 hydrophilic modification by glucuronidation and glucosidation.75,83 Enarodustat is also metabolized by CYP enzymes,72 whereas molidustat and vadadustat are primarily metabolized by uridine 5′-diphospho-glucuronosyltransferases.74,76
Changes in renal function are unlikely to affect elimination rates of nonmodified daprodustat and roxadustat as their renal clearance is low (Table 1).73,75 Where reported, coadministration with meals did not substantially affect the pharmacokinetics of roxadustat and daprodustat,75,81 nor did the coadministration of the phosphate binder lanthanum carbonate75 or the proton-pump inhibitor omeprazole84 affect roxadustat pharmacokinetics.
Potential drug-drug interactions
Because of the high prevalence of comorbidities and concomitant medication use in CKD patients, HIF-PHIs should be carefully screened for potential drug-drug interactions. Given that HIF-PHIs are metabolized by CYP enzymes, studies have assessed potential interactions between roxadustat or daprodustat and other CYP substrates or inhibitors, such as warfarin and gemfibrozil. These studies suggest that warfarin does not require dose adjustment when coadministered with roxadustat,84 whereas gemfibrozil, a strong irreversible CYP2C8 inhibitor, significantly increased daprodustat plasma levels and reduced its rate of elimination.79 Data with gemfibrozil and roxadustat have not been published. Daprodustat exposure was also mildly increased (∼1.5 fold) by trimethoprim (a weak CYP2C8 inhibitor).85 The pharmacokinetics of pioglitazone, a CYP2C8 substrate, and rosuvastatin, a substrate of organic anion transporter P1B1 (which is inhibited by daprodustat), were not affected by daprodustat coadministration.85
HIF-PHIs in Clinical Trials
HIF-PHIs stimulate erythropoiesis in a dose-dependent manner and have consistently shown clinical efficacy in patients with anemia of non–dialysis-dependent (NDD) and dialysis-dependent (DD) CKD in phase 2 and 3 studies. Data from completed phase 3 trials are discussed herein and summarized in Tables 286, 87, 88, 89, 90, 91, 92, 93, 94 and 3.95, 96, 97, 98, 99 Currently ongoing phase 3 studies evaluating efficacy and major adverse cardiovascular event (MACE) end points are summarized in Table 4.
Table 2.
Summary of clinical efficacy data from phase 3 clinical trials of hypoxia-inducible factor–prolyl hydroxylase inhibitors in patients with anemia and non–dialysis-dependent chronic kidney disease
| Study | Study design; no. of patients | Treatment, duration | Hb response ratea | Mean ΔHb from baseline |
|---|---|---|---|---|
| Daprodustat | ||||
| Kimura et al., 201915 (NCT02791763) | R, OL, AC; ESA-naïve and ESA-treated; n = 299 | DAPRO QDb vs. CERA, 52 wk | Hb at target (11–13 g/dl) during weeks 40–52: DAPRO: 92% CERA: 92% |
Difference in mean Hb (weeks 40–52): 0.10 (95% CI, –0.07 to 0.28) g/dl |
| Enarodustat | ||||
| Akizawa et al., 201978 | R, OL, AC; ESA status NR; n = 216 | ENARO QDc vs. DPO, 24 wk | Hb at target (10–12 g/dl) during weeks 1–24: ENARO: 89.6% DPO: 90.6% |
Difference in mean Hb (weeks 20–24): 0.09 (95% CI, –0.07 to 0.26) g/dl |
| Molidustat | ||||
| MIYABI ND-C86 (NCT03350321) | R, OL, AC; ESA-naïve; n = 161 | MOLI 25 mg QDd vs. DPO, 52 wk | NR | LSM difference in mean Hb (weeks 30–36): –0.38 (95% CI, –0.67 to –0.08) g/dl |
| MIYABI ND-M87 (NCT03350347) | R, OL, AC; ESA-treated; n = 164 | MOLI QDe vs. DPO, 52 wk | Hb at target (11–13 g/dl) during weeks 30–36: MOLI: 80.5% DPO: NR |
LSM difference in mean Hb (weeks 30–36): 0.13 (95% CI, –0.15 to 0.40) g/dl |
| Roxadustat | ||||
| Chen et al., 201923 (NCT02652819) | R, DB, PC; ESA-naïve; n = 152 | ROXA 70 or 100 mg TIWf vs. PBO, 8 wk DB, then 18 wk OL | At week 9: ROXA: 84% PBO: 0% |
During weeks 7–9: ROXA: 1.9 g/dl PBO: –0.4 g/dl (P < 0.001) |
| Akizawa et al., 202088 (NCT02964936) | R, OL, NC; ESA-naïve; n = 99 | ROXA 50 or 70 mg TIWc, 24 wk | From baseline to EOT: Hb at target ≥10 g/dl: ROXA (50 mg): 97.0% ROXA (70 mg): 100.0% Hb at target ≥10.5 g/dl: ROXA (50 mg): 94.9% ROXA (70 mg): 98.0% |
During weeks 18–24: ROXA (50 mg): 1.34 g/dl ROXA (70 mg): 1.30 g/dl |
| Akizawa et al., 202089 | R, OL, AC; ESA-treated; n = 262 | ROXA vs. DPO, 52 wk | Mean Hb during weeks 18–24: ROXA: 11.14 g/dl |
Difference in mean Hb (weeks 18–24): –0.07 g/dl |
| ALPS90 (NCT01887600) | R, DB, PC; ESA-naïve; n = 594 | ROXA vs. PBO, 52–104 wk | NR | During weeks 28–52: ROXA: 1.99 g/dl PBO: 0.41 g/dl (P < 0.001) |
| ANDES91 (NCT01750190) | R, DB, PC; ESA-naïve; n = 922 | ROXA TIWc vs. PBO, 52 wk | During weeks 1–24: ROXA: 86.0% PBO: 6.6% (P = 0.0007) |
During weeks 28–52: ROXA: 2.00 g/dl PBO: 0.16 g/dl (P < 0.0001) |
| OLYMPUS92 (NCT02174627) | R, DB, PC; ESA-naïve; n = 2781 | ROXA 70 mg TIWg vs. PBO, 52 wk | During weeks 1–24: ROXA: 77.0% PBO: 8.5% (P < 0.001) |
During weeks 28–52: ROXA: 1.75 g/dl PBO: 0.40 g/dl (P < 0.001) |
| DOLOMITES93 (NCT02021318) | R, OL, AC; ESA-naïve; n = 616 | ROXA TIW vs. DPO, 104 wk | During weeks 1–24h: ROXA: 89.5% DPO: 78.0% |
NR |
| Vadadustat | ||||
| Nangaku et al., 201919 (NCT03329196) | R, OL, AC; ESA-naïve and ESA-treated; n = 304 | VADA 300 mg QD, then 150–600 mg QDd vs. DPO, 52 wk | NR | LSM of average Hb (weeks 20 and 24): VADA: 11.66 g/dl DPO: 11.93 g/dl |
| PRO2TECT94 (NCT02648347) | R, OL, AC; ESA-naïve; n = 1751 | VADA QD vs. DPO, 52 wk | NR | Difference (VADA vs. DPO): weeks 24–36: 0.05 g/dl weeks 40–52: 0.04 g/dl |
| PRO2TECT94 (NCT02680574) | R, OL, AC; ESA-treated; n = 1725 | VADA QD vs. DPO, 52 wk | NR | Difference (VADA vs. DPO): weeks 24–36: –0.01 g/dl weeks 40–52: 0.00 g/dl |
AC, active controlled; CERA, continuous erythropoietin receptor activator (epoetin beta pegol); CI, confidence interval; DAPRO, daprodustat; DB, double blind; DPO, darbepoetin alfa; ENARO, enarodustat; EOT, end of treatment; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; LSM, least-squares mean; MOLI, molidustat; NC, noncomparative; NR, not reported; OL, open label; PBO, placebo; PC, PBO controlled; QD, once daily; R, randomized; ROXA, roxadustat; TIW, 3 times weekly; VADA, vadadustat.
ESA-naïve is defined as no use of ESA for a study-defined time period before start of study.
Defined as the proportion of patients with an increase in Hb from baseline of ≥1 g/dl, unless defined otherwise.
Started at 2 and 4 mg QD in ESA-naïve patients with baseline Hb 9 to <11 and 8 to <9 g/dl, respectively, and 4 mg QD in ESA-treated patients with baseline Hb 9 to 13 g/dl; dose adjusted to maintain Hb levels of 11 to 12 g/dl.
Dose adjusted to achieve and maintain Hb levels of 10 to 12 g/dl.
Dose adjusted to achieve and maintain Hb levels of 11 to 13 g/dl.
Starting dose based on prior ESA dose; dose adjusted to achieve and maintain Hb levels of 11 to 13 g/dl.
Weight-based dosing (>40–60 or ≥60 kg), adjusted every 4 weeks to maintain Hb levels of 10 to 12 g/dl.
Tiered, weight-based dosing.
Hb response defined as Hb ≥11 g/dl and an increase in Hb from baseline of ≥1 g/dl in patients with baseline Hb >8 g/dl or ≥2 g/dl in patients with baseline Hb ≤8 g/dl.
Table 3.
Summary of clinical efficacy data from phase 3 clinical trials of hypoxia-inducible factor–prolyl hydroxylase inhibitors in patients with anemia and dialysis-dependent chronic kidney disease
| Study | Study design; no. of patients | Treatment, duration | Hb response ratea | Mean ΔHb from baseline |
|---|---|---|---|---|
| Daprodustat | ||||
| Tsubakihara et al., 201913 (NCT02829320) | OL, NC; ESA-naïve, I-HD and M-HD; n = 28 | DAPRO 4 mg QDb, 24 wk | During weeks 1–4: 39% |
After 4 weeks: 0.79 g/dl |
| Akizawa et al., 202014 (NCT02969655) | R, DB, AC; ESA-treated, M-HD; n = 271 | DAPRO 4 mg QDb vs. DPO, 52 wk | Hb within target range (10–12 g/dl) during weeks 40–52: DAPRO: 88% DPO: 90% |
During weeks 40–52: DAPRO: 0.0 g/dl DPO: 0.0 g/dl Adjusted difference vs. DPO: 0.1 (95% CI, –0.1 to 0.2) g/dl |
| Enarodustat | ||||
| Akizawa et al., 201977 | R, DB, AC; ESA-treated, M-HD; n = 173 | ENARO QDc vs. DPO; 24 wk | Hb within target range (10–12 g/dl) during weeks 1–24: ENARO: 78.2% DPO: 88.8% |
Difference in mean Hb (weeks 20–24): –0.12 (–0.33 to 0.10) g/dl |
| Molidustat | ||||
| MIYABI HD-M95 (NCT03543657) | R, DB, AC; ESA-treated, M-HD; n = 229 | MOLI 5–200 mg QDb vs. DPO; 52 wk | NR | LSM difference in Hb change from baseline (weeks 33–36): –0.13 g/dl |
| Roxadustat | ||||
| Chen et al., 201922 (NCT02652806) | R, OL, AC; ESA-treated, M-DD; n = 304 | ROXA 100 or 120 mg TIWd vs. Epoetin alfa, 26 wk | During weeks 23–27: ROXA: 92.5% Epoetin alfa: 92.5% |
During weeks 23–27: ROXA: 0.7 g/dl Epoetin alfa: 0.5 g/dl Difference vs. Epoetin alfa: 0.2 (95% CI, –0.02 to 0.5) g/dl |
| Akizawa et al., 202016 (NCT02779764, NCT02780141) | R, OL, NC; ESA-naïve (I-HD) and ESA-treated (M-HD); n = 239 | ESA-naïve: ROXA 50 or 70 mg TIWd, 24 wk ESA-treated: ROXA 70 or 100 mg TIWe, 52 wk |
From baseline to EOTf: ESA-naïve: 87.8% ESA-treated: NR |
During weeks 18–24: ESA-naïve: 2.26 g/dl ESA-treated: –0.03 g/dl During weeks 46–52: ESA-treated: 0.12 g/dl |
| Akizawa et al., 202017 (NCT02780726) | R, OL, NC; ESA-naïve and ESA-treated, PD (>4 wk); n = 56 | ROXA 50 or 70 mg TIW (ESA-naïve) or ROXA 70 or 100 mg (ESA-treated), 24 wk | Hb within target range (10–12 g/dl) during weeks 18–24: ESA-naïve: 92.3% ESA-treated: 74.4% |
During weeks 18–24: ESA-naïve: 1.69 g/dl ESA-treated: 0.14 g/dl |
| Akizawa et al., 202018 (NCT02952092) | R, DB, AC; ESA-treated, M-HD; n = 303 | ROXA 70 or 100 mg TIW vs. DPO QW, 24 wk | Hb within target range (10–12 g/dl) during weeks 18–24: ROXA: 95.2% DPO: 91.3% |
During weeks 18–24: ROXA: –0.04 g/dl DPO: –0.03 g/dl Difference vs. DPO: –0.02 (95% CI, –0.18 to 0.15) g/dl |
| HIMALAYAS96 (NCT02052310) | R, OL, AC, ESA-naïve and ESA-limited use, I-DD; n = 1043 | ROXA TIW vs. Epoetin alfa, 52 wk | During weeks 1–24: ROXA: 88.2% Epoetin alfa: 84.4% |
During weeks 28–52: ROXA: 2.57 g/dl Epoetin alfa: 2.36 g/dl (P< 0.001 vs. Epoetin alfa) |
| PYRENEES90 (NCT02278341) | R, OL, AC, ESA-treated, M-DD; n = 836 | ROXA 100, 150, or 200 mg TIWe vs. ESA, 52–104 wk | NR | During weeks 28–52: ROXA: 0.40 g/dl ESA: 0.18 g/dl (P < 0.001 vs. ESA) |
| ROCKIES97 (NCT02174731) | R, OL, AC; ESA-naïve and ESA-treated, M-DD and I-DD (n = 416); total n = 2133 | ROXA TIWg vs. Epoetin alfa, 52 wk | Proportion of time with Hb ≥10 g/dl during weeks 28–52: ROXA: 79% Epoetin alfa: 76% |
During weeks 28–52: ROXA: 0.77 g/dl Epoetin alfa: 0.68 g/dl (P < 0.05 vs. Epoetin alfa) |
| SIERRAS98 (NCT02273726) | R, OL, AC; ESA-treated, M-DD and I-DD (n = 371); total n = 741 | ROXA TIWh vs. Epoetin alfa, 52 wk | NR | During weeks 28–52: ROXA: 0.39 g/dl Epoetin alfa: –0.09 g/dl (P < 0.0001 vs. Epoetin alfa) |
| Vadadustat | ||||
| Nangaku et al., 201920 (NCT03439137) | R, DB, AC; ESA-treated, M-HD; n = 323 | VADA 300 mg QD, then 150–600 mgb vs. DPO, 52 wk | Hb within target range (10–12 g/dl) at week 24: VADA: 75.4% DPO: 75.7% |
LSM of average Hb (weeks 20 and 24): VADA: 10.61 g/dl DPO: 10.65 g/dl |
| INNO2VATE99 (NCT02865850) | R, DB, AC; ESA-naïve and ESA-treated; I-DD; n = 369 | VADA vs. DPO, 52 wk | NR | LSM difference in Hb change: During weeks 24–36: –0.3 g/dl During weeks 40–52: –0.07 g/dl |
| INNO2VATE99 (NCT02892149) | R, DB, AC; ESA-naïve and ESA-treated; M-DD; n = 3554 | VADA vs. DPO, 52 wk | NR | LSM difference in Hb change: During weeks 24–36: –0.17 g/dl During weeks 40–52: –0.18 g/dl |
AC, active controlled; CI, confidence interval; DAPRO, daprodustat; DB, double blind; DPO, darbepoetin alfa; ENARO, enarodustat; EOT, end of treatment; ESA, erythropoietin-stimulating agent; Hb, hemoglobin; I-DD, incident dialysis (hemodialysis and PD); I-HD, incident hemodialysis; LSM, least-squares mean; M-DD, maintenance/stable dialysis (hemodialysis and PD); M-HD, maintenance/stable hemodialysis; MOLI, molidustat; NC, noncomparative; NR, not reported; OL, open label; PD, peritoneal dialysis; QD, once daily; QW, once weekly; R, randomized; ROXA, roxadustat; TIW, 3 times weekly; VADA, vadadustat.
Defined as the proportion of patients with an increase in Hb from baseline of ≥1.0 g/dl, unless defined otherwise.
Titrated to maintain Hb levels of 10 to 12 g/dl.
Weight-based dosing (>45–60 or ≥60 kg), adjusted to maintain Hb levels of 10 to 12 g/dl.
Starting dose based on study site, weight, and 2 most recent Hb measurements, adjusted to maintain Hb levels of 10 to 12 g/dl.
Dosed according to average weekly dose of prior ESA therapy, adjusted to maintain Hb levels of 10 to 12 g/dl.
Hb response defined as the proportion of patients with Hb of ≥10 g/dl and an increase in Hb from baseline of ≥1.0 g/dl.
Titrated to achieve an Hb level of 11 g/dl and to maintain Hb levels of 10 to 12 g/dl.
Initially dosed according to prior dose of erythropoietin therapy.
Table 4.
Summary of ongoing NCT-registered clinical studies of hypoxia-inducible factor–prolyl hydroxylase inhibitors in patients with anemia of CKD
| Study design (identifier) | Patients; target no. | Comparator | Primary end point(s) | Completion date |
|---|---|---|---|---|
| Daprodustat | ||||
| Phase 2, R, OL, AC, CO (ASCEND: Fe; NCT03457701) | NDD-CKD; n = 12 | rhEPO | Fractional iron absorption | Mar 19, 2021 |
| Phase 2, R, OL, AC, PG (ASCEND-BP; NCT03029247) | DD-CKD (M-HD); n = 62 | Epoetin alfa | Mean 6-h postdose SBP at day 57 | Jul 16, 2020 |
| Phase 3, R, DB, PC, PG (ASCEND-NHQ; NCT03409107) | NDD-CKD; n = 600 | PBO | Mean change from baseline in Hb at weeks 24–28 | Oct 6, 2020 |
| Phase 3, R, OL, AC, PG (ASCEND-ND; NCT02876835) | NDD-CKD; n = 4500 | DPO Iron |
|
Mar 17, 2022 |
| Phase 3, R OL, AC, PG (ASCEND-ID; NCT03029208) | DD-CKD (I-DD); n = 300 | DPO Iron |
Mean change from baseline in Hb at weeks 28–52 | Oct 8, 2020 |
| Phase 3, R, DB, AC, PG (ASCEND-TD; NCT03400033) | DD-CKD (M-HD); n = 407 | Epoetin alfa | Mean change from baseline in Hb at weeks 28–52 | Jun 19, 2020 |
| Phase 3, R, OL, AC, PG (ASCEND-D; NCT02879305) | DD-CKD (M-DD); n = 2986 | rhEPO |
|
Nov 25, 2021 |
| Desidustat | ||||
| Phase 3, R, OL, AC, PG (DREAM-ND; NCT04012957) | NDD-CKD; n = 588 | DPO | Mean change from baseline in Hb at week 24 | Dec 30, 2020 |
| Phase 3, R, OL, AC, PG (DREAM-D; NCT04215120) | DD-CKD (M-HD); n = 392 | Epoetin alfa | Mean change from baseline in Hb at week 24 | Nov 30, 2021 |
| Molidustat | ||||
| Phase 3, R, DB, DD, AC, PG (MIYABI HD-M; NCT03543657) | DD-CKD (M-HD); n = 220 | DPO |
|
Dec 24, 2019 |
| Roxadustat | ||||
| Phase 3, R, OL, AC, PG (NCT02988973) | NDD-CKD; n = 334 | DPO | Change from baseline in mean Hb at weeks 18–24 | Mar 26, 2020 |
| Phase 4, R, OL, AC, PG (NCT04134026) | DD-CKD (I-HD); n = 400 | Epoetin alfa |
|
Oct 19, 2023 |
| Phase 4, R, OL, NC, PG (NCT04059913) | DD-CKD (HD or PD); n = 306 | None | Part 1 (weeks 1–20): ESA-naïve: % of patients who achieve Hb ≥11.0 g/dl ESA-treated: % of patients who achieve mean Hb ≥10.0 g/dl Part 2 (weeks 21–36): Mean Hb at weeks 33–37 |
Nov 2021 |
| Vadadustat | ||||
| Phase 1, R, OL, AC, PG (NCT03992066) | DD-CKD (M-HD); n = 35 | DPO Epoetin alfa |
|
Jul 2020 |
| Phase 2, R, OL, AC, PG (FO2RWARD-2; NCT03799627) | DD-CKD (M-HD); n = 125 | Epoetin alfa | Mean change from baseline in Hb at weeks 10–12 | Jul 2020 |
AC, active controlled; AUC∞, area under the concentration-time curve from 0 to infinity; AUClast, area under the concentration-time curve from 0 to last quantifiable concentration; CKD, chronic kidney disease; CL/F, apparent clearance; Cmax, peak plasma concentration; CV, cardiovascular; DB, double blind; DPO, darbepoetin alfa; DD, dialysis dependent; ESA, erythropoietin-stimulating agent; Fe, iron; Hb, hemoglobin; HD, hemodialysis; I-DD, incident dialysis (HD or PD); I-HD, incident HD; MACE, major adverse CV event; M-DD, maintenance/stable dialysis (HD or PD); M-HD, maintenance HD; NC, noncomparative; NDD, non–dialysis dependent; OL, open label; PBO, placebo; PC, PBO controlled; PD, peritoneal dialysis; PG, parallel group; R, randomized; rhEPO, recombinant human erythropoietin; SBP, systolic blood pressure; t½, terminal half-life; Tmax, time to Cmax; Vd/F, apparent volume of distribution.
Non–dialysis-dependent CKD
Dose-dependent increases in Hb levels were observed in phase 2 studies of orally administered daprodustat,100, 101, 102 desidustat,103 enarodustat,104 molidustat,80,105 roxadustat,106, 107, 108, 109 and vadadustat110, 111, 112 over 4- to 30-week treatment periods in recombinant human EPO-naïve patients or patients with prior exposure to ESA. Of the HIF-PHIs in development, phase 3 data in NDD CKD patients are reported for daprodustat,15 enarodustat,78 molidustat,86,87 roxadustat,23,88, 89, 90, 91, 92, 93 and vadadustat19,94 (Table 2). Roxadustat, orally administered 3 times weekly, effectively corrected Hb levels in a small double-blind, placebo-controlled phase 3 study in China (n = 154; 8-week duration)23 and in a 2-arm, randomized, open-label, noncomparative study in Japan (n = 99; 24-week duration),88 and was noninferior to darbepoetin alfa in preliminary results from a 52-week, randomized, open-label, active-comparator study in Japan (n = 262).89 Preliminary data from the larger international, double-blind, placebo-controlled ALPS (n = 594),90 ANDES (n = 922),91 and OLYMPUS (n = 2781)92 trials indicated that roxadustat was efficacious in correcting and maintaining Hb levels over 52 weeks; and in a preliminary report from the open-label, active-controlled DOLOMITES trial (n = 616), roxadustat was noninferior to darbepoetin alfa in providing Hb response over 24 weeks.93 Preliminary data from Japanese studies reported that daprodustat was noninferior to epoetin beta pegol (n = 299),15 whereas enarodustat (n = 216),78 molidustat (n = 161 and 164),86,87 and vadadustat (n = 304)19 were noninferior to darbepoetin alfa for maintaining target Hb levels. In the large global PRO2TECT trial, vadadustat achieved noninferiority compared with darbepoetin alfa in both the correction (n = 1751) and the conversion (n = 1725) arms.113
HIF-PHI administration in NDD CKD patients was associated with an increase in total iron binding capacity in most phase 2 and phase 3 studies (mostly placebo controlled).23,80,88,100,101,103, 104, 105, 106, 107, 108, 109,111 Some studies directly measured and reported increases in serum TF,23,88,102,109 which most likely resulted from HIF-induced stimulation of TF transcription. As predicted, decreases in serum hepcidin and ferritin were reported for all HIF-PHIs,23,80,88,100,101,103, 104, 105, 106, 107, 108, 109,111 which most likely resulted from increased erythropoietic activity and iron utilization in the bone marrow.51,114 One study observed relatively greater decreases in serum hepcidin and ferritin with molidustat versus darbepoetin alfa,105 suggesting that HIF-PHIs may have effects on iron metabolism resulting from distinct, nonerythropoietic mechanisms. Superiority with regard to first i.v. iron use was reported for roxadustat compared with darbepoetin alfa in preliminary results from the DOLOMITES trial.92 Further investigations that control for iron supplementation and stratify for serum iron markers are needed to corroborate these findings.
Dialysis-dependent CKD
The clinical efficacy of HIF-PHIs in correcting and maintaining Hb levels in patients with DD CKD has been consistently demonstrated in phase 2 and 3 studies. Phase 2 studies of up to 30 weeks’ duration included ESA-converted patients who maintained Hb levels with daprodustat,100,102,115, 116, 117 enarodustat,118 molidustat,80 roxadustat,107,119 or vadadustat,120 or EPO-naïve incident dialysis patients (dialysis duration, <4 months) who corrected Hb levels with roxadustat.121 Some phase 2 studies included patients who received placebo for a limited time period of 4 to 6 weeks.112,115, 116, 117, 118
Clinical efficacy data from phase 3 trials for daprodustat,13,14 enarodustat,77 molidustat,95 roxadustat,16, 17, 18,22,90,96, 97, 98 and vadadustat20,99 are summarized in Table 3. Noninferiority to darbepoetin alfa with regard to maintaining target Hb was reported for daprodustat (n = 271),14 enarodustat (n = 173),77 molidustat (n = 229),95 and vadadustat, in both a study of maintenance hemodialysis patients (n = 323)20 and the INNO2VATE studies of patients on incident (n = 369) and maintenance (n = 3554) hemodialysis.99 Noninferiority criteria, compared with ESAs, were also met by roxadustat in Chinese maintenance dialysis patients (n = 256) versus epoetin alfa,22 in Japanese maintenance hemodialysis patients (n = 303) versus darbepoetin alfa,18 and in a larger international, randomized, phase 3 study of incident dialysis patients (n = 1043) versus epoetin alfa (HIMALAYAS; preliminary data).96 In addition, HIMALAYAS reported superiority of roxadustat over epoetin alfa with regard to mean Hb change from baseline; the study included epoetin alfa–naïve patients and patients with limited prior epoetin alfa use.96 Preliminary data from 3 other randomized phase 3 studies of dialysis patients (PYRENEES [n = 836],90 ROCKIES [n = 2133],97 and SIERRAS [n = 741]98) also indicated that roxadustat was associated with greater improvements in mean Hb than ESA therapy in treating anemia, including in patients with elevated C-reactive protein.97 Furthermore, reduced i.v. iron use was reported in the PYRENEES and ROCKIES trials,90,97 while the SIERRAS study and a pooled analysis of the 3 trials reported reduced transfusion rates.98,122
Similar to NDD CKD patients, consistent decreases in serum hepcidin and ferritin levels and increases in total iron binding capacity and serum TF were observed in DD CKD patients in phase 2 and 3 trials, in line with stimulated erythropoiesis and iron utilization.13,16, 17, 18,22,80,100,102,107,115, 116, 117, 118, 119, 120, 121
Therapeutic Benefits
HIF-PHIs induce robust Hb response in patients with anemia of CKD. Because HIF transcription factors regulate a broad spectrum of hypoxia responses, HIF-PHIs are predicted to have clinical effects beyond the stimulation of endogenous EPO (Figure 251). Validation of predicted non-EPO benefits still requires well-designed and properly controlled clinical trials. Specifically, it will be important to: (i) corroborate the predicted ferrokinetic properties of HIF-PHIs and determine their impact on i.v. iron supplementation needs; (ii) establish whether patients need to be iron replete and which iron parameters should be met before HIF-PHIs can be safely initiated; (iii) determine whether HIF-PHIs can be effectively and safely used in patients with inflammation; (iv) determine whether HIF-PHI therapy impacts cardiovascular risk and mortality; (v) determine whether HIF-PHI therapy impacts CKD progression; and (vi) determine which CKD subgroups are most likely to benefit from HIF-PHIs. Furthermore, a better understanding is needed of the differences between individual HIF-PHIs with regard to pharmacokinetics, therapeutic window, nonerythropoietic actions (e.g., cardiovascular, metabolic, and blood pressure effects), and adverse effects.
Stimulation of endogenous EPO
When administered i.v., ESA therapy may lead to supraphysiological plasma EPO concentrations,123 which are associated with increased cardiovascular risk and mortality in CKD patients.124 Compared with ESA-treated dialysis patients, substantially lower peak serum EPO concentrations were measured in patients treated with HIF-PHIs.88,114 The impact of lower serum EPO concentrations with HIF-PHIs on cardiovascular outcome is not clear. Possible explanations for lower serum EPO levels in patients treated with HIF-PHIs include direct effects of HIF-PHIs on erythroid progenitors and bone marrow environment facilitating erythropoiesis, beneficial effects on iron utilization, and potential differences in the biochemical properties of recombinant versus endogenous EPO, such as differences in ligand receptor interaction and glycosylation patterns.
Although the kidney is the main source of EPO in adults under normal physiological conditions, the liver produces differentially glycosylated forms of EPO that contribute to the plasma EPO pool under hypoxic conditions, and can be biochemically differentiated from kidney-derived EPO.50,125 In patients with CKD, biochemical analysis suggested that hepatic EPO production correlated inversely with declining estimated glomerular filtration rate.126 In addition to renal EPO production, hepatic EPO production can be substantially stimulated by pharmacologic HIF-PHD inhibition in rodent models of renal anemia.49,53,71,127 Although liver EPO production has not been specifically examined in current clinical trials, a single-dose phase 1 study demonstrated that FG-2216, a compound related to roxadustat, resulted in increased plasma EPO concentrations in anephric patients.128 Whether higher doses of HIF-PHI will be needed for efficacious treatment of anemia in anephric patients is not clear and will have to be addressed in future studies. Of interest in this context is a novel HIF-PHI compound (TP0463518), which appears to specifically target hepatic EPO production.129
Iron metabolism
As discussed by Agarwal130 in this supplement, the potential benefits of HIF-PHIs include hepatic hepcidin suppression and upregulation of iron metabolism and transport genes, such as DCYTB, DMT1, and TF (Figure 2).53 These HIF-PHI responses are predicted to provide improvements in iron mobilization and utilization.53 Although HIF-PHI administration in clinical trials was consistently associated with decreased serum hepcidin levels and increased total iron binding capacity and/or serum TF, there is no direct evidence from iron absorption studies or direct measurement of intestinal iron metabolism gene expression in patients with CKD.
Dedicated studies are needed to establish the degree by which HIF-PHIs impact clinical iron management, especially in patients with inflammation. Because of general iron loading in many patients to avoid functional iron deficiency, the degree to which iron supplementation needs are lower in HIF-PHI–treated patients is difficult to quantify. Nevertheless, several studies have included patients who (i) did not receive iron supplementation during HIF-PHI treatment; (ii) were not iron replete at baseline (i.e., iron replete defined as ferritin >100 ng/ml and TF saturation >20%), but received oral iron supplementation; or (iii) were on hemodialysis and received oral or i.v. iron.13,22,23,105,107,108,115,121
In incident dialysis patients, roxadustat treatment without iron supplementation resulted in an initial Hb increase; however, Hb started to plateau at a lower Hb level after 7 weeks of the 12-week study period compared with iron-treatment groups.121 Similar to ESAs, HIF-PHIs can produce a state of relative iron deficiency in which the sudden increase in bone marrow iron requirements surpasses enteral iron uptake and mobilization from internal stores, necessitating iron supplementation in patients with borderline iron stores.131,132 In the same study, supplementation with either oral (50–195 mg/d elemental) or i.v. (50–62.5 mg/wk elemental) iron was equally effective in raising and maintaining Hb levels.121 As oral iron is inferior to i.v. iron in ESA-treated dialysis patients,2 this suggests that HIF-PHIs may have beneficial effects on enteral iron uptake and/or utilization. This notion is further supported by data from a phase 3 study in Chinese patients on hemodialysis who were successfully treated with roxadustat in conjunction with oral iron only,22 and preliminary data from the roxadustat PYRENEES and ROCKIES trials90,97 and a phase 3 daprodustat study in Japanese hemodialysis patients,14 which reported i.v. iron-sparing effects in comparison with ESA therapy. In NDD CKD patients treated with roxadustat who were permitted oral but not i.v. iron, mean Hb increases and response rates with roxadustat were similar between patients who were iron replete and not iron replete at baseline.108 However, this study did not control for iron supplementation, and patients were not adequately stratified.
Patients with inflammation
As discussed by Raichoudhury and Spinowitz133 in this issue of the supplement, inflammation suppresses erythropoiesis via cytokine-mediated effects on bone marrow, iron metabolism (elevated serum hepcidin levels), EPO responsiveness and synthesis, and other mechanisms.55 Preclinical data indicate that HIF-PHIs have the potential to correct Hb levels under inflammatory conditions. In a rodent model of inflammatory anemia, increases in hematocrit and renal EPO expression, and decreases in liver hepcidin and renal monocyte chemotactic protein 1 transcription, have been demonstrated in response to molidustat administration.127 Comparable erythropoietic effects were seen with JNJ-42905343 (HIF-PHI in preclinical development) and roxadustat in experimental inflammation.53,134 In addition to proerythropoietic effects, HIF-PHIs have been shown to have anti-inflammatory effects in several disease models, such as acute ischemic injury and sepsis. The underlying mechanisms are complex, highly context dependent, and likely to involve interactions between the HIF pathway and proinflammatory nuclear factor-κB signaling, direct and/or indirect effects of cytokine production, and modulation of innate and adaptive immune responses.135,136
In a single-arm study, 29% of ESA-hyporesponsive patients responded to daprodustat and increased or maintained Hb levels over 16 weeks.137 However, the small study population limited any conclusions as to whether daprodustat was efficacious in this patient subgroup.137 Nevertheless, roxadustat and vadadustat appear to be efficacious in patients with elevated baseline C-reactive protein, and dose increases were not required to reach Hb target levels.18,22,92,97,108,119,121,138 Further studies are needed to confirm the efficacy, dose requirements, and safety of HIF-PHIs in patients with underlying inflammation over a wider range of C-reactive protein levels and/or ESA hyporesponsiveness.
Safety
Because HIF-1 and HIF-2 control multiple biologic processes, systemic HIF-PHD inhibition may potentially produce adverse on-target effects. These effects will most likely depend on the dosing and pharmacokinetics of the HIF-PHI agent. However, because regulation of EPO expression is highly sensitive to hypoxia compared with other HIF targets, such as VEGF,68,139 HIF-PHIs may achieve desirable proerythropoietic effects at doses that do not elicit a broader spectrum of HIF responses in CKD patients, including stimulation of VEGF-dependent pathways.140
To date, HIF-PHIs have been generally well tolerated, and major signals of serious risk have not been reported in healthy volunteers69,70,73 or in clinical trials (Table 5141,142). Serious adverse events (AEs), reported in phase 3 studies, have not been considered to be drug related and fell within the range of expected AE frequencies in CKD patients. However, the Japanese Pharmaceutical and Medical Devices Agency prescribing information includes a safety warning regarding the potential risk for thromboembolism, cerebral and myocardial infarction, pulmonary embolism, and deep vein and vascular access thrombosis with HIF-PHIs.141 A higher incidence of thromboembolic events (11.3% vs. 3.9%) was reported with roxadustat versus darbepoetin alfa in the safety analysis of pooled phase 3 trials in hemodialysis patients.141
Table 5.
Overview of SAEs and most common AEs reported in phase 3 clinical trials of hypoxia-inducible factor–prolyl hydroxylase inhibitors in patients with anemia and CKD
| Variable | NDD-CKD patients | DD-CKD patients |
|---|---|---|
| SAEs | ||
|
|
|
|
|
|
|
|
|
|
||
| Most common AEs | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AE, adverse event; AV, arteriovenous; CKD, chronic kidney disease; CV, cardiovascular; DD, dialysis-dependent; DPO, darbepoetin alfa; EPO, erythropoietin; ESA, erythropoietin-stimulating agent; ESRD, end-stage renal disease; GFR, glomerular filtration rate; GI, gastrointestinal; HD, hemodialysis; I-DD-CKD, incident DD-CKD; M-DD-CKD, maintenance DD CKD; NDD, non–dialysis-dependent; PBO, placebo; PD, peritoneal dialysis; PMDA, Japanese Pharmaceutical and Medical Device Agency; SAE, serious AE; UTI, urinary tract infection.
Study results available from https://clinicaltrials.gov/ct2/show/NCT02174627.
Study results available from https://clinicaltrials.gov/ct2/show/NCT02278341.
Study results available from https://clinicaltrials.gov/ct2/show/NCT02174731.
Severity of treatment-emergent AE was not specified.
Cardiovascular safety
Patients with CKD are at increased risk for cardiovascular events (e.g., myocardial infarction and stroke).143,144 Although preclinical data have suggested that short-term systemic HIF activation provides protection from ischemic injury and may be beneficial in several renal and cardiovascular disease models,11,145 rigorous cardiovascular outcome analysis is needed to determine whether long-term treatment of patients with CKD anemia is safe.
Preliminary data from 3 pooled phase 3 studies with roxadustat in dialysis patients (HIMALAYAS, ROCKIES, and SIERRAS trials; including both maintenance and incident dialysis patients) reported no increase in the risk for all-cause mortality and MACE (i.e., death, myocardial infarction, or stroke) for roxadustat compared with epoetin alfa, whereas the risk of MACE+ (i.e., MACE plus heart failure or unstable angina, requiring hospitalization) was significantly reduced (MACE+ hazard ratio [HR] 0.84; 95% confidence interval [CI], 0.73–0.97; P = 0.02).122 In the analysis of incident dialysis patients, both the risks of MACE and MACE+ were significantly reduced with roxadustat versus epoetin alfa (MACE HR, 0.70 [95% CI, 0.51–0.97] [P = 0.03]; and MACE+ HR, 0.66 [95% CI, 0.50–0.89] [P = 0.005]),122 suggesting an improvement in cardiovascular safety compared with the current standard of care. However, safety data from the smaller PYRENEES trial (in maintenance DD CKD patients only) were not included in this pooled analysis (see Table 5 for AE rates). The INNO2VATE trial, a large global study of vadadustat in dialysis patients, reported noninferiority compared with darbepoetin alfa with regard to MACE and all-cause mortality.99,142 Preliminary cardiovascular safety data for NDD CKD patients have been reported for both roxadustat and vadadustat. In the pooled analysis of OLYMPUS, ALPS, and ANDES, the risks of MACE and MACE+ were not significantly different between the roxadustat and placebo groups.122 In the global PRO2TECT study, which used a different trial design and compared cardiovascular outcomes with vadadustat versus darbepoetin alfa in patients with NDD CKD, vadadustat did not meet the primary safety MACE end point for noninferiority (MACE HR 1.14 [95% CI, 0.99–1.32]; all-cause mortality HR 0.99 [95% CI, 0.77–1.27]).94,113 This increase in the HR for MACE appeared to be largely driven by cardiovascular events in the non-US study population (MACE HR in the US population, 1.01 [95% CI, 0.83–1.23] vs. MACE HR in non-US population, 1.29 [95% CI, 1.03–1.60]).94,113 Additional subgroup analysis is needed to understand the differences in cardiovascular safety outcomes between US and non-US populations and to identify the patient groups with the greatest risk of MACE.
Nonerythropoietic actions with potential impact on cardiovascular outcomes have been consistently reported in clinical studies of roxadustat and daprodustat, including reductions in total serum cholesterol, low-density and high-density lipoprotein cholesterol, and triglycerides.13,22,23,116 These lipid-lowering effects can be explained by HIF-dependent increases in lipoprotein uptake and reductions in cholesterol synthesis via enhanced degradation of 3-hydroxy-3-methyl-glutaryl-CoA reductase.146,147
HIF-PHIs do not appear to cause QT interval prolongation, even at supratherapeutic doses,148 or changes in echocardiographic parameters.101,115 In general, HIF-PHI treatment was not associated with significant changes in systolic or diastolic blood pressure.22,80,103,111 However, almost twice as many daprodustat-treated patients had an increase in antihypertensive medications, although the mean increase in systolic blood pressure was lower with daprodustat in a 24-week hemodialysis study compared with control.115
Renal safety
The effects of HIF-PHIs on CKD progression are unclear, and peer-reviewed data are not yet available. However, preliminary data from the MolIdustat once dailY improves renal Anaemia By Inducing erythropoietin (MIYABI) Haemodialysis-Correction (HD-C) and Haemodialysis-Maintenance (HD-M) trials (n = 325) in NDD CKD patients with previously untreated or ESA-treated anemia indicated that worsening of CKD occurred more frequently with molidustat (13.4% and 12.2%, respectively) than darbepoetin alfa (6.3% and 7.3%, respectively).86,87 In contrast, a preliminary pooled safety analysis of roxadustat-treated NDD CKD patients (n = 2438) suggested beneficial effects on glomerular filtration rate with roxadustat (1-year decline in estimated glomerular filtration rate of –2.8 vs. –4.4 ml/min with placebo).149
Hyperkalemia was reported more frequently with roxadustat in Chinese phase 3 studies in both NDD CKD and DD CKD patients.22,23 Although the underlying mechanisms are unclear, metabolic acidosis was reported in 12% of roxadustat-treated NDD CKD patients.23 Hyperkalemia was also observed in phase 2 trials in patients treated with other HIF-PHIs.106,111,115
Other safety considerations
Despite concerns regarding proangiogenic effects of HIF-PHIs in patients with vascular retinopathy,150 there was no increase in the incidences of retinal hemorrhage, macular edema, or changes in intraocular pressure or visual acuity in clinical studies with roxadustat or daprodustat.14,18,101,115 In Japanese hemodialysis patients, ophthalmologic evaluation reported new or worsening retinal hemorrhage in 32.4% of roxadustat-treated versus 36.6% of darbepoetin alfa–treated patients overall, and in 19.1% of roxadustat-treated versus 25.0% of darbepoetin alfa–treated patients without prior retinal lesions.18
Several concerns regarding the long-term safety of HIF-PHIs have remained unanswered, and extended clinical studies and/or postmarketing investigations will be needed. These concerns are based on observations made in genetically modified animals with HIF-activating mutations, physiological and pathologic responses to high altitude,151 and clinical manifestations in patients with genetic mutations in the HIF pathway, such as patients with Chuvash polycythemia or certain neuroendocrine tumors.152
There are theoretical concerns that systemic HIF activation may be pro-oncogenic, promote tumor growth, or facilitate metastasis given that HIF activation is evident in many cancers, and that growing tumors experience hypoxia and co-opt the HIF pathway for metabolic adaptation and angiogenesis.153 To date, animal studies have shown no evidence that prolonged exposure to HIF-PHIs is pro-oncogenic.140,154,155 Although preliminary data from phase 3 roxadustat studies indicated that the rates of neoplasm-related AEs were not increased compared with placebo or epoetin alfa in patients with NDD CKD or DD CKD,156 long-term observations in humans are needed to rule out any possible pro-oncogenic properties of HIF-PHIs. Given the multiple theoretical safety concerns and the exclusion of patients with a history of cancer (<2–5 years) from clinical trials, the use of HIF-PHIs in patients with cancer should not be recommended, as safety data are not available.
Other concerns include the potential risk for pulmonary arterial hypertension, as HIF activation increases vascular tone in pulmonary arteries157, 158, 159; thromboembolic events, which have been observed in patients with Chuvash polycythemia160,161; promotion of renal cyst growth162; AEs on glucose and liver metabolism51; profibrogenic effects in kidney and other organs163; and AEs on vascular calcifications and fibroblast growth factor 23 levels.164,165 There are also concerns regarding HIF-PHI use in patients with autoimmune diseases, viral hepatitis, and other infections.51
Other Indications
In addition to the management of anemia of CKD, clinical studies are investigating the efficacy and safety of HIF-PHIs in other diseases, including anemia associated with myelodysplastic syndrome (NCT03263091 and NCT03303066), chemotherapy-induced anemia (NCT04076943), wound healing (NCT01831804), diabetic foot ulcers (NCT03153007), sarcopenia of aging (NCT03371134), peripheral vascular disease (NCT02135848), and inflammatory bowel disease (NCT02914262).
Conclusions
The efficacy of HIF-PHIs for the treatment of anemia of CKD has been well established in clinical trials, with 4 compounds being under license for marketing in Asia. Going forward, several questions and concerns regarding the use of HIF-PHIs in clinical practice need to be addressed. Aside from establishing long-term safety in extended trials and postmarketing analysis, evidence-based guidance will be required as to the laboratory and clinical parameter criteria for the safe use of HIF-PHI therapy. It will be important to differentiate between CKD patients who will clearly benefit from HIF-PHIs and those in whom treatment should not be started. This will require rigorous comparisons to current standard of care in both CKD patients on and not on dialysis.
Disclosure
VHH has received consulting fees from Akebia Therapeutics, AstraZeneca, FibroGen, and Rockwell Medical.
Acknowledgements
This article is published as part of a supplement supported by AstraZeneca. Medical writing assistance was provided by Sarah Greig, PhD (Auckland, New Zealand) and Meri Pozo, PhD, CMPP (New York, NY, USA), of inScience Communications (and funded by AstraZeneca).
Roxadustat is being developed for clinical use by an alliance of FibroGen, Astellas, and AstraZeneca.
VHH apologizes to those colleagues whose original publications could not be cited because of space limitations. VHH is supported by the Krick-Brooks Chair in Nephrology at Vanderbilt University. More information about research performed in the Haase laboratory can be found at https://www.haaselab.org.
Author Contributions
VHH meets the International Committee of Medical Journal Editors criteria for authorship for this article and takes responsibility for the integrity of the work as a whole. VHH wrote the article with the assistance of medical writers from inScience Communications and is fully accountable for all aspects of the work.
References
- 1.Kidney Disease: Improving Global Outcomes Notice. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 2.Besarab A., Coyne D.W. Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol. 2010;6:699–710. doi: 10.1038/nrneph.2010.139. [DOI] [PubMed] [Google Scholar]
- 3.Besarab A., Bolton W.K., Browne J.K. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein F.O., Story K., Firanek C. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:33–38. doi: 10.2215/CJN.00630208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parfrey P.S., Lauve M., Latremouille-Viau D. Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: a meta-analysis. Clin J Am Soc Nephrol. 2009;4:755–762. doi: 10.2215/CJN.02730608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finkelstein F.O., Finkelstein S.H. The impact of anemia treatment on health-related quality of life in patients with chronic kidney disease in the contemporary era. Adv Chronic Kidney Dis. 2019;26:250–252. doi: 10.1053/j.ackd.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Singh A.K., Szczech L., Tang K.L. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 8.Drueke T.B., Locatelli F., Clyne N. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer M.A., Burdmann E.A., Chen C.Y. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 10.Semenza G.L. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 11.Schodel J., Ratcliffe P.J. Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol. 2019;15:641–659. doi: 10.1038/s41581-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 12.Loenarz C., Schofield C.J. Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol. 2008;4:152–156. doi: 10.1038/nchembio0308-152. [DOI] [PubMed] [Google Scholar]
- 13.Tsubakihara Y., Akizawa T., Nangaku M. A 24-week anemia correction study of daprodustat in Japanese dialysis patients. Ther Apher Dial. 2020;24:108–114. doi: 10.1111/1744-9987.12962. [DOI] [PubMed] [Google Scholar]
- 14.Akizawa T., Nangaku M., Yonekawa T. Efficacy and safety of daprodustat compared with darbepoetin alfa in Japanese hemodialysis patients with anemia: a randomized, double-blind, phase 3 trial. Clin J Am Soc Nephrol. 2020;15:1155–1165. doi: 10.2215/CJN.16011219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura T., Nangaku M., Hamano T. ERA-EDTA 2019 Congress. 2019. Efficacy and safety of daprodustat compared with epoetin beta pegol in Japanese non-dialysis patients with anemia of chronic kidney disease: a 52-week, open-label, randomized controlled phase 3 trial [late-breaking clinical trial] Budapest, Hungary. [Google Scholar]
- 16.Akizawa T., Ueno M., Shiga T. Oral roxadustat three times weekly in ESA-naive and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: results from two phase 3 studies. Ther Apher Dial. 2020;24:628–641. doi: 10.1111/1744-9987.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akizawa T., Otsuka T., Reusch M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open-label study. Ther Apher Dial. 2020;24:115–125. doi: 10.1111/1744-9987.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akizawa T., Iwasaki M., Yamaguchi Y. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nangaku M., Kondo K., Kokado Y. Randomized, open-label, active-controlled (darbepoetin alfa), phase 3 study of vadadustat for treating anemia in non-dialysis-dependent CKD patients in Japan [abstract SA-PO229] J Am Soc Nephrol. 2019;30:823. [Google Scholar]
- 20.Nangaku M., Kondo K., Ueta K. Randomized, double-blinded, active-controlled (darbepoetin alfa), phase 3 study of vadadustat in CKD patients with anemia on hemodialysis in Japan [abstract TH-OR024] J Am Soc Nephrol. 2019;30:6. [Google Scholar]
- 21.Japan Tobacco Inc. JT receives manufacturing and marketing approval of Enaroy®tablets 2 mg・4 mg for the treatment of anemia associated with chronic kidney disease in Japan. https://www.jt.com/media/news/2020/pdf/20200925_E1.pdf Available at:
- 22.Chen N., Hao C., Liu B.C. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med. 2019;381:1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 23.Chen N., Hao C., Peng X. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019;381:1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 24.Miyake T., Kung C.K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252:5558–5564. [PubMed] [Google Scholar]
- 25.Jacobs K., Shoemaker C., Rudersdorf R. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 26.Lin F.K., Suggs S., Lin C.H. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg M.A., Glass G.A., Cunningham J.M. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci U S A. 1987;84:7972–7976. doi: 10.1073/pnas.84.22.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza G.L., Nejfelt M.K., Chi S.M. Hypoxia-inducible nuclear factors bind to an enhancer element located 3' to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G.L., Semenza G.L. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 31.Wang G.L., Jiang B.H., Rue E.A. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G.L., Semenza G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell P.H., Pugh C.W., Ratcliffe P.J. Inducible operation of the erythropoietin 3' enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firth J.D., Ebert B.L., Pugh C.W. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3' enhancer. Proc Natl Acad Sci U S A. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy A.P., Levy N.S., Wegner S. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 36.Jaakkola P., Mole D.R., Tian Y.M. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 37.Ivan M., Kondo K., Yang H. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 38.Yu F., White S.B., Zhao Q. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivan M., Haberberger T., Gervasi D.C. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99:13459–13464. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J., Zhao Q., Mooney S.M. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277:39792–39800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein A.C., Gleadle J.M., McNeill L.A. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 42.Bruick R.K., McKnight S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 43.Fandrey J., Schodel J., Eckardt K.U. Now a nobel gas: oxygen. Pflugers Arch. 2019;471:1343–1358. doi: 10.1007/s00424-019-02334-8. [DOI] [PubMed] [Google Scholar]
- 44.McIntosh B.E., Hogenesch J.B., Bradfield C.A. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol. 2010;72:625–645. doi: 10.1146/annurev-physiol-021909-135922. [DOI] [PubMed] [Google Scholar]
- 45.Kaelin W.G., Jr., Ratcliffe P.J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Mahon P.C., Hirota K., Semenza G.L. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lando D., Peet D.J., Gorman J.J. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rankin E.B., Biju M.P., Liu Q. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapitsinou P.P., Liu Q., Unger T.L. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koury M.J., Haase V.H. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015;11:394–410. doi: 10.1038/nrneph.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanghani N.S., Haase V.H. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26:253–266. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semenza G.L. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 53.Barrett T.D., Palomino H.L., Brondstetter T.I. Prolyl hydroxylase inhibition corrects functional iron deficiency and inflammation-induced anaemia in rats. Br J Pharmacol. 2015;172:4078–4088. doi: 10.1111/bph.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemeth E., Tuttle M.S., Powelson J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 55.Weiss G., Ganz T., Goodnough L.T. Anemia of inflammation. Blood. 2019;133:40–50. doi: 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganz T., Olbina G., Girelli D. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 57.van Swelm R.P.L., Wetzels J.F.M., Swinkels D.W. The multifaceted role of iron in renal health and disease. Nat Rev Nephrol. 2020;16:77–98. doi: 10.1038/s41581-019-0197-5. [DOI] [PubMed] [Google Scholar]
- 58.Ganz T., Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicolas G., Chauvet C., Viatte L. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pak M., Lopez M.A., Gabayan V. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashby D.R., Gale D.P., Busbridge M. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2010;95:505–508. doi: 10.3324/haematol.2009.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talbot N.P., Lakhal S., Smith T.G. Regulation of hepcidin expression at high altitude. Blood. 2012;119:857–860. doi: 10.1182/blood-2011-03-341776. [DOI] [PubMed] [Google Scholar]
- 63.Ganz T. Erythropoietic regulators of iron metabolism. Free Radic Biol Med. 2019;133:69–74. doi: 10.1016/j.freeradbiomed.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arezes J., Foy N., McHugh K. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132:1473–1477. doi: 10.1182/blood-2018-06-857995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volke M., Gale D.P., Maegdefrau U. Evidence for a lack of a direct transcriptional suppression of the iron regulatory peptide hepcidin by hypoxia-inducible factors. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Q., Davidoff O., Niss K. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest. 2012;122:4635–4644. doi: 10.1172/JCI63924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mastrogiannaki M., Matak P., Mathieu J.R. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica. 2012;97:827–834. doi: 10.3324/haematol.2011.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeh T.L., Leissing T.M., Abboud M.I. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem Sci. 2017;8:7651–7668. doi: 10.1039/c7sc02103h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hara K., Takahashi N., Wakamatsu A. Pharmacokinetics, pharmacodynamics and safety of single, oral doses of GSK1278863, a novel HIF-prolyl hydroxylase inhibitor, in healthy Japanese and Caucasian subjects. Drug Metab Pharmacokinet. 2015;30:410–418. doi: 10.1016/j.dmpk.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Kansagra K.A., Parmar D., Jani R.H. Phase I clinical study of ZYAN1, a novel prolyl-hydroxylase (PHD) inhibitor to evaluate the safety, tolerability, and pharmacokinetics following oral administration in healthy volunteers. Clin Pharmacokinet. 2018;57:87–102. doi: 10.1007/s40262-017-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukui K., Shinozaki Y., Kobayashi H. JTZ-951 (enarodustat), a hypoxia-inducibe factor prolyl hydroxylase inhibitor, stabilizes HIF-alpha protein and induces erythropoiesis without effects on the function of vascular endothelial growth factor. Eur J Pharmacol. 2019;859:172532. doi: 10.1016/j.ejphar.2019.172532. [DOI] [PubMed] [Google Scholar]
- 72.Pai S.M., Connaire J., Yamada H. A mass balance study of (14) C-labeled JTZ-951 (enarodustat), a novel orally available erythropoiesis-stimulating agent, in patients with end-stage renal disease on hemodialysis. Clin Pharmacol Drug Dev. 2020;9:728–741. doi: 10.1002/cpdd.752. [DOI] [PubMed] [Google Scholar]
- 73.Bottcher M., Lentini S., Arens E.R. First-in-man-proof of concept study with molidustat: a novel selective oral HIF-prolyl hydroxylase inhibitor for the treatment of renal anaemia. Br J Clin Pharmacol. 2018;84:1557–1565. doi: 10.1111/bcp.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beck H., Jeske M., Thede K. Discovery of molidustat (BAY 85-3934): a small-molecule oral HIF-prolyl hydroxylase (HIF-PH) inhibitor for the treatment of renal anemia. ChemMedChem. 2018;13:988–1003. doi: 10.1002/cmdc.201700783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shibata T., Nomura Y., Takada A. Evaluation of food and spherical carbon adsorbent effects on the pharmacokinetics of roxadustat in healthy nonelderly adult male Japanese subjects. Clin Pharmacol Drug Dev. 2019;8:304–313. doi: 10.1002/cpdd.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chavan A.B., Paulson S.K., Burke L. Effect of moderate hepatic impairment on pharmacokinetics of vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) [abstract TH-PO369] J Am Soc Nephrol. 2019;30:210. doi: 10.1002/cpdd.927. [DOI] [PubMed] [Google Scholar]
- 77.Akizawa T., Maeda K., Miyazawa Y. Phase 3 study to compare the efficacy and safety of enarodustat (JTZ-951), an oral HIF-PH inhibitor, with darbepoetin alfa in anemic patients with CKD receiving maintenance hemodialysis [abstract TH-PO1186] J Am Soc Nephrol. 2019;30:B4. [Google Scholar]
- 78.Akizawa T., Matsui A., Miyazawa Y. Phase 3 study to compare the efficacy and safety of enarodustat (JTZ-951), an oral HIF-PH inhibitor, with darbepoetin alfa in anemic patients with CKD not requiring dialysis [abstract TH-PO1185] J Am Soc Nephrol. 2019;30:B4. [Google Scholar]
- 79.Caltabiano S., Cizman B., Burns O. Effect of renal function and dialysis modality on daprodustat and predominant metabolite exposure. Clin Kidney J. 2019;12:693–701. doi: 10.1093/ckj/sfz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Macdougall I.C., Akizawa T., Berns J.S. Effects of molidustat in the treatment of anemia in CKD. Clin J Am Soc Nephrol. 2019;14:28–39. doi: 10.2215/CJN.02510218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson B.M., Stier B.A., Caltabiano S. Effect of food and gemfibrozil on the pharmacokinetics of the novel prolyl hydroxylase inhibitor GSK1278863. Clin Pharmacol Drug Dev. 2014;3:109–117. doi: 10.1002/cpdd.83. [DOI] [PubMed] [Google Scholar]
- 82.Shibata T., Nomura Y., Takada A. Evaluation of the effect of lanthanum carbonate hydrate on the pharmacokinetics of roxadustat in non-elderly healthy adult male subjects. J Clin Pharm Ther. 2018;43:633–639. doi: 10.1111/jcpt.12729. [DOI] [PubMed] [Google Scholar]
- 83.Eichner D., Van Wagoner R.M., Brenner M. lmplementation of the prolyl hydroxylase inhibitor roxadustat (FG-4592) and its main metabolites into routine doping controls. Drug Test Anal. 2017;9:1768–1778. doi: 10.1002/dta.2202. [DOI] [PubMed] [Google Scholar]
- 84.Groenendaal-van de Meent D., den Adel M., van Dijk J. Effect of multiple doses of omeprazole on the pharmacokinetics, safety, and tolerability of roxadustat in healthy subjects. Eur J Drug Metab Pharmacokinet. 2018;43:685–692. doi: 10.1007/s13318-018-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caltabiano S., Mahar K.M., Lister K. The drug interaction potential of daprodustat when coadministered with pioglitazone, rosuvastatin, or trimethoprim in healthy subjects. Pharmacol Res Perspect. 2018;6 doi: 10.1002/prp2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akizawa T., Iekushi K., Matsuda Y. P1866 to investigate the efficacy and safety of molidustat in non-dialysis patients with renal anemia who are not treated with erythropoiesis-stimulating agents: Miyabi Nd-C. Nephrol Dial Transplant. 2020;35:iii2177. [Google Scholar]
- 87.Akizawa T., Iekushi K., Matsuda Y. P1868 to investigate the efficacy and safety of molidustat in non-dialysis patients with renal anemia who are treated with erythropoiesis-stimulating agents: Miyabi Nd-M. Nephrol Dial Transplant. 2020;35:iii2179. [Google Scholar]
- 88.Akizawa T., Yamaguchi Y., Otsuka T. A phase 3, multicenter, randomized, two-arm, open-label study of intermittent oral dosing of roxadustat for the treatment of anemia in Japanese erythropoiesis-stimulating agent-naive chronic kidney disease patients not on dialysis. Nephron. 2020;144:372–382. doi: 10.1159/000508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akizawa T., Iwasaki M., Otsuka T. A phase 3, multicenter, randomized, open-label, active comparator conversion study of roxadustat in non-dialysis-dependent (NDD) patients with anemia in CKD [abstract PO0269] J Am Soc Nephrol. 2020;31:134. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esposito C., Csiky B., Tataradze A. Two phase 3, multicenter, randomized studies of intermittent oral roxadustat in anemic CKD patients on (PYRENEES) and not on (ALPS) dialysis [abstract SA-PO225] J Am Soc Nephrol. 2019;30:822. [Google Scholar]
- 91.Coyne D.W., Roger S.D., Shin S.K. ANDES: a phase 3, randomized, double-blind, placebo controlled study of the efficacy and safety of roxadustat for the treatment of anemia in CKD patients not on dialysis [abstract SA-PO228] J Am Soc Nephrol. 2019;30:822. [Google Scholar]
- 92.Fishbane S., El-Shahawy M.A., Pecoit-Filho R. OLYMPUS: a phase 3, randomized, double-blind, placebo-controlled, international study of roxadustat efficacy in patients with non-dialysis-dependent (NDD) CKD and anemia [abstract TH-OR023] J Am Soc Nephrol. 2019;30:6. [Google Scholar]
- 93.Barratt J., Andre B., Tataradze A. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a phase 3, randomised, open-label, active-controlled study. Nephrol Dial Transplant. 2020;35:iii101–iii102. doi: 10.1093/ndt/gfab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chertow G.M., PRO2TECT Author Group Global phase 3 clinical trials of vadadustat vs. darbepoetin alfa for treatment of anemia in patients with non-dialysis-dependent CKD [abstract FR-OR54] J Am Soc Nephrol. 2020;31:B2. [Google Scholar]
- 95.Akizawa T., Yamada H., Nobori K. Results from a phase 3 study comparing the efficacy and safety of molidustat vs. darbepoetin alfa in patients receiving hemodialysis and treated with erythropoiesis-stimulating agents (ESAs) [abstract PO2623] J Am Soc Nephrol. 2020;31:B4. [Google Scholar]
- 96.Provenzano R., Evgeny S., Liubov E. HIMALAYAS: a phase 3, randomized, open-label, active-controlled study of the efficacy and safety of roxadustat in the treatment of anemia in incident-dialysis patients [abstract TH-OR021] J Am Soc Nephrol. 2019;30:5. [Google Scholar]
- 97.Fishbane S., Pollock C.A., El-Shahawy M.A. ROCKIES: an international, phase 3, randomized, open-label, active-controlled study of roxadustat for anemia in dialysis-dependent CKD patients [abstract TH-OR022] J Am Soc Nephrol. 2019;30:6. [Google Scholar]
- 98.Charytan C., Manllo-Karim R., Martin E.R. SIERRAS: a phase 3, open-label, randomized, active-controlled study of the efficacy and safety of roxadustat in the maintenance treatment of anemia in subjects with ESRD on stable dialysis [abstract SA-PO227] J Am Soc Nephrol. 2019;30:822. [Google Scholar]
- 99.Eckardt K.U., INNO2VATE Author Group Global phase 3 clinical trials of vadadustat vs darbepoetin alfa for treatment of anemia in patients with dialysis-dependent CKD [abstract TH-OR01] J Am Soc Nephrol. 2020;31:1. [Google Scholar]
- 100.Brigandi R.A., Johnson B., Oei C. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: a 28-day, phase 2A randomized trial. Am J Kidney Dis. 2016;67:861–871. doi: 10.1053/j.ajkd.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 101.Holdstock L., Cizman B., Meadowcroft A.M. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J. 2019;12:129–138. doi: 10.1093/ckj/sfy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holdstock L., Meadowcroft A.M., Maier R. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27:1234–1244. doi: 10.1681/ASN.2014111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parmar D.V., Kansagra K.A., Patel J.C. Outcomes of desidustat treatment in people with anemia and chronic kidney disease: a phase 2 study. Am J Nephrol. 2019;49:470–478. doi: 10.1159/000500232. [DOI] [PubMed] [Google Scholar]
- 104.Akizawa T., Nangaku M., Yamaguchi T. A placebo-controlled, randomized trial of enarodustat in patients with chronic kidney disease followed by long-term trial. Am J Nephrol. 2019;49:165–174. doi: 10.1159/000496929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akizawa T., Macdougall I.C., Berns J.S. Iron regulation by molidustat, a daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor, in patients with chronic kidney disease. Nephron. 2019;143:243–254. doi: 10.1159/000502012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Besarab A., Provenzano R., Hertel J. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30:1665–1673. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen N., Qian J., Chen J. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017;32:1373–1386. doi: 10.1093/ndt/gfx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Provenzano R., Besarab A., Sun C.H. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol. 2016;11:982–991. doi: 10.2215/CJN.06890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akizawa T., Iwasaki M., Otsuka T. Roxadustat treatment of chronic kidney disease-associated anemia in Japanese patients not on dialysis: a phase 2, randomized, double-blind, placebo-controlled trial. Adv Ther. 2019;36:1438–1454. doi: 10.1007/s12325-019-00943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin E.R., Smith M.T., Maroni B.J. Clinical trial of vadadustat in patients with anemia secondary to stage 3 or 4 chronic kidney disease. Am J Nephrol. 2017;45:380–388. doi: 10.1159/000464476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pergola P.E., Spinowitz B.S., Hartman C.S. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90:1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 112.Nangaku M, Farag YMK, deGoma E, et al. Vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, for treatment of anemia of chronic kidney disease: two randomized phase 2 trials in Japanese patients [epub ahead of print]. Nephrol Dial Transplant. 10.1093/ndt/gfaa060. Accessed February 9, 2021. [DOI] [PubMed]
- 113.Akebia Therapeutics Akebia presents results from its PRO2TECT global phase 3 program of vadadustat for the treatment of anemia due to chronic kidney disease in adult patients not on dialysis during late-breaking session at American Society of Nephrology Kidney Week 2020 Reimagined. https://ir.akebia.com/news-releases/news-release-details/akebia-presents-results-its-pro2tect-global-phase-3-program Available at:
- 114.Kautz L., Jung G., Valore E.V. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meadowcroft A.M., Cizman B., Holdstock L. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J. 2019;12:139–148. doi: 10.1093/ckj/sfy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akizawa T., Tsubakihara Y., Nangaku M. Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol. 2017;45:127–135. doi: 10.1159/000454818. [DOI] [PubMed] [Google Scholar]
- 117.Bailey C.K., Caltabiano S., Cobitz A.R. A randomized, 29-day, dose-ranging, efficacy and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis. BMC Nephrol. 2019;20:372. doi: 10.1186/s12882-019-1547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akizawa T., Nangaku M., Yamaguchi T. Enarodustat, conversion and maintenance therapy for anemia in hemodialysis patients: a randomized, placebo-controlled phase 2b trial followed by long-term trial. Nephron. 2019;143:77–85. doi: 10.1159/000500487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Provenzano R., Besarab A., Wright S. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis. 2016;67:912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 120.Haase V.H., Chertow G.M., Block G.A. Effects of vadadustat on hemoglobin concentrations in patients receiving hemodialysis previously treated with erythropoiesis-stimulating agents. Nephrol Dial Transplant. 2019;34:90–99. doi: 10.1093/ndt/gfy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Besarab A., Chernyavskaya E., Motylev I. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Provenzano R., Fishbane S., Wei L.J. Pooled efficacy and cardiovascular (CV) analyses of roxadustat in the treatment of anemia in CKD patients on and not on dialysis [abstract FR-OR131] J Am Soc Nephrol. 2019;30:B1. [Google Scholar]
- 123.Fishbane S., Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2:1274–1282. doi: 10.2215/CJN.02380607. [DOI] [PubMed] [Google Scholar]
- 124.Wagner M., Alam A., Zimmermann J. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1573–1579. doi: 10.2215/CJN.00380111. [DOI] [PubMed] [Google Scholar]
- 125.Lonnberg M., Garle M., Lonnberg L. Patients with anaemia can shift from kidney to liver production of erythropoietin as shown by glycoform analysis. J Pharm Biomed Anal. 2013;81–82:187–192. doi: 10.1016/j.jpba.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 126.de Seigneux S., Lundby A.K., Berchtold L. Increased synthesis of liver erythropoietin with CKD. J Am Soc Nephrol. 2016;27:2265–2269. doi: 10.1681/ASN.2015050508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Flamme I., Oehme F., Ellinghaus P. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernhardt W.M., Wiesener M.S., Scigalla P. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shinfuku A., Shimazaki T., Fujiwara M. Novel compound induces erythropoietin secretion through liver effects in chronic kidney disease patients and healthy volunteers. Am J Nephrol. 2018;48:157–164. doi: 10.1159/000492181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Agarwal A.K. Iron metabolism and management: focus on chronic kidney disease. Kidney Int Suppl. 2021;11:46–57. doi: 10.1016/j.kisu.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Macdougall I.C., Hutton R.D., Cavill I. Poor response to treatment of renal anaemia with erythropoietin corrected by iron given intravenously. BMJ. 1989;299:157–158. doi: 10.1136/bmj.299.6692.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rutherford C.J., Schneider T.J., Dempsey H. Efficacy of different dosing regimens for recombinant human erythropoietin in a simulated perisurgical setting: the importance of iron availability in optimizing response. Am J Med. 1994;96:139–145. doi: 10.1016/0002-9343(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 133.Raichoudhury R., Spinowitz B.S. Treatment of anemia in difficult-to-manage patients with chronic kidney disease. Kidney Int Suppl. 2021;11:26–34. doi: 10.1016/j.kisu.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Del Balzo U., Signore P.E., Walkinshaw G. Nonclinical characterization of the hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat, a novel treatment of anemia of chronic kidney disease. J Pharmacol Exp Ther. 2020;374:342–353. doi: 10.1124/jpet.120.265181. [DOI] [PubMed] [Google Scholar]
- 135.Eltzschig H.K., Bratton D.L., Colgan S.P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taylor C.T., Doherty G., Fallon P.G. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J Clin Invest. 2016;126:3716–3724. doi: 10.1172/JCI84433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cizman B., Sykes A.P., Paul G. An exploratory study of daprodustat in erythropoietin-hyporesponsive subjects. Kidney Int Rep. 2018;3:841–850. doi: 10.1016/j.ekir.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haase V.H., Khawaja Z., Chan J. Vadadustat maintains hemoglobin (Hb) levels in dialysis-dependent chronic kidney disease (DD-CKD) patients independent of systemic inflammation or prior dose of erythropoiesis-stimulating agent (ESA) [abstract TH-PO960] J Am Soc Nephrol. 2016;27:318A. [Google Scholar]
- 139.Sandner P., Gess B., Wolf K. Divergent regulation of vascular endothelial growth factor and of erythropoietin gene expression in vivo. Pflugers Arch. 1996;431:905–912. doi: 10.1007/s004240050084. [DOI] [PubMed] [Google Scholar]
- 140.Seeley T.W., Sternlicht M.D., Klaus S.J. Induction of erythropoiesis by hypoxia-inducible factor prolyl hydroxylase inhibitors without promotion of tumor initiation, progression, or metastasis in a VEGF-sensitive model of spontaneous breast cancer. Hypoxia. 2017;5:1–9. doi: 10.2147/HP.S130526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.PMDA Japanese Pharmaceutical and Medical Devices Agency report on roxadustat. https://www.pmda.go.jp/files/000234811.pdf Available at:
- 142.Akebia Therapeutics Akebia presents results from its INNO2VATE global phase 3 program; demonstrated efficacy and cardiovascular safety of vadadustat for the treatment of anemia due to chronic kidney disease in adult patients on dialysis. https://ir.akebia.com/news-releases/news-release-details/akebia-presents-results-its-inno2vate-global-phase-3-program Available at:
- 143.Cozzolino M., Mangano M., Stucchi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33:iii28–iii34. doi: 10.1093/ndt/gfy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 145.Kapitsinou P.P., Haase V.H. Molecular mechanisms of ischemic preconditioning in the kidney. Am J Physiol Renal Physiol. 2015;309:F821–F834. doi: 10.1152/ajprenal.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hwang S., Nguyen A.D., Jo Y. Hypoxia-inducible factor 1alpha activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem. 2017;292:9382–9393. doi: 10.1074/jbc.M117.788562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shen G.M., Zhao Y.Z., Chen M.T. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem J. 2012;441:675–683. doi: 10.1042/BJ20111377. [DOI] [PubMed] [Google Scholar]
- 148.Caltabiano S., Collins J., Serbest G. A randomized, placebo- and positive-controlled, single-dose, crossover thorough QT/QTc study assessing the effect of daprodustat on cardiac repolarization in healthy subjects. Clin Pharmacol Drug Dev. 2017;6:627–640. doi: 10.1002/cpdd.342. [DOI] [PubMed] [Google Scholar]
- 149.FibroGen FibroGen announces positive phase 3 pooled roxadustat safety and efficacy results for treatment of anemia in chronic kidney disease. http://investor.fibrogen.com/news-releases/news-release-details/fibrogen-announces-positive-phase-3-pooled-roxadustat-safety-and Available at:
- 150.Lin M., Chen Y., Jin J. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Muller cells. Diabetologia. 2011;54:1554–1566. doi: 10.1007/s00125-011-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.West J.B., Schoene R.B., Milledge J.S. 4th ed. Hodder Arnold; London, England: 2007. High Altitude Medicine and Physiology. [Google Scholar]
- 152.Semenza G.L. The genomics and genetics of oxygen homeostasis. Annu Rev Genomics Hum Genet. 2020;21:183–204. doi: 10.1146/annurev-genom-111119-073356. [DOI] [PubMed] [Google Scholar]
- 153.Chappell J.C., Payne L.B., Rathmell W.K. Hypoxia, angiogenesis, and metabolism in the hereditary kidney cancers. J Clin Invest. 2019;129:442–451. doi: 10.1172/JCI120855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beck J., Henschel C., Chou J. Evaluation of the carcinogenic potential of roxadustat (FG-4592), a small molecule inhibitor of hypoxia-inducible factor prolyl hydroxylase in CD-1 mice and Sprague Dawley rats. Int J Toxicol. 2017;36:427–439. doi: 10.1177/1091581817737232. [DOI] [PubMed] [Google Scholar]
- 155.Adams D.F., Watkins M.S., Durette L. Carcinogenicity assessment of daprodustat (GSK1278863), a hypoxia-inducible factor (HIF)-prolyl hydroxylase inhibitor. Toxicol Pathol. 2020;48:362–378. doi: 10.1177/0192623319880445. [DOI] [PubMed] [Google Scholar]
- 156.Coyne D.W., Fishbane S., Pergola P.E. Roxadustat is not associated with an increased risk of neoplasm in patients with CKD and anemia [abstract TH-OR04] J Am Soc Nephrol. 2020;31:1. [Google Scholar]
- 157.Cowburn A.S., Crosby A., Macias D. HIF2alpha-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci U S A. 2016;113:8801–8806. doi: 10.1073/pnas.1602978113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kapitsinou P.P., Rajendran G., Astleford L. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol. 2016;36:1584–1594. doi: 10.1128/MCB.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shimoda L.A., Yun X., Sikka G. Revisiting the role of hypoxia-inducible factors in pulmonary hypertension. Curr Opin Physiol. 2019;7:33–40. doi: 10.1016/j.cophys.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kaneko M., Minematsu T., Yoshida M. Compression-induced HIF-1 enhances thrombosis and PAI-1 expression in mouse skin. Wound Repair Regen. 2015;23:657–663. doi: 10.1111/wrr.12312. [DOI] [PubMed] [Google Scholar]
- 161.Gordeuk V.R., Prchal J.T. Vascular complications in Chuvash polycythemia. Semin Thromb Hemost. 2006;32:289–294. doi: 10.1055/s-2006-939441. [DOI] [PubMed] [Google Scholar]
- 162.Kraus A., Peters D.J.M., Klanke B. HIF-1alpha promotes cyst progression in a mouse model of autosomal dominant polycystic kidney disease. Kidney Int. 2018;94:887–899. doi: 10.1016/j.kint.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 163.Liu J., Wei Q., Guo C. Hypoxia, HIF, and associated signaling networks in chronic kidney disease. Int J Mol Sci. 2017;18:950. doi: 10.3390/ijms18050950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mokas S., Lariviere R., Lamalice L. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int. 2016;90:598–609. doi: 10.1016/j.kint.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 165.Flamme I., Ellinghaus P., Urrego D. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186979. [DOI] [PMC free article] [PubMed] [Google Scholar]