Abstract
Background:
Familial sitosterolemia is a rare Mendelian disorder characterized by hyperabsorption and decreased biliary excretion of dietary sterols. Affected individuals typically have complete genetic deficiency – homozygous loss-of-function (LoF) variants – in the ATP-binding cassette transporter G5 (ABCG5) or G8 (ABCG8) genes and have substantially elevated plasma sitosterol and low-density lipoprotein cholesterol (LDL-C) levels. The impact of partial genetic deficiency of ABCG5 or ABCG8 – as occurs in heterozygous carriers of LoF variants – on LDL-C and risk of coronary artery disease (CAD) has remained uncertain.
Methods:
We first recruited nine sitosterolemia families, identified causative LoF variants in ABCG5 or ABCG8, and evaluated the associations of these ABCG5 or ABCG8 LoF variants with plasma phytosterols and lipid levels. We next assessed for LoF variants in ABCG5 or ABCG8 in CAD cases (n=29,321) versus controls (n=357,326). We tested the association of rare LoF variants in ABCG5 or ABCG8 with blood lipids and risk for CAD. Rare LoF variants were defined as protein-truncating variants with minor allele frequency less than 0.1% in ABCG5 or ABCG8.
Results:
In sitosterolemia families, seven pedigrees harbored causative LoF variants in ABCG5 and two pedigrees in ABCG8. Homozygous LoF variants in either ABCG5 or ABCG8 led to marked elevations in sitosterol and LDL-C. Of those sitosterolemia families, heterozygous carriers of ABCG5 LoF variants exhibited increased sitosterol and LDL-C levels compared to non-carriers. Within large-scale CAD case-control cohorts, prevalence of rare LoF variants in ABCG5 and in ABCG8 were approximately 0.1% each. ABCG5 heterozygous LoF variant carriers had significantly elevated LDL-C levels (25 mg/dL; 95% confidence interval [CI] 14 to 35; P=1.1×10−6) and were at two-fold increased risk of CAD (odds ratio 2.06, 95% CI 1.27 to 3.35; P=0.004). By contrast, ABCG8 heterozygous LoF carrier status was not associated with increased LDL-C or risk of CAD.
Conclusions:
Although familial sitosterolemia is traditionally considered as a recessive disorder, we observed that heterozygous carriers of a LoF variant in ABCG5 had significantly increased sitosterol and LDL-C levels and a two-fold increase in risk of CAD.
Keywords: Lipids and Cholesterol, Cardiovascular Disease, Genetics, coronary artery disease, lipids, cholesterol, sitosterol, population genetics
Introduction
Familial sitosterolemia (OMIM #210250) is a rare Mendelian disorder characterized by tendonous xanthomas, high plasma plant sterols and cholesterol levels, and increased risk of premature myocardial infarction.1–4 The ATP-binding cassette transporters G5 (ABCG5) and G8 (ABCG8) are the primary causal genes of familial sitosterolemia. ABCG5, ABCG8, and N-terminal Niemann-Pick C1 Like 1 (NPC1L1) determine the efflux and absorption of sterols on the surface of intestine and bile duct.5–8 NPC1L1 regulates sterol absorption whereas ABCG5 and ABCG8 form obligate heterodimers9 and coordinately control the excretion at both the brush border membrane of enterocyte and the apical membrane of hepatocytes.5, 10–12
Complete deficiency due to homozygous or compound heterozygous loss-of-function (LoF) variants in ABCG5 and/or ABCG8 causes markedly increased sitosterolemia and cholesterol levels, and potentially accelerated atherosclerotic disease as well.1–4 Genome-wide association studies also demonstrated that common genetic variants in the ABCG5-ABCG8 gene region were associated with phytosterols, low-density lipoprotein cholesterol (LDL-C),13 and risk of coronary artery disease (CAD).14 However, it is uncertain whether partial deficiency of ABCG5 or ABCG8 as conferred by LoF variants in the heterozygous state are also associated with higher cholesterol levels and an increased risk of CAD.
Here, we explored the metabolic and clinical consequences of ABCG5 or ABCG8 deficiency. We recruited probands and relatives in sitosterolemia families and assessed whether observed ABCG5 or ABCG8 causative LoF variants were associated with increased plasma phytosterols and LDL-C. We then analyzed exome sequences from 93,513 participants and genotype data from an additional 293,134 individuals to test whether carriers of rare heterozygous LoF variants in ABCG5 or ABCG8 had elevated blood lipids and risk of CAD.
Methods
The detail methods of this study are available in the Supplemental Material. The data that support the findings of this study are available from the corresponding author upon reasonable request. All participants in each study provided written informed consent for genetic studies. The institutional review board at Partners HealthCare (Boston, MA, USA) and each participating institution approved the study protocol. Analyses conducted using the UK Biobank Resource were conducted under Application Number 7089.
Results
ABCG5 or ABCG8 causative LoF variants, blood phytosterol and cholesterol levels in sitosterolemia families
We recruited nine Japanese families with sitosterolemia and sequenced the exons of the ABCG5 and ABCG8 genes in 47 individuals from these families (Supplemental Figure). Among the individuals within these families, 9 carried a homozygous or compound heterozygous ABCG5 or ABCG8 causative LoF variants while 26 carried a heterozygous ABCG5 or ABCG8 LoF causative variants. Of those, 10 out of 11 LoF variants were classified as pathogenic protein truncating or missense variants and one as likely pathogenic according to the American College of Medical Genetics variant classification guidelines (Supplemental Table 1).15 As expected, ABCG5 or ABCG8 homozygote or compound heterozygous LoF variant carriers showed very high sitosterol / Total Cholesterol (TC) ratios and LDL-C levels compared to non-carriers. Regarding heterozygous state, carriers of ABCG5 or ABCG8 heterozygous LoF variant carriers exhibited increased sitosterol / TC ratio compared with non-carriers. Moreover, ABCG5 heterozygous LoF variant carrier status was associated with an increased LDL-C level. (Table 1 and Figure 1).
Table 1.
Clinical characteristics by ABCG5 and ABCG8 variant carrier status in sitosterolemia families.
| Non-carrier | ABCG5 LoF variant | ABCG8 LoF Variant | |||
|---|---|---|---|---|---|
| Heterozygote | Homozygote | Heterozygote | Homozygote | ||
| N | 12 | 21 | 8 | 5 | 1 |
| Age, mean (SD) | 42.1 (19) | 40.2 (21) | 12.9 (20) | 45.2 (19) | 1 |
| Male sex, n (%) | 3 (25) | 12 (57) | 3 (38) | 2 (40) | 0 |
| Lipid profile | |||||
| Total cholesterol, mg/dL, median (IQR) | 181 (166–207) | 217 (185–276)* | 539 (247–700) | 293 (223–307) | 968 |
| LDL cholesterol, mg/dL, median (IQR) | 100 (84–143) | 145 (126–176)* | 408 (166–594) | 169 (121–169) | 832 |
| HDL cholesterol, mg/dL, median (IQR) | 58 (52–76) | 50 (40–71) | 47 (40–54) | 65 (46–65) | 46 |
| Triglycerides, mg/dL, median (IQR) | 85 (65–95) | 91 (55–151) | 188 (140–248)* | 154 (73–154) | 71 |
| Lipoproteins | |||||
| Apolipoprotein A1, mg/dL, median (IQR) | 148 (139–164) | 139 (126–150) | 106 (97–129) | NA | NA |
| Apolipoprotein B, mg/dL, median (IQR) | 73 (63–109) | 104 (90–118)* | 262 (198–303) | NA | NA |
| Non–cholesterol sterols | |||||
| Sitosterol, μg/mL, median (IQR) | 2.3 (1.8–2.8) | 7.8 (6.0–11)* | 102 (74–125)* | 9.9 (8.2–12)* | 36.5 |
| Campesterol, μg/mL, median (IQR) | 3.7 (3.3–5.2) | 13 (11–14)* | 70 (65–95)* | NA | NA |
| Sitosterol / TC, μg/mg, median (IQR) | 1.3 (1.1–1.4) | 3.5 (2.7–4.7)* | 22 (14–30)* | 4.4 (3.9–5.3)* | 3.8 |
| Campesterol / TC, μg/mg, median (IQR) | 2.6 (1.8–2.6) | 5.4 (4.8–6.6)* | 13 (7.5–18) | NA | NA |
P value < 0.025 compared to non-carrier controls. P values were calculated by linear regression adjusted by kinship matrix within each family using the log-transformed values.
Abbreviations: HDL, high-density lipoprotein; IQR, interquartile range; LDL, low density lipoprotein; SD, standard deviation; TC, total cholesterol.
Figure 1. Sitosterol to total cholesterol ratio and LDL-C levels among individuals with homozygous or heterozygous sitosterolemia, and unaffected controls in sitosterolemia families.
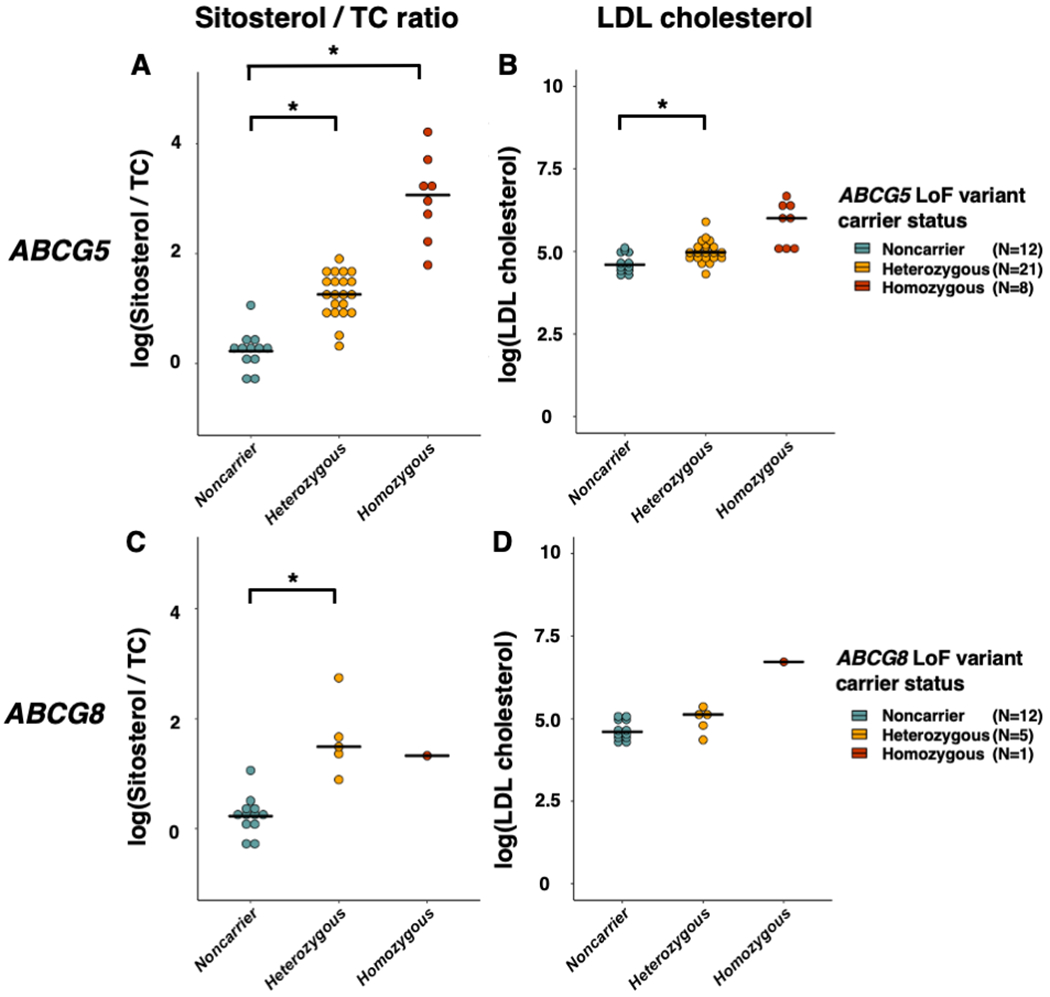
Each dot indicates an individual’s value. Each horizontal line represents a mean value for each carrier status. *: P value < 0.025.
ABCG5 or ABCG8 rare heterozygous LoF variation, blood lipids and risk for CAD in large cohorts
Next, we examined whether rare heterozygous LoF variant carrier status in ABCG5 or ABCG8 associated with higher blood lipids and elevated risk of CAD. We sequenced the protein coding regions of ABCG5 and ABCG8 in 93,513 individuals from three datasets: 48,576 participants from MIGen, 43,223 participants from UK Biobank and 1,714 participants from TSCA (Table 2). We detected 108 individuals harboring rare ABCG5 LoF alleles and the prevalence of ABCG5 heterozygous carrier status was 0.12%. (Supplemental Table 2). We also discovered 142 individuals who harbored rare ABCG8 LoF alleles, a heterozygous carrier prevalence also around 0.15% (Supplemental Table 3).
Table 2.
Clinical characteristics of participants in MIGen, UK Biobank and TSCA.
| MIGen | UK Biobank (Sequencing and Genotyping) |
TSCA | |
|---|---|---|---|
| N = 48,576 | N = 336,357 | N = 1,714 | |
| Age, years (SD) | 54 (10) | 57 (8) | 57.2 (13) |
| Male gender, n (%) | 41,203 (71) | 156,112 (46) | 1,140 (67) |
| BMI (SD), kg/m2 | 27 (5) | 27 (5) | 29 (6) |
| Current smoker, n (%) | 18,173 (33) | 25,802 (8) | 639 (37) |
| Medical history | |||
| Coronary artery disease, n (%) | 16,106 (33) | 12,073 (3) | 1,184 (69) |
| Hypertension, n (%) | 16,839 (35) | 153,535 (46) | 798 (47) |
| Type 2 diabetes, n (%) | 11,245 (22) | 15,770 (5) | 277 (16) |
| Lipid profile | |||
| Total cholesterol, mg/dL (SD) | 178 (60) | 222 (41) | 182 (51) |
| LDL cholesterol, mg/dL† (SD) | 109 (45) | 138 (34) | 108 (41) |
| HDL cholesterol, mg/dL (SD) | 36 (14) | 56 (15) | 43 (7) |
| Triglycerides, mg/dL (SD) | 154 (127) | 155 (90) | 135 (82) |
Abbreviations: MIGen, Myocardial Infarction Genetics consortium; SD, standard deviation; TSCA, TruSeq Custom Amplicon target resequencing studies.
Individuals carrying ABCG5 LoF variants had significantly increased TC (17 mg/dL; 95% confidence interval [CI], 13 to 32; P = 6.9×10−6) and LDL-C levels (25 mg/dL, 95% CI, 13 to 32; P = 1.1×10−6, Figure 2).
Figure 2. Effects of loss-of-function variants in ABCG5 on blood lipid profiles from MIGen and UK Biobank.
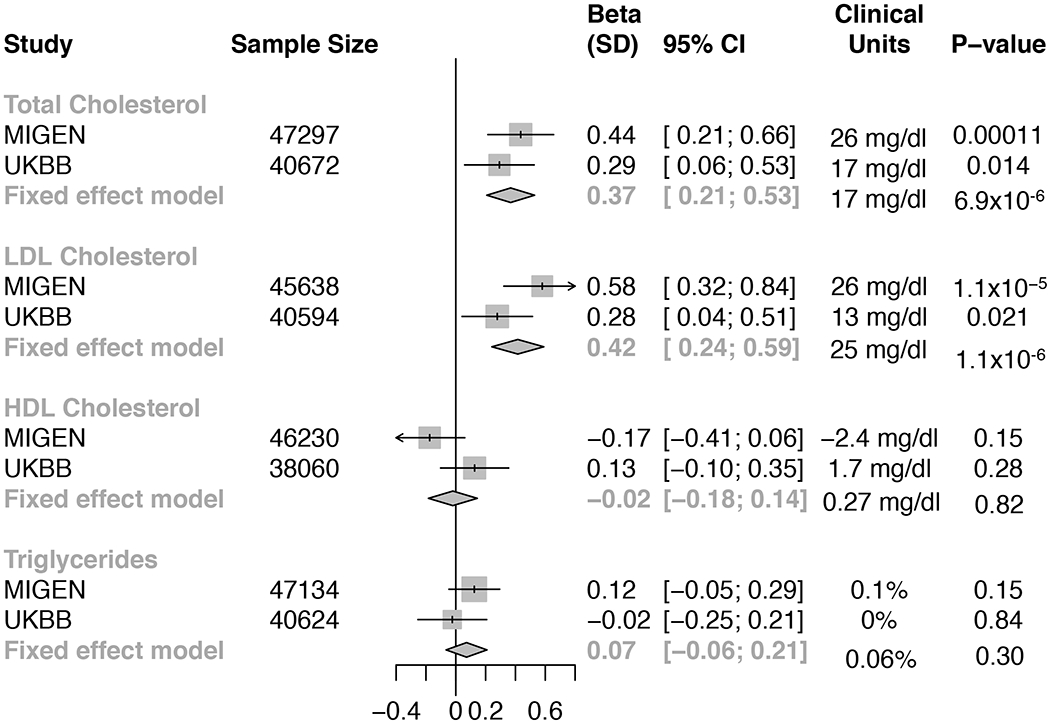
Effect sizes were calculated using linear regression adjusted by age, gender, study, case-control status, and first five principal components of ancestry. Triglycerides was natural log-transformed before analysis. Fixed-effects meta-analysis was applied to combine results.
Abbreviation: UKBB, UK biobank.
We investigated the association between rare ABCG5 heterozygous LoF variant carrier status and CAD risk using more than 380,000 participants from the three sequencing cohorts and additional UK biobank genotyping array-based cohort. We identified 34 carriers of ABCG5 heterozygous LoF variants among 29,321 CAD cases (0.12%) and 63 among 357,326 controls (0.018%). In a Cochran-Mantel-Haenszel fixed-effect meta-analysis, individuals carrying ABCG5 heterozygous LoF variants were at two-fold risk of CAD (Odds ratio [OR], 2.06; 95% CI, 1.27 to 3.35; P value = 0.004) (Figure 3). A similar effect estimate was noted in a meta-analysis of adjusted odds ratios derived using logistic regression (OR 2.04; 95% CI 1.28 to 3.26; P = 0.003).
Figure 3. Effect of LoF variants in ABCG5 on CAD.
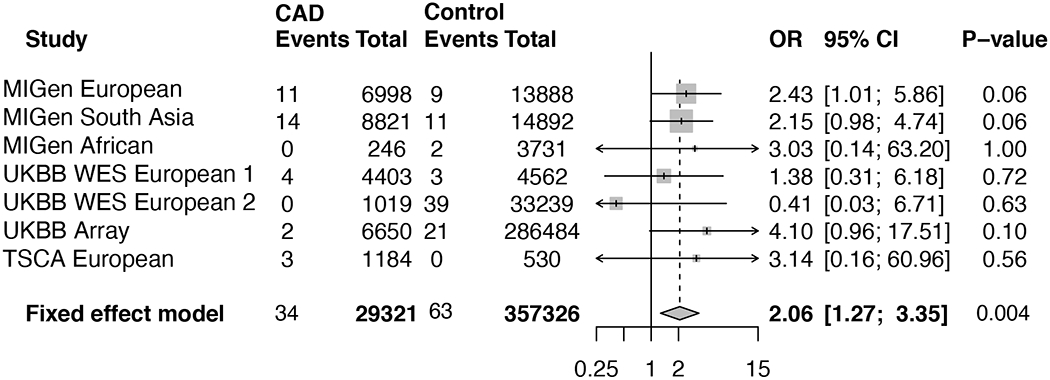
A meta-analysis across studies was performed using the Cochran–Mantel–Haenszel statistics for stratified 2-by-2 tables. MIGen, Myocardial Infarction Genetics Consortium.
Abbreviations: CAD, coronary artery disease; CI, confidence interval; LoF, loss-of-function; OR, odds ratio; TSCA, TruSeq Custom Amplicon target resequencing studies; UKBB, UK biobank.
In contrast to ABCG5, carriers of rare ABCG8 heterozygous LoF variants did not exhibit significant increase in any of blood lipids including LDL-C level (beta, 0.06; 95% CI, −0.09 to 0.22; P = 0.47) (Supplemental Table 4). Moreover, ABCG8 heterozygous LoF variant carrier status was not at elevated risk for CAD (OR, 0.79; 95% CI, 0.47 to 1.31; P = 0.36) (Supplemental Table 4).
We also explored whether the effect size of ABCG5 LoF variants on CAD risk was consistent with the impact on LDL-C. We observed a linear dose-response relationship between CAD risk and LDL-C change conferred by DNA sequence variants in LDLR, PCSK9, ABCG5, or ABCG8 (Supplemental Table 5). The effect of ABCG5 LoF variants on CAD (a doubling of in risk) was consistent with the estimate based on the impact in LDL-C (25 mg/dL) (Figure 4).
Figure 4. For LoF variants at the ABCG5, ABCG8, PCSK9 or LDLR genes, relationship between impact on LDL cholesterol levels and CAD risk.
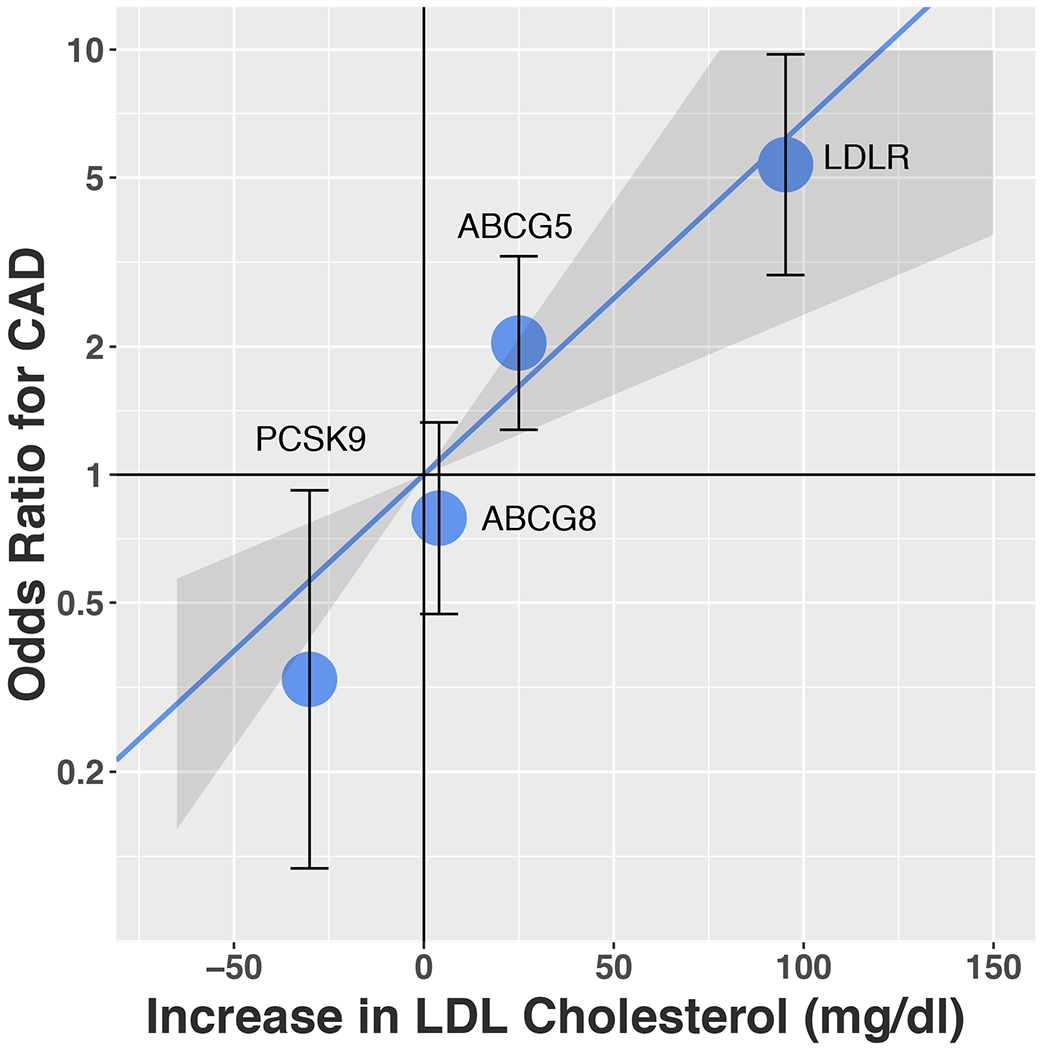
Solid line indicates a dose-response reference line with the 95% CI indicated by shadow. Each dot represents the effects of LoF variants in that gene on both LDL-C and CAD risk with 95% CI.
Abbreviations; CAD, coronary artery disease; LDL, low-density lipoprotein; LoF, loss-of-function.
Discussion
In this study, we evaluated whether rare heterozygous LoF variations in ABCG5 or ABCG8 were associated with blood lipid levels and CAD risk. We used two different approaches — sitosterolemia family-based analysis and population-based analysis from over 380,000 individuals — to test whether rare heterozygous LoF variants in ABCG5 or ABCG8 associated with phytosterols, lipids and CAD. We found that when compared to non-carriers, carriers of heterozygous LoF variants in ABCG5 had higher sitosterol and ~25 mg/dL higher LDL-C and were at two-fold risk of CAD.
These results permit several conclusions. First, individuals who carry rare heterozygous LoF variants in ABCG5 (but not ABCG8) have significantly elevated LDL-C levels and are at elevated risk for CAD. Although there have been reports of premature atherosclerosis among sitosterolemia patients with homozygous causative LoF variant carriers,2–4 it had been unclear if ABCG5 or ABCG8 partial deficiency also increases blood lipid levels and CAD risk. These findings imply that ABCG5 LoF variant carriers may derive clinical benefit from LDL-C lowering therapy. Importantly, the NPC1L1 inhibitor ezetimibe is known to reduce intestinal cholesterol and phytosterol absorption in patients with sitosterolemia, and could have increased efficacy in individuals with partial ABCG5 deficiency.16 Although both ABCG5 and ABCG8 are part of a heterodimer complex involved in the excretion of sterols from intestine to the lumen and from hepatocytes into the biliary tree, heterozygous ABCG5 deficiency seems to affect plasma LDL-C and CAD whereas heterozygous ABCG8 deficiency does not. Additional functional studies are needed to explain this new finding.17
Second, it has been unclear whether elevated plant sterol levels or elevated blood cholesterol levels cause atherosclerosis among patients with sitosterolemia.14 The impact of heterozygous LoF carriers status on risk of CAD was proportional to the effect on LDL-C elevation, suggesting that LDL-C rather than sitosterol itself is the key driver of the accelerated atherosclerosis. These findings were also consistent with a recent meta-analysis that did not observe a significant association between circulating sitosterol levels and risk of cardiovascular disease.18 Moreover, the effect size of ABCG5 heterozygous LoF variant carrier status on both blood lipids and CAD risk was consistent with predictions based on known familial hypercholesterolemia and hypobetalipoproteinemia variants (Figure 4, Supplemental Table 5).
This study has several limitations. First, detailed functional analyses of each observed variant predicted to cause LoF were not performed. Second, the number of ABCG8 causative LoF variant carriers in sitosterol families was relatively small and thus, our statistical power to evaluate an effect of heterozygous ABCG8 deficiency was more limited. Third, lipid measurements and CAD definition were different among study cohorts. However, the effect direction among studies was largely consistent and we observed little heterogeneity in the meta-analysis (I-squared of 0% for CAD).
In conclusion, approximately 0.1% of population carried rare LoF variants in ABCG5 and compared to non-carriers, ABCG5 heterozygous LoF variant carriers had elevated sitosterol and LDL-C levels and were at two-fold risk for CAD.
Supplementary Material
Acknowledgements
We would like to express our gratitude to all the participants and staff of KUMD, MIGen consortium, TSCA, and UK biobank for their outstanding contributions.
Source of Funding
This study was funded by the National Institutes of Health (R01 HL127564 and 5UM1HG008895). Exome sequencing in ATVB, PROCARDIS, OHS, PROMIS, Leicester, Lubeck was supported by 5U54HG003067 to SG. The ATVB Study was supported by a grant from RFPS-2007–3-644382 and Programma di ricerca Regione-Università 2010–2012 Area 1–Strategic Programmes–Regione Emilia-Romagna. Funding for the ESP-EOMI was provided by RC2 HL103010 (HeartGO), RC2 HL102923 (LungGO), and RC2 HL102924 (WHISP). Exome sequencing was performed through RC2 HL102925 (BroadGO) and RC2 HL102926 (SeattleGO). The JHS is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD). The authors also wish to thank the staffs and participants of the JHS. REGICOR study was supported by the Spanish Ministry of Economy and Innovation through the Carlos III Health Institute (Red Investigación Cardiovascular RD12/0042, PI09/90506), European Funds for Development (ERDF-FEDER), and by the Catalan Research and Technology Innovation Interdepartmental Commission (2014SGR240). Samples for the Leicester cohort were collected as part of projects funded by the British Heart Foundation (British Heart Foundation Family Heart Study, RG2000010; UK Aneurysm Growth Study, CS/14/2/30841) and the National Institute for Health Research (NIHR Leicester Cardiovascular Biomedical Research Unit Biomedical Research Informatics Centre for Cardiovascular Science, IS_BRU_0211_20033). GMP is supported by K01HL125751 and R03HL141439.
Disclosures
A.N. received consulting fees from CureApp Inc. and speaker fees from Daiichi Sankyo, Kowa, and Otsuka pharmaceutical. He is a co-founder of CureApp Institute. C.A.E. reports personal fees from Navitor Pharma and Novartis. H.T. received honoraria from Astellas Pharma, Amgen Astellas BioPharma, Bayer Japan, Boehringer Ingelheim, Daiichi Sankyo, Kowa, Mitsubishi Tanabe Pharma Corporation, MSD, Sanofi, Sanwa Kagaku Kenkyusho, Sumitomo Dainippon Pharma, and Takeda Pharmaceutical. M.K. received honoraria from Amgen Astellas Biopharma, Astellas Pharma, Daiichi Sankyo, Kowa Pharmaceutical, MSD, Pfizer Japan and Sanofi. A.V.K. has served as a consultant or received honoraria from Color Genomics, Illumina, and Navitor Pharmaceuticals, received grant support from the Novartis Institute for Biomedical Research and IBM Research, and reports a patent related to a genetic risk predictor (20190017119). S.K. is an employee of Verve Therapeutics. He is a founder of Maze Therapeutics, Verve Therapeutics, and San Therapeutics. He holds equity in Catabasis and San Therapeutics. He is a member of the scientific advisory boards for Regeneron Genetics Center and Corvidia Therapeutics; served as a consultant for Acceleron, Eli Lilly, Novartis, Merck, Novo Nordisk, Novo Ventures, Ionis, Alnylam, Aegerion, Huag Partners, Noble Insights, Leerink Partners, Bayer Healthcare, Illumina, Color Genomics, MedGenome, Quest, and Medscape; and reports patents related to a method of identifying and treating a person having a predisposition to or afflicted with cardiometabolic disease (20180010185) and a genetic risk predictor (20190017119). The remaining authors have nothing to disclose.
Non-standard Abbreviations and Acronyms
- ABCG5
ATP-binding cassette transporters G5
- ABCG8
ATP-binding cassette transporters G8
- CAD
coronary artery disease
- CI
confidence interval
- LDL-C
low-density lipoprotein cholesterol
- LoF
Loss-of-function
- NPC1L1
N-terminal Niemann-Pick C1 Like 1
- OR
odds ratio
- TC
total cholesterol
Footnotes
: These authors contributed equally
References
- 1.Bhattacharyya AK and Connor WE. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J Clin Invest. 1974;53:1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salen G, Horak I, Rothkopf M, Cohen JL, Speck J, Tint GS, Shore V, Dayal B, Chen T, Shefer S. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J Lipid Res. 1985;26:1126–33. [PubMed] [Google Scholar]
- 3.Katayama T, Satoh T, Yagi T, Hirose N, Kurita Y, Anzai T, Asakura Y, Yoshikawa T, Mitamura H, Ogawa S. A 19-year-old man with myocardial infarction and sitosterolemia. Intern Med. 2003;42:591–4. [DOI] [PubMed] [Google Scholar]
- 4.Tada H, Nohara A, Inazu A, Sakuma N, Mabuchi H, Kawashiri MA. Sitosterolemia, Hypercholesterolemia, and Coronary Artery Disease. J Atheroscler Thromb. 2018;25:783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altmann SW, Davis HR Jr., Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. [DOI] [PubMed] [Google Scholar]
- 6.Davis HR Jr., Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–92. [DOI] [PubMed] [Google Scholar]
- 7.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R and Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–5. [DOI] [PubMed] [Google Scholar]
- 8.Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–82. [DOI] [PubMed] [Google Scholar]
- 10.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidambi S, Patel SB. Sitosterolaemia: pathophysiology, clinical presentation and laboratory diagnosis. J Clin Pathol. 2008;61:588–94. [DOI] [PubMed] [Google Scholar]
- 13.Webb TR, Erdmann J, Stirrups KE, Stitziel NO, Masca NG, Jansen H, Kanoni S, Nelson CP, Ferrario PG, Konig IR, et al. Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. J Am Coll Cardiol. 2017;69:823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teupser D, Baber R, Ceglarek U, Scholz M, Illig T, Gieger C, Holdt LM, Leichtle A, Greiser KH, Huster D, et al. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ Cardiovasc Genet. 2010;3:331–9. [DOI] [PubMed] [Google Scholar]
- 15.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B, Multicenter Sitosterolemia Study Group. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Mitsche MA, Lutjohann D, Cohen JC, Xie XS, Hobbs HH. Relative roles of ABCG5/ABCG8 in liver and intestine. J Lipid Res. 2015;56:319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genser B, Silbernagel G, De Backer G, Bruckert E, Carmena R, Chapman MJ, Deanfield J, Descamps OS, Rietzschel ER, Dias KC, et al. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2012;33:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


