Abstract
Global attention on early child development, inclusive of those with disability, has the potential to translate into improved action for the millions of children with developmental disability living in low- and middle-income countries. Nurturing care is crucial for all children, arguably even more so for children with developmental disability. A high proportion of survivors of neonatal conditions such as prematurity and neonatal encephalopathy are affected by early child developmental disability. The first thousand days of life is a critical period for neuroplasticity and an important window of opportunity for interventions, which maximize developmental potential and other outcomes. Since 2010, our group has been examining predictors, outcomes, and experiences of neonatal encephalopathy in Uganda. The need for an early child intervention program to maximize participation and improve the quality of life for children and families became apparent. In response, the “ABAaNA early intervention program,” (now re-branding as ‘Baby Ubuntu’) a group participatory early intervention program for young children with developmental disability and their families, was developed and piloted. Piloting has provided early evidence of feasibility, acceptability, and impact and a feasibility trial is underway. Future research aims to develop programmatic capacity across diverse settings and evaluate its impact at scale.
Keywords: cerebral palsy, developmental disability, early intervention, neonatal encephalopathy, neurodevelopment, Uganda
THE GLOBAL STRATEGY for Women's, Children's and Adolescent's Health (2016-2030) highlights the need for all newborns to thrive beyond survival and advocates for early child development (ECD) interventions to achieve this (United Nations, 2015). Furthermore, the Sustainable Development Goals' commitment to “leaving no one behind” mandates that all children have access to quality ECD interventions (United Nations, 2016). With such high-level attention, ECD for survivors of newborn conditions at high risk of disability is now a global health priority.
Since 1990 and the start of the Millennium Development Goal era, there has been substantial progress for under-five child mortality, with a reduction from 12.6 million deaths in 1990 to 5.3 million in 2018, but progress for neonatal deaths has been much slower (UN Inter-Agency Group for Child Mortality Estimation, 2019). Neonatal deaths account for 47% of under-five child mortality, with huge disparities between regions and countries; the greatest burden lying in sub-Saharan Africa where 41% of the world's newborn deaths occur (UN Inter-Agency Group for Child Mortality Estimation, 2019). Until recently, newborn health was neglected on the global health agenda. A “trickle down” effect was expected through strengthening maternal and child health programs; however, the relative burden of newborn deaths continued to rise. The Sustainable Development Goals adopted in 2015 include the first explicit global target for newborn survival and aim to address inequitable progress (United Nations, 2016).
The Global Strategy's three core pillars “Survive,” “Thrive,” and “Transform” have moved the focus beyond improving mortality rates alone to now encompass longer term developmental outcomes (United Nations, 2015). Approximately 30 million neonates each year experience complications around the time of birth, which can potentially impact long-term health and development in survivors (World Health Organization, 2018a). A systematic review estimated that 40% of survivors of intrauterine or neonatal insults suffered neurodevelopmental impairment (NDI) in at least one domain (Mwaniki, Atieno, Lawn, & Newton, 2012). The three leading causes of newborn deaths—preterm birth complications, intrapartum-related events (deaths related to neonatal encephalopathy [NE]), and sepsis—also contribute to a high burden of adverse neurodevelopmental outcomes (Blencowe et al., 2012; Kohli-Lynch et al., 2017; Lee et al., 2013; Liu et al., 2016; Seale et al., 2013).
GLOBAL PERSPECTIVES ON EARLY CHILD DEVELOPMENT AND DISABILITY
The World Health Organization Nurturing Care Framework comprises five components to support ECD: good health, adequate nutrition, security and safety, opportunities for early learning, and responsive caregiving (World Health Organization, 2018b). It lays out three “levels” of support for ECD: (1) “universal,” delivered at the societal level, (2) “targeted,” for children at risk of developmental delay/disability, and (3) “indicated,” specialized for children with specific additional needs (World Health Organization, 2018b). Although “universal” and “targeted” interventions have gained traction worldwide with governmental and nongovernmental organizations alike, “indicated” interventions have not received the same attention (Arregoces et al., 2019). The Archives of Diseases in Childhood series “Informing design and implementation for early child development programmes” presents data from the Saving Brains portfolio on ECD interventions and highlights the lack of attention placed on “indicated” interventions for children with disabilities (Arregoces et al., 2019; Banerjee, Britto, Daelmans, Goh, & Peterson, 2019; Boggs et al., 2019; Cavallera et al., 2019; Kohli-Lynch, Tann, & Ellis, 2019; Milner, Bernal Salazar, et al., 2019; Milner, Bhopal, et al., 2019; World Health Organization, 2012). A conservative estimate of the number of children younger than 5 years with developmental disabilities, published in 2019, is 53 million globally (Olusanya, Davis, & Kassebaum, 2019; Olusanya et al., 2018). Ninety-five percent of those children live in low- and middle-income countries (LMICs) (Olusanya et al., 2018).
EARLY DETECTION OF DEVELOPMENTAL DELAY AND DISABILITY
To deliver effective interventions, early and accurate detection of developmental delay and disability is required. A review of early diagnosis of cerebral palsy (CP) supported the use of the Hammersmith Infant Neurological Examination (HINE) to detect disabilities from 3 months, although data were lacking from LMICs (Novak et al., 2017). It is also recommended that high-risk infants are screened with a child development assessment screening tool (Bellman, Byrne, & Sege, 2013). However, although a plethora of tools are available, the predictive validity of such tools for infants in LMICs is not yet clear (Boggs et al., 2019). Moreover, if an infant “screens positive” for developmental delay, a formal comprehensive developmental assessment should be undertaken, but challenges exist in delivering this service. In addition, training in administration of assessments is resource-intensive and costs of assessment kits are often very high (Bellman et al., 2013; Boggs et al., 2019). Lack of standardized and validated norms in LMIC settings means that even when comprehensive assessments are performed, results can be difficult to interpret.
EARLY INTERVENTION FOR DEVELOPMENTAL DISABILITY
The first thousand days of life is a critical period for neuroplasticity and an important window of opportunity for interventions, which maximize developmental potential (Guralnick, 2019; Shonkoff & Meisels, 2000). Nurturing care is crucial for all children, arguably even more so for children with developmental delay or disability, as ECD interventions have the potential to limit impairments following newborn brain injury (Banks, Kuper, & Polack, 2017; Morgan, Novak, & Badawi, 2013; Wallander et al., 2010). For example, in high-income countries, early environmental enrichment has been found to improve motor function in young children (Morgan et al., 2013; Palmer, Shapiro, & Wachter, 1993). However, there is little evidence on interventions developed specifically for children with developmental disabilities—called early childhood interventions (ECIs)—in LMICs. Given the focus of the article, we will primarily discuss ECI for child developmental disability rather than delay. Some small studies on ECI in LMICs have been done, but intervention content and outcome measures vary considerably, and the studies generally focus on a wider population of “at-risk” children, rather than children with developmental disability (Bao, Sun, Yu, & Sun, 1997; de Souza, Sardessai, Joshi, Joshi, & Hughes, 2006; Wallander et al., 2014). More and larger studies on ECI in LMICs specifically addressing the five components of the Nurturing Care Framework are urgently needed.
EPIDEMIOLOGY OF CHILDHOOD DEVELOPMENTAL DISABILITY
Quantifying disability is complex yet vitally important (Durkin, 2002; Kostanjsek et al., 2013; McDermott & Turk, 2011). Estimates rely on population-based surveys, multinational databases, systematic reviews, and hospital records from countries able to gather such data. Thereafter, data are frequently extrapolated for countries where fewer, smaller, or poorer quality datasets are available (Olusanya et al., 2019). The datasets upon which extrapolations are based are helpful but do have drawbacks. Reliance on small studies with cross-sectional data leads to an underestimate of very young children with severe developmental disabilities due to the high mortality rate in this population (Durkin, 2002). Underreporting of developmental disabilities such as neurodevelopmental disorders, and emotional and behavioural disorders in LMICs, also affects estimates (Damiano & Forssberg, 2019). Furthermore, lack of routine hearing and vision assessments in preschool children in LMICs may lead to underestimation of sensorineural impairments (Courtright, Hutchinson, & Lewallen, 2011; Olusanya, 2012). Another challenge with quantifying childhood developmental disability in LMICs is that the scale of disability, including physical, emotional, social, and financial consequences, experienced by the child and their family may not be adequately captured by the severity of their functional impairment alone (Nakamanya, Siu, Lassman, Seeley, & Tann, 2015). For example, a child living in rural Uganda with the same physical impairment as a child living in the U.K. may experience greater disability due to environmental, social, and economic conditions. Despite these challenges, accurate counting of children with developmental disabilities, and the effect on families and the wider community, is crucial. Epidemiological data must be improved, not least to enable reliable measurement of quality, coverage, and outcomes of ECIs.
EARLY CHILD DEVELOPMENT AND DISABILITY IN UGANDA
In Uganda neonatal mortality has fallen by two thirds over the past 20 years (UNICEF, 2018). However, the prevalence of developmental disability remains difficult to quantify. It is estimated that 3.5% of all children aged 2–4 years and 7.5% of children aged 5–17 years are living with a disability in Uganda (Uganda Bureau of Statistics, 2019). However, only 10% of these children are estimated to have access to rehabilitative services (UNICEF, 2014). Empowering mothers to access care and promote inclusion and participation is vital to encourage early development; in Uganda 51% of women reported full participation in household decision-making, an improvement from 38% in 2011 (Uganda Bureau of Statistics [UBOS] and ICF, 2018). Although there has been a strong focus on newborn survival at local and governmental levels, until recently the longer-term outcomes of newborn conditions have received less attention. Recognition of the need for integrated policy on ECD, which requires a multisectoral and multidisciplinary approach including health, education, sanitation, empowerment, and safeguarding, has also been evidenced (Ministry for Gender, 2016; Ministry of Health, Government of Uganda, 2015).
CEREBRAL PALSY IN UGANDA
Kakooza-Mwesige et al. (2017) reported on a population-based study examining CP prevalence and presumed time of injury in children in a rural population in eastern Uganda. They reported a prevalence of CP of 2.9 per 1,000 children in a rural region of Uganda; however, for diagnostic precision this estimate did not include children younger than 2 years. Given the significant proportion (63%) of children with CP who were younger than 2 years in a study based in Kampala (Hassell, Tann, Idro, & Robertson, 2018), the true prevalence of childhood CP in Uganda is likely to be much higher than 2.9 per 1,000. The etiology of CP in Uganda is also incompletely understood. Seventy-three percent of Kakooza-Mwesige and colleagues' cohort were described as “neonatal full-term cases,” with no further detail provided on the children's risk factors for CP; for example, NE or newborn infections. In an observational study of 130 children with CP attending outpatient clinics and pediatric wards at Mulago National Referral Hospital in Uganda's capital, Kampala, 88 caregivers (68%) reported a history consistent with NE or sepsis, whereas 76% attributed their child's CP to severe neonatal illness (Hassell et al., 2018). Another study by Kakooza-Mwesige, investigating CT scan images in 78 Ugandan children with CP aged 2–12 years, found that 44% had primary gray matter injuries, a pattern seen in perinatal complications, whereas 4% had primary white pattern injuries, more frequently seen in preterm newborns (Kakooza-Mwesige et al., 2016). It is likely that the etiology of CP in Uganda differs from high-income countries, with a higher proportion attributable to intrapartum-related events or newborn infections as opposed to preterm birth, but more data are needed to confirm this.
NEONATAL ENCEPHALOPATHY RESEARCH: RISKS, OUTCOMES, AND MATERNAL EXPERIENCES IN UGANDA
Deaths relating to NE are the third leading cause of mortality in children younger than 5 years and contribute substantially to long-term neurological morbidity worldwide (Lawn et al., 2014). Globally, approximately 1.15 million infants every year develop NE and of these, more than 400,000 survive with NDI; around three-quarters of whom live in sub-Saharan Africa (Lee et al., 2013). As obstetric and neonatal care improves in LMICs, the number of children surviving the neonatal period is increasing. Neonates affected by a perinatal insult typically present with NE, a descriptive term for a clinical constellation of neurological dysfunctions in the term infant (Lee et al., 2013). Long-term sequelae among NE survivors include CP, global developmental delay (GDD), vision and hearing impairments, and seizure disorders (Dixon et al., 2002). However, despite its major contribution to global neonatal mortality and morbidity, information on risks, outcomes, and interventions for NE in the lowest resource settings in Africa is lacking.
Risk factors for neonatal encephalopathy and the role of infection
Previous studies have examined risk factors for NE, largely, but not exclusively, in high-resource settings (Badawi et al., 1998a, 1998b; Ellis, Manandhar, Manandhar, & Costello, 2000; Martinez-Biarge, Diez-Sebastian, Wusthoff, Mercuri, & Cowan, 2013). Clinical and preclinical studies have suggested strong linkages between perinatal infections, NE, and CP (Fleiss et al., 2015); however, it remained unclear whether these effects are mediated through, or mediators of, established risk factors, or whether they act independently through alternative pathways (Fleiss et al., 2015; Hagberg et al., 2015). If implicated however, the high prevalence of perinatal infections in African settings means that their contribution to CP may be substantial and potentially amenable to intervention.
To investigate this, between 2010 and 2013, our group conducted a case–control study examining the role of infection in NE in Uganda to determine risk factors for NE in a low-resource African setting, known as the “ABAaNA study” (Associations Between Birth Asphyxia and infection amongst Newborns in Africa: Perinatal risk factors for neonatal encephalopathy in Uganda—“Abaana” meaning “Children” in the local language Luganda) (Tann, Nakakeeto, et al., 2018). We aimed to improve the understanding of the causal chain of predisposing factors, exposures and events, and specifically the role of common perinatal infections, in NE development (Tann, Nakakeeto, et al., 2018).
Between September 2011 and October 2012, 210 case and 409 control infants were recruited. Cases were term infants with NE (defined by a Thompson score of >5; Thompson et al., 1997), and controls were contemporaneous randomly selected unaffected term babies. Data from the antepartum, intrapartum, and postpartum periods were collected with a repository of biological samples (placenta, maternal, and neonatal blood) used to identify perinatal infections and quantify infection markers (Tann, Nakakeeto, et al., 2018). Laboratory work included placental histological diagnosis of chorioamnionitis and funisitis (fetal vasculitis) and a newly developed panel of species-specific bacterial real-time polymerase chain reaction to identify neonatal blood pathogens (Tann et al., 2014). Amongst cases, 8.9% showed evidence of early bacteremia using a combination of blood culture and molecular biological techniques. (Tann, Nakakeeto, et al., 2018). Findings showed strong associations with large effect measure between perinatal infection and/or, inflammation exposures such as early neonatal bacteremia and funisitis and NE. These associations remained strong even after sequential adjustment for potential confounding factors from the preconception, antepartum, and intrapartum periods, suggesting an independent risk association with NE in this population (Tann, Nakakeeto, et al., 2018). Recent retrospective studies from Europe mirrored our findings, suggesting they are not specific to African settings. A high prevalence of placental infection and inflammation was reported among infants with NE in the Netherlands (Harteman et al., 2013), and a further study from Ireland found funisitis to be a significant risk factor for moderate–severe NE (risk ratio 9.29 [95% confidence interval, 1.36, 63.06]) (Hayes et al., 2013).
Nature and timing of brain injury
In sub-Saharan Africa, information on the timing and nature of neonatal brain injury from intrapartum events and their relation to mortality is lacking. Among the ABAaNA study participants, early cranial ultrasound (cUS) imaging was performed to examine the nature and timing of perinatal brain injury (Tann et al., 2016). Among neonates with NE, 184 were scanned at <36 hr of age (median age 11.5 hr). In this facility-based African population, signs of major evolving injury, suggestive of a relatively recent as opposed to an established antepartum brain insult, affected one in five encephalopathic infants (21.2%). Because changes seen on cUS are recognized to take some time to develop after the original brain insult (Eken, Toet, Groenendaal, & de Vries, 1995), this may imply that the injury pathway begins several hours before delivery, or a very severe acute exposure to hypoxia–ischemia. There was no significant increase in white matter injury seen on early cUS among encephalopathic infants with neonatal infection. In infants with encephalopathy, the presence of a major evolving brain injury tripled the likelihood of dying in the neonatal period (Tann et al., 2016). Abnormalities on cUS performed later on day 4 or 5 were also found to be associated with moderate–severe NDI at 2 years of age (Tann, Webb, et al., 2018).
Early childhood outcomes after neonatal encephalopathy and the need for early intervention
Retrospective cohort studies in low-income countries estimate 40%–50% of CP is secondary to “birth asphyxia” (Belonwu, Gwarzo, & Adeleke, 2009; Singhi & Saini, 2013; Singhi, Ray, & Suri, 2002); however, no prospective studies had described comprehensive 2-year neurodevelopmental outcomes among survivors of NE in low-resource settings in Africa. Participants from the ABAaNA case–control study were followed up as a cohort through infancy and early childhood. To the best of our knowledge, this represents the largest cohort study to follow, comprehensively assess, and describe early childhood outcomes among infants with NE in sub-Saharan Africa.
The primary aim of the prospective cohort study was to determine the neurodevelopmental status of survivors of NE at 27–30 months of age in Uganda compared with a comparison cohort without NE and to describe the type and severity of NDI in NE survivors (Tann, Webb, et al., 2018). In addition, we aimed to describe nutritional status and identify clinical predictors for impairment outcomes and important early clinical risk factors for death and disability after NE in this low-resource sub-Saharan African setting.
This prospective cohort study documented high mortality (40.3%) to 27 months among children with NE, with most deaths occurring in the neonatal period (Tann, Webb, et al., 2018). Only 41.6% survived and were disability free at 2 years. A third of survivors were affected by NDIs (29.3%), including CP (around two thirds of NDI) and severe GDD without motor impairment (around one third of NDI). Bilateral spastic CP was common and strongly associated with multidomain impairment, vision and hearing loss, microcephaly, undernutrition, and childhood seizures. However, even children without overt impairment had an increased risk of delay in motor functioning compared with non-NE children. The high burden of early mortality seen among children with severe impairment in this setting means that the contribution of NE to childhood disability may be underestimated. Simple clinical risk factors for NDI were identified, including continued difficulty with feeding at neonatal discharge and a small head circumference at 1 year that may facilitate targeted follow-up of infants at greatest risk (Tann, Webb, et al., 2018).
The study highlighted the urgent need to develop prevention strategies for NE, as well as programs for early intervention for those with neurodisability to maximize functional and nutritional outcomes and quality of life (QoL) for affected children and their caregivers. Longer-term follow-up studies to determine whether further problems emerge as educational, motor, and social demands increase are also urgently needed for us to understand the true impact of NE on children of school age and beyond.
Maternal experiences of caring
During the early phases of the ABAaNA early outcomes cohort study, it became apparent that the maternal experience of caring for a young child with early signs of developmental disability presented a set of unique and difficult challenges for affected families. In response, and to understand the caregiver experience better, a qualitative study was conducted among the cohort families, examining the experiences of caring for a child affected by NDI after NE in Uganda (Nakamanya et al., 2015). Between September 2011 and October 2012, small group and individual in-depth interviews were conducted with mothers recruited to the ABAaNA study and data were analyzed thematically (Nakamanya et al., 2015). Mothers reported that caring for an infant with a disability was often complicated by substantial social, emotional, and financial difficulties and was associated with high levels of emotional stress, feelings of isolation, and stigma. Improved social support and the opportunity to share experiences with other similarly affected mothers were associated with a more positive maternal caring experience. High transport costs, lack of paternal support, and poor availability of counseling and support services were all found to be barriers to maternal health care seeking. The tremendous need for an early child intervention program to maximize participation and improve the QoL for affected mothers and their infants became apparent.
DEVELOPING AN EARLY INTERVENTION PROGRAM FOR YOUNG CHILDREN WITH DEVELOPMENTAL DISABILITY IN UGANDA
To fill this gap, a new parent training program called “Getting to Know Cerebral Palsy” was developed and launched through a partnership between the London School of Hygiene and Tropical Medicine (LSHTM) and “CBM” (Christian Blind Mission)—an international disability and development organization (Ubuntu Hub, 2019). The program aims to increase parental knowledge and skills and promotes a participatory learning approach with an emphasis on the empowerment of caregivers across a broad spectrum of impairment for children aged 2–12 years (Zuurmond et al., 2018). To date, more than 200 individuals and organizations from more than 60 countries have downloaded the material, and 300 members participate in a community of practice for working with children with CP.
In 2014, funding was secured to develop and pilot an intervention program targeting much younger infants (<2 years) with NDI. This new program went on to be developed around the principles of the “Getting to Know Cerebral Palsy” program of promoting family empowerment through shared experiences and peer support to maximize child developmental potential, optimize health, and improve QoL. The primary aim of this formative work was to develop and evaluate a modular, facilitated, participatory early intervention program (EIP) and examine for early evidence of feasibility, acceptability, and impact on child and family QoL, using a mixed-methods evaluation.
Term survivors of NE were identified from a register of neonates with NE at Mulago National Referral Hospital. Infants with moderate–severe NDI at 6 months, defined as Griffiths Mental Developmental Scales II (Amod, Cockcroft, & Soellaart, 2007), developmental quotient (DQ) <70 and/or a HINE score of <60 (Romeo, Cioni, Palermo, Cilauro, & Romeo, 2013), were enrolled to the EIP after caregivers provided written informed consent. A convenience sample of 35 infants was enrolled. Data were collected using standardized case report forms and double entered into an Microsoft Access database. Pre- and post-EIP comparisons in QoL and nutritional status evaluated intervention impact. HINE assessments were videoed and reviewed to ensure correctly classification of impairment. Mid-upper arm circumference (MUAC), MUAC z-scores, weight-for-age z-scores, and height-for-age z-score assessed nutritional status, and family QoL was assessed using the Pediatric Quality of Life Inventory tool 2.0 (PedsQL), Family Impact Module (Varni, Burwinkle, Seid, & Skarr, 2003).
In parallel, feasibility, acceptability, and impact on family QoL were evaluated among recruited caregivers, program facilitators, and study staff. Impact of disability, confidence level of the parents, level of participation in family and community life, and experience of stigma and discrimination were evaluated through pre and postprogram in-depth interviews (16 purposively selected participants) and focus group discussions among caregivers completing the EIP (n = 28) in the local language. A thematic coding framework was developed, and transcriptions were coded and summarized. Ethical approval was obtained from Ugandan local, national, and U.K. research ethics committees.
The ABAaNA early intervention program
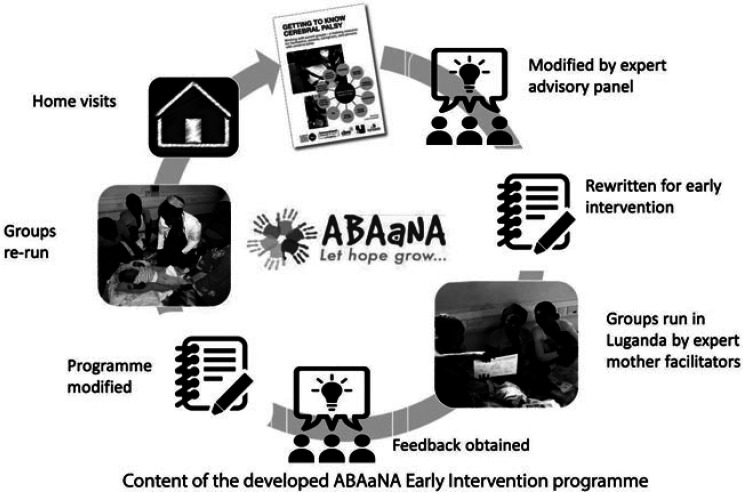
A 10-modular program was developed, adapted from Getting to Know Cerebral Palsy and supported by an Expert Advisory Group (EAG) which included parents of children with developmental disability, Disabled Persons Organisations, and experts in early intervention and child development. Program development was guided by recommendations on the development of complex interventions (Campbell et al., 2000) and included fast-cycle learning through participant, facilitator and EAG feedback to inform each round of development (Figure 1).
Figure 1.
Iterative process of program development.
The developed EIP is a community-based, peer-led group program with caregivers at a community level, using a participatory approach driven by adult learning theory (Knowles, 1984). The following themes run through the program:
Promoting inclusion and participation of children with disability within the family and community
Maximizing a child's developmental potential, optimizing health and QoL
Promoting empowerment of caregivers through information sharing and peer support
Understanding the child and family experience, and particularly addressing stigma
Promoting the human rights of children with disability
Enrolled families are encouraged to share experiences through discussion and reflection, prioritize problems, and identify solutions together. “Expert parents” act as facilitators of the group sessions and are themselves parents of children with a developmental disability. EIP facilitators undergo 5 days of training followed by regular supervision and mentorship with existing master facilitators, themselves usually trained therapists. Each EIP group involves 6–10 families; groups are selected depending on locality for ease of attendance. The training is divided into 10 modules covering understanding disability, positioning and carrying, feeding, mobilizing, communication, play, everyday activities, and experiences in the local community (Nampijja et al., 2019). Modules are delivered weekly or fortnightly, and last 2–3 hours including time for facilitated discussion. The entire program is designed to be delivered over 6 months including at least one home visit conducted by the expert parent facilitator. The program manual is freely available to download (https://www.ubuntu-hub.org).
Early evidence of feasibility, acceptability, and impact
In total, 112 infants were screened of which 35 were eligible to enter the program and 28 completed pre- and postprogram evaluation (2 withdrew, 2 moved away, 2 attended <7 EIP modules, and 1 died prior to reassessment). Of the 28 completing both evaluations, 16 (57%) were male. Mean age at recruitment was 6.7 months (SD of 0.7 months) and at postintervention assessment was 11.1 (SD of 1.6) months. A mean of 8.7 modules were attended (range 7–10).
Table 1 describes neurodevelopmental status, nutritional parameters, and family QoL at baseline and post-intervention. The majority of infants recruited to join the EIP had severe NDI (DQ <55) (75% at baseline and 86% at post-intervention). No significant change was seen in DQ postintervention; however, a statistically significant increase in HINE score was seen (Table 1). Feeding difficulties among recruited infants were common (32%). At baseline, three infants (11%) had MUAC consistent with acute malnutrition (two moderate, <12.5 cm, one severe, <11.5 cm), around a third were underweight, and a third stunted (Table 1).
Table 1. Comparison of Total Pediatric Evaluation of Quality-of-Life Family Impact Total Score and Subscores, Neurodevelopmental Assessments, and Nutritional Status Before and After Intervention (n = 28).
| PedsQL Family Impact Module Scale | Median Score (IQR) Pre-EIP | Median Score (IQR) Post-EIP | Median Difference (IQR) | p Value* |
|---|---|---|---|---|
| Total PedsQL score | 63.2 (50-83.7) | 79.2 (72.4-94.1) | +15.6 (0.9, 30.4) | .001 |
| Subscores | ||||
| Physical functioning | 65.8 (54.2-82.3) | 77.1 (62.5-91.7) | +14.6 (−3.1, 29.2) | .034 |
| Emotional Functioning | 37.5 (16.3-63.8) | 67.5 (45.0-100.0) | +30.0 (7.5, 45.0) | <.001 |
| Social functioning | 75.0 (40.6-92.2) | 78.1 (57.8-100) | +12.5 (−10.9, 25.0) | .13 |
| Cognitive functioning | 85 (65-98.8) | 97.5 (76.3-100) | 0 (−8.8, 27.5) | .10 |
| Communication | 66.7 (33.3-100) | 83.3 (66.7-100) | +12.5 (0, 41.7) | .014 |
| Worry | 57.5 (40-97.5) | 87.5 (66.3-100) | +22.5 (0, 40.0) | .002 |
| Daily activities | 75 (41.7-100) | 95.8 (50-100) | +4.2 (0, 25.0) | .07 |
| Family relationships | 77.5 (51.3-100) | 100 (86.3-100) | +2.5 (0, 43.8) | .015 |
| Neurodevelopment | Median Score Pre-EIP | Median Score Post-EIP | Median Difference (IQR) | p Value* |
| HINE (IQR) | 37.3 (29.3-46.4) | 39.7 (31.4-54.8) | +4.0 (−1.4, 12.5) | .02 |
| Global DQ (IQR) | 27.3 (12.3-55.9) | 26.9 (15.2-45.0) | +0.4 (−9.5, 4.3) | .52 |
| Nutritional Status | Pre-EIP | Post-EIP | Mean Difference [95% CI] | p Value |
| MUAC (cm), M (SD) | 14.0 (1.2) | 14.5 (1.2) | 0.5 [0.04, 0.9] | .03** |
| MUAC <12.5 cm, moderate acute malnutrition (%) | 3 (10.7%) | 0 (0%) | .12*** | |
| MUAC <11.5 cm, severe acute malnutrition (%) | 1 (3.6%) | 0 (0%) | .50*** | |
| MUAC-for-age z-score, M (SD) | −0.2 (1.1) | 0.0 (1.0) | −0.2 [−0.6, 0.1] | .15** |
| MUAC-for-age z-score <−2 (%) | 2 (7%) | 0 (0%) | .16*** | |
| Weight-for-age z-score, M (SD) | −1.6 (1.3) | −1.9 (1.2) | −0.3 [−0.6, 0.0] | .07** |
| Weight-for-age z-score <−2 (%) | 10 (36%) | 13 (46%) | .08*** | |
| Height-for-age z-score, M (SD) | −1.3 (1.5) | −2.0 (1.7) | −0.7 [−1.1, −0.2] | .009** |
| Height-for-age z-score <−2 (%) | 10 (36%) | 17 (61%) | .01*** |
Note. CI = confidence interval; DQ = developmental quotient; EIP = early intervention program; HINE = Hammersmith Infant Neurological Examination; IQR = interquartile range; MUAC = mid-upper arm circumference; PedsQL = Pediatric Quality of Life Inventory.
*Wilcoxon signed rank test.
**Paired t-test.
***McNemar's test.
Total PedsQL score increased significantly (p < .05) post-intervention with specific improvements in physical functioning, emotional functioning, worry, communication, and family relationships (see Table 1). Although no children met the MUAC criteria for acute malnutrition post-intervention, the proportion who were underweight and with stunting significantly increased (Table 1), with nearly one half underweight and two thirds stunted at postintervention.
Qualitative findings
The qualitative data show that caregivers and facilitators reported the program to be feasible and acceptable. Evidence of feasibility of the EIP included timely recruitment of infants and caregivers to the program and positive reports from caregivers on the timing, location, and group format of the modules. High levels of attendance provided evidence of acceptability for families, with all participants attending seven or more modules of the 10-module program.
Caregivers described a high desire for knowledge about their child's condition and valued the peer support provided by other caregivers and the facilitators in the group format of the program. All participants had experienced negative psychosocial effects including exclusion, hopelessness, blame, and regret. Biological symptoms such as poor sleep, low appetite, and exhaustion were also frequently reported. Caregivers commonly described that sharing their experiences, and hearing those of others, relieved their stress and isolation, built their confidence in parenting and their hope for the future. Barriers to attendance however were also reported and these included lack of financial support, often relating to fathers' abandonment, and competing family and work commitments. Regarding program content and delivery, caregivers responded positively to the participatory nature of the training. Particular value was placed on sessions relating to positioning, feeding, communication, and understanding their child's underlying condition. Improved understanding led to reductions in self-stigma and provided caregivers with a shared narrative that reduced the stigma they experienced from others. Caregivers frequently felt that the content led to improvement in their child's progress and encouraged them to continue to practice at home as well as to share information with other families with children with a disability.
In summary, early evaluation of this facilitated, participatory, EIP for infants at high-risk of NDI showed significant improvements in family QoL in a pre-/postevaluation and was feasible and acceptable to families and facilitators. Caregivers reported increased knowledge, improved family relationships, reduced self-stigma, raised hope, and enhanced emotional well-being. The absence of a fall in post-EIP DQ and small but significant increase in HINE may be considered encouraging because declines in developmental attainment are commonly seen over this period for children with severe neurodevelopmental difficulties. Although no participants fulfilled criteria for acute malnutrition post-intervention, there was a significant increase in the proportion who were underweight and, or, stunted post-intervention, highlighting the early nutritional risks to children with developmental disability. The findings, and particularly those regarding impact, were limited by the small sample size and pre-/poststudy design. Randomized studies are needed to fully assess intervention feasibility, acceptability, and effectiveness.
Subsequent development and future plans
A pilot feasibility trial of the EIP is currently underway across both urban and rural settings in Uganda (Nampijja et al., 2019). Data collection is complete, and analysis of the trial data underway. In autumn 2019, the ABAaNA EIP was launched on the Ubuntu-Hub web platform (https://www.ubuntu-hub.org/resources/abaana/). In early 2021 the EIP will be harmonised with the Ubuntu programs for older children and will be re-branded as the ‘Baby Ubuntu’ program. An 11th program module has subsequently been developed and launched to support families and communities in addressing discrimination and exclusion.
Programmatic roll-out in parts of central and western Uganda and eastern Rwanda is underway in partnership with local nongovernmental organizations, Partners in Health Rwanda and the Rwandan Ministry of Health. In early 2020, delivery of the EIP was disrupted by the coronavirus-2019 (COVID-19), pandemic, which has had far-reaching implications for children and families. The indirect effect of “lockdown” restrictions in LMICs has been substantial, and has disproportionally affected the most vulnerable, including children with developmental disability. Social distancing, transport restrictions, and reduced employment opportunities (exacerbating poverty) have substantially impacted program access for families. In Rwanda, to ensure continued access to the EIP, a risk assessment and recovery plan was instituted to enable restarting of the program and provide vital support to families. African CDC guidance (Africa Centres for Disease Control and Prevention, 2020a, 2020b) and facilitated discussions among EIP facilitators and families were used to develop and successfully implement an integrated package of physical distancing, hand and equipment hygiene, and personal protective equipment (face masks) to the EIP groups to minimize the risk of transmission of COVID-19.
During the feasibility trial and early program roll-out, important barriers to EIP scale-up were identified. These included poverty, gender inclusion, and poor levels of literacy among caregivers. In response, the program is being strengthened through addition of a livelihood component to support families financially and development of an enhanced media platform aims to improve accessibility, promote paternal engagement, and protect program fidelity at scale. This new, enhanced programmatic package aims to be complete by early 2021.Ongoing and future research aims to develop programmatic capacity across diverse settings in Africa and evaluate impact at scale.
Footnotes
Portions of this article were based on a Keynote Presentation at the International Society on Early Intervention Conference, Sydney, Australia, June 2019.
We thank the parents, guardians, and children who participated in the studies; the ABAaNA study team and investigators specifically Nicola Robertson, Alison Elliott, Jennifer Kurinczuk, Margaret Nakakeeto, Cornelia Hagmann, Miriam Martinez-Biarge, and Frances Cowan; all on the special care unit and labor ward at Mulago Hospital including Jamiir Mugalu, Jolly Nankunda, Natasha Nyombi, Flaviah Namiiro, and Anita Muhumuza; the ABAaNA EIP expert advisory group including Himali DaSilva, Maya Asir, Hannah Kuper, Maria Zuurmond, Sue Fry, Mel Adams, Tina Gerwicke, Tracey Smythe, Cathy Morgan, Angelina Kakooza, Julius Ssekyewa, and our lead EIP Facilitators and Expert Mothers Lucy Mbabazi and Betty Nyangoma. Thank you also to the MRC/UVRI & LSHTM Uganda Research Unit, Entebbe, for their administrative and laboratory support as well as the Wellcome Bloomsbury Centre, LSHTM, and Nigel Klein, Kathryn Harris, and the Camelia Botnar Laboratories at Great Ormond Street Hospital NHS Trust. We are immensely grateful for the support and collaboration of the International Centre for Evidence in Disability in their support of the development of the Early Intervention Programme & UCLH Charities in supporting its roll out. Thanks also go to the UCL Institute for Women's Health for hosting the early outcomes study, Adara Development & Kiwoko Hospital for their belief and investment in our program and our funders Grand Challenges Canada, Cerebral Palsy Alliance Research Institute, the Wellcome Trust, and the Tropical Health Education Trust.
The first author (C.T.) has received funding from Cerebral Palsy Alliance Australia for studies examining neurodevelopmental outcomes after neonatal encephalopathy cohorts (career development IRG 2012-CT) and to develop the ABAaNA Early Intervention (PG2214). The ABAaNA Early Intervention Trial is funded by Saving Brains, Grand Challenges Canada (grant number 1707-08687). C.T. has previously received funding from a Wellcome Trust/Bloomsbury Research Training Fellowship (094016/Z/10/Z) to conduct the original ABAaNA case–control study and the early neurodevelopmental follow-up of the cohorts.
The authors declare no conflict of interest.
REFERENCES
- Africa Centres for Disease Control and Prevention (Africa CDC). (2020a). Guidance on community physical distancing during COVID-19 pandemic: Physical distancing, social support. Retrieved from https://africacdc.org/download/guidance-on-community-social-distancing-during-covid-19-outbreak/
- Africa Centres for Disease Control and Prevention (Africa CDC). (2020b). Simple instructions on how to use a face mask. Retrieved from https://africacdc.org/download/simple-instructions-on-how-to-use-a-face-mask/
- Amod Z., Cockcroft K., Soellaart B. (2007). Use of the 1996 Griffiths Mental Development Scales for infants: A pilot study with a Black, South African sample. Journal of Child and Adolescent Mental Health, 19, 123–130. doi:10.2989/17280580709486647 [DOI] [PubMed] [Google Scholar]
- Arregoces L., Hughes R., Milner K. M., Ponce Hardy V., Tann C, Upadhyay A., Lawn J. E. (2019). Accountability for funds for nurturing care: What can we measure? Archives of Disease in Childhood, 104(Suppl. 1), S34–S42. doi:10.1136/archdischild-2018-315429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi N., Kurinczuk J. J., Keogh J. M., Alessandri L. M., O'Sullivan F., Burton P. R., Stanley F. J. (1998a). Antepartum risk factors for newborn encephalopathy: The Western Australian case-control study. BMJ, 317(7172), 1549–1553. doi:10.1136/bmj.317.7172.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi N., Kurinczuk J. J., Keogh J. M., Alessandri L. M., O'Sullivan F., Burton P. R., Stanley F. J. (1998b). Intrapartum risk factors for newborn encephalopathy: The Western Australian case-control study. BMJ, 317(7172), 1554–1558. doi:10.1136/bmj.317.7172.1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Britto P. R., Daelmans B., Goh E., Peterson S. (2019). Reaching the dream of optimal development for every child, everywhere: What do we know about “how to”? Archives of Disease in Childhood, 104(Suppl. 1), S1–S2. doi:10.1136/archdischild-2019-317087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L. M., Kuper H., Polack S. (2017). Poverty and disability in low- and middle-income countries: A systematic review. PLoS One, 12(12), e0189996. doi:10.1371/journal.pone.0189996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X., Sun S., Yu R., Sun J. (1997). Early intervention improves intellectual development in asphyxiated newborn infants. Intervention of Asphyxiated Newborn Infants Cooperative Research Group. Chinese Medical Journal (Engl.), 110(11), 875–878. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9772422 [PubMed] [Google Scholar]
- Bellman M., Byrne O., Sege R. (2013). Developmental assessment of children. BMJ, 346, e8687. doi:10.1136/bmj.e8687 [DOI] [PubMed] [Google Scholar]
- Belonwu R. O., Gwarzo G. D., Adeleke S. I. (2009). Cerebral palsy in Kano, Nigeria—a review. Nigerian Journal of Medicine, 18, 186–189. doi:10.4314/njm.v18i2.45062 [DOI] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Oestergaard M. Z., Chou D., Moller A. B., Narwal R., Lawn J. E. (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet, 379(9832), 2162–2172. doi:10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- Boggs D., Milner K. M., Chandna J., Black M., Cavallera V., Dua T., Lawn J. E. (2019). Rating early child development outcome measurement tools for routine health programme use. Archives of Disease in Childhood, 104(Suppl. 1), S22–S33. doi:10.1136/archdischild-2018-315431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M., Fitzpatrick R., Haines A., Kinmonth A. L., Sandercock P., Spiegelhalter D., Tyrer P. (2000). Framework for design and evaluation of complex interventions to improve health. BMJ, 321, 694–696. doi:10.1136/bmj.321.7262.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallera V., Tomlinson M., Radner J., Coetzee B., Daelmans B., Hughes R., Dua T. (2019). Scaling early child development: What are the barriers and enablers? Archives of Disease in Childhood, 104(Suppl. 1), S43–S50. doi:10.1136/archdischild-2018-315425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtright P., Hutchinson A. K., Lewallen S. (2011). Visual impairment in children in middle- and lower-income countries. Archives of Disease in Childhood, 96(12), 1129–1134. doi:10.1136/archdischild-2011-300093 [DOI] [PubMed] [Google Scholar]
- Damiano D., Forssberg H. (2019). Poor data produce poor models: Children with developmental disabilities deserve better. Lancet Global Health, 7(2), e188. doi:10.1016/S2214-109X(18)30498-4 [DOI] [PubMed] [Google Scholar]
- de Souza N., Sardessai V., Joshi K., Joshi V., Hughes M. (2006). The determinants of compliance with an early intervention programme for high-risk babies in India. Child Care Health Dev, 32(1), 63–72. doi:10.1111/j.1365-2214.2006.00576.x [DOI] [PubMed] [Google Scholar]
- Dixon G., Badawi N., Kurinczuk J. J., Keogh J. M., Silburn S. R., Zubrick S. R., Stanley F. J. (2002). Early developmental outcomes after newborn encephalopathy. Pediatrics, 109(1), 26–33. doi:10.1542/peds.109.1.26 [DOI] [PubMed] [Google Scholar]
- Durkin M. (2002). The epidemiology of developmental disabilities in low-income countries. Mental Retardation and Developmental Disabilities Research Reviews, 8, 206–211. doi:10.1002/mrdd.10039 [DOI] [PubMed] [Google Scholar]
- Eken P., Toet M. C., Groenendaal F., de Vries L. S. (1995). Predictive value of early neuroimaging, pulsed Doppler and neurophysiology in full term infants with hypoxic-ischaemic encephalopathy. Archives of Disease in Childhood Fetal and Neonatal Edition, 73(3), F75–F80. doi:10.1136/fn.73.2.f75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M., Manandhar N., Manandhar D. S., Costello A. M. (2000). Risk factors for neonatal encephalopathy in Kathmandu, Nepal, a developing country: Unmatched case-control study. BMJ, 320, 1229–1236. doi:10.1136/bmj.320.7244.1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss B., Tann C. J., Degos V., Sigaut S., Van Steenwinckel J., Schang A. L., Gressens P. (2015). Inflammation-induced sensitization of the brain in term infants. Developmental Medicine and Child Neurology, 57(Suppl. 3), 17–28. doi:10.1111/dmcn.12723 [DOI] [PubMed] [Google Scholar]
- Guralnick M. J. (2019). Effective early intervention: The developmental systems approach. Baltimore, MD: Brookes Publishing Co. [Google Scholar]
- Hagberg H., Mallard C., Ferriero D. M., Vannucci S. J., Levison S. W., Vexler Z. S., Gressens P. (2015). The role of inflammation in perinatal brain injury. Nature Reviews: Neurology, 11(4), 192–208. doi:10.1038/nrneurol.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteman J. C., Nikkels P. G., Benders M. J., Kwee A., Groenendaal F., de Vries L. S. (2013). Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. Journal of Pediatrics, 163(4), 968–995.e2. doi:10.1016/j.jpeds.2013.06.010 [DOI] [PubMed] [Google Scholar]
- Hassell J., Tann C., Idro R., Robertson N. J. (2018). Contribution of perinatal conditions to cerebral palsy in Uganda. Lancet Global Health, 6, e248–e249. doi:10.1016/S2214-109X(18)30041-X [DOI] [PubMed] [Google Scholar]
- Hayes B. C., Cooley S., Donnelly J., Doherty E., Grehan A., Madigan C., King M. D. (2013). The placenta in infants >36 weeks gestation with neonatal encephalopathy: A case control study. Archives of Disease in Childhood Fetal and Neonatal Edition, 98(3), F233–F239. doi:10.1136/archdischild-2012-301992 [DOI] [PubMed] [Google Scholar]
- Kakooza-Mwesige A., Andrews C., Peterson S., Wabwire Mangen F., Eliasson A. C., Forssberg H. (2017). Prevalence of cerebral palsy in Uganda: A population-based study. Lancet Global Health, 5(12), e1275–e1282. doi:10.1016/S2214-109X(17)30374-1 [DOI] [PubMed] [Google Scholar]
- Kakooza-Mwesige A., Byanyima R. K., Tumwine J. K., Eliasson A. C., Forssberg H., Flodmark O. (2016). Grey matter brain injuries are common in Ugandan children with cerebral palsy suggesting a perinatal aetiology in full-term infants. Acta Paediatrica, 105(6), 655–664. doi:10.1111/apa.13352 [DOI] [PubMed] [Google Scholar]
- Knowles M. S. (1984). The adult learner: A neglected species (ed. 3rd). Houston, TX: Gulf Pub. Co., Book Division. [Google Scholar]
- Kohli-Lynch M., Russell N. J., Seale A. C., Dangor Z., Tann C. J., Baker C. J., Lawn J. E. (2017). Neurodevelopmental impairment in children after group b streptococcal disease worldwide: Systematic review and meta-analyses. Clinical Infectious Diseases, 65(Suppl. 2), S190–S199. doi:10.1093/cid/cix663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli-Lynch M., Tann C. J., Ellis M. E. (2019). Early intervention for children at high risk of developmental disability in low- and middle-income countries: A narrative review. International Journal of Environmental Research and Public Health, 16(22), 4449. doi:10.3390/ijerph16224449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostanjsek N., Good A., Madden R. H., Ustun T. B., Chatterji S., Mathers C. D., Officer A. (2013). Counting disability: Global and national estimation. Disability and Rehabilitation, 35(13), 1065–1069. doi:10.3109/09638288.2012.720354 [DOI] [PubMed] [Google Scholar]
- Lawn J. E., Blencowe H., Oza S., You D., Lee A. C., Waiswa P., . . . Lancet Every Newborn Study Group. (2014). Every newborn: Progress, priorities, and potential beyond survival. Lancet, 384(9938), 189–205. doi:10.1016/S0140-6736(14)60496-7 [DOI] [PubMed] [Google Scholar]
- Lee A. C., Kozuki N., Blencowe H., Vos T., Bahalim A., Darmstadt G. L., Lawn J. E. (2013). Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatric Research, 74(Suppl. 1), 50–72. doi:10.1038/pr.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., Black R. E. (2016). Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet, 388(10063), 3027–3035. doi:10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Biarge M., Diez-Sebastian J., Wusthoff C. J., Mercuri E., Cowan F. M. (2013). Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics, 132(4), e952–959. doi:10.1542/peds.2013-0511 [DOI] [PubMed] [Google Scholar]
- McDermott S., Turk M. A. (2011). The myth and reality of disability prevalence: Measuring disability for research and service. Disability and Health Journal, 4(1), 1–5. doi:10.1016/j.dhjo.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Milner K. M., Bernal Salazar R., Bhopal S., Brentani A., Britto P. R., Dua T., Lawn J. E. (2019). Contextual design choices and partnerships for scaling early child development programmes. Archives of Disease in Childhood, 104(Suppl. 1), S3–S12. doi:10.1136/archdischild-2018-315433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner K. M., Bhopal S., Black M., Dua T., Gladstone M., Hamadani J., Lawn J. E. (2019). Counting outcomes, coverage and quality for early child development programmes. Archives of Disease in Childhood, 104(Suppl. 1), S13–S21. doi:10.1136/archdischild-2018-315430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Government of Uganda. (2015). Health sector development plan 2015/16-2019/20. Retrieved from http://www.npa.go.ug/development-plans/national-development-plan-ndp/ [Google Scholar]
- Morgan C., Novak I., Badawi N. (2013). Enriched environments and motor outcomes in cerebral palsy: Systematic review and meta-analysis. Pediatrics, 132(3), e735–e746. doi:10.1542/peds.2012-3985 [DOI] [PubMed] [Google Scholar]
- Mwaniki M. K., Atieno M., Lawn J. E., Newton C. R. (2012). Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet, 379(9814), 445–452. doi:10.1016/S0140-6736(11)61577-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamanya S., Siu G. E., Lassman R., Seeley J., Tann C. J. (2015). Maternal experiences of caring for an infant with neurological impairment after neonatal encephalopathy in Uganda: A qualitative study. Disability and Rehabilitation, 37(16), 1470–1476. doi:10.3109/09638288.2014.972582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampijja M., Webb E., Nanyunja C., Sadoo S., Nalugya R., Nyonyintono J., Tann C. J. (2019). Randomised controlled pilot feasibility trial of an early intervention programme for young infants with neurodevelopmental impairment in Uganda: A study protocol. BMJ Open, 9(10), e032705. doi:10.1136/bmjopen-2019-032705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I., Morgan C., Adde L., Blackman J., Boyd R. N., Brunstrom-Hernandez J., Badawi N. (2017). Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatrics, 171(9), 897–907. doi:10.1001/jamapediatrics.2017.1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusanya B. O. (2012). Neonatal hearing screening and intervention in resource-limited settings: An overview. Archives of Disease in Childhood, 97, 654–659. doi:10.1136/archdischild-2012-301786 [DOI] [PubMed] [Google Scholar]
- Olusanya B. O., Davis A. C., Kassebaum N. J., & Global Research on Developmental Disabilities Collaborators (GRDDC). (2019). Poor data produce poor models: Children with developmental disabilities deserve better—authors' reply. Lancet Glob Health, 7(2), e189. doi:10.1016/S2214-109X(18)30496-0 [DOI] [PubMed] [Google Scholar]
- Olusanya B. O., Davis A. C., Wertlieb D., Boo N. Y., Nair M. K. C., Halpern R., ... Global Research on Developmental Disabilities Collaborators. (2018). Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Global Health, 6(10), e1100–e1121. doi:10.1016/S2214-109x(18)30309-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer F. B., Shapiro B. L., Wachter R. C. (1993). The effects of physical therapy on cerebral palsy: A controlled trial in infants with spastic diplegia. Pediatric Physical Therapy, 5, 93–94. doi:10.1097/00001577-199300520-00019 [DOI] [PubMed] [Google Scholar]
- Romeo D. M., Cioni M., Palermo F., Cilauro S., Romeo M. G. (2013). Neurological assessment in infants discharged from a neonatal intensive care unit. European Journal of Paediatric Neurology, 17, 192–198. doi:10.1016/j.ejpn.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Seale A. C., Blencowe H., Zaidi A., Ganatra H., Syed S., Engmann C., ... Neonatal Infections Estimation Team. (2013). Neonatal severe bacterial infection impairment estimates in South Asia, sub-Saharan Africa, and Latin America for 2010. Pediatric Research, 74(Suppl. 1), 73–85. doi:10.1038/pr.2013.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J. P., Meisels S. J. (2000). Handbook of early childhood intervention (2nd ed.). Cambridge: Cambridge University Press. [Google Scholar]
- Singhi P. D., Ray M., Suri G. (2002). Clinical spectrum of cerebral palsy in north India—an analysis of 1,000 cases. Journal of Tropical Pediatrics, 48(3), 162–166. doi:10.1093/tropej/48.3.162 [DOI] [PubMed] [Google Scholar]
- Singhi P., Saini A. G. (2013). Changes in the clinical spectrum of cerebral palsy over two decades in North India—an analysis of 1212 cases. Journal of Tropical Pediatrics, 59(6), 434–440. doi:10.1093/tropej/fmt035 [DOI] [PubMed] [Google Scholar]
- Tann C. J., Nakakeeto M., Hagmann C., Webb E. L., Nyombi N., Namiiro F., Cowan F. M. (2016). Early cranial ultrasound findings among infants with neonatal encephalopathy in Uganda: An observational study. Pediatric Research, 80(2), 190–196. doi:10.1038/pr.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tann C. J., Nakakeeto M., Willey B. A., Sewegaba M., Webb E. L., Oke I., Robertson N. J. (2018). Perinatal risk factors for neonatal encephalopathy: An unmatched case-control study. Archives of Disease in Childhood Fetal and Neonatal Edition, 103(3), F250–F256. doi:10.1136/archdischild-2017-312744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tann C. J., Nkurunziza P., Nakakeeto M., Oweka J., Kurinczuk J. J., Were J., Harris K. A. (2014). Prevalence of bloodstream pathogens is higher in neonatal encephalopathy cases vs. controls using a novel panel of real-time PCR assays. PLoS One, 9(5), e97259. doi:10.1371/journal.pone.0097259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tann C. J., Webb E. L., Lassman R., Ssekyewa J., Sewegaba M., Musoke M., Cowan F. M. (2018). Early childhood outcomes after neonatal encephalopathy in Uganda: A cohort study. EClinicalMedicine, 6, 26–35. doi:10.1016/j.eclinm.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. M., Puterman A. S., Linley L. L., Hann F. M., van der Elst C. W., Molteno C. D., Malan A. F. (1997). The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatrica, 86, 757–761. doi:10.1111/j.1651-2227.1997.tb08581.x [DOI] [PubMed] [Google Scholar]
- Ubuntu Hub. (2019). Getting to know cerebral palsy. Retrieved from https://www.ubuntu-hub.org/resources/gtkcp/
- Uganda Bureau of Statistics (UBOS) and ICF. (2018). Uganda demographic and health survey 2016. Retrieved from https://dhsprogram.com/pubs/pdf/FR333/FR333.pdf
- Uganda Bureau of Statistics. (2019) Situation analysis of children in Uganda—2019. Retrieved from https://www.unicef.org/uganda/media/5181/file/Situation%20Analysis%20of%20Children%20in%20Uganda%202019-FINAL.pdf
- Uganda Ministry for Gender. (2016). The national integrated every child development policy action plan of Uganda. Retrieved from https://www.health.go.ug/sites/default/files/FINAL_National_Integrated_ECD_ACTION_PLAN_Approved.pdf
- UN Inter-Agency Group for Child Mortality Estimation. (2019). Levels & trends in child mortality: Report 2019, estimates developed by the United Nations Inter-agency group for child mortality estimation. Retrieved from https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019 [DOI] [PMC free article] [PubMed]
- UNICEF. (2014). Research study on children with disabilities living in Uganda. Retrieved from https://eprcug.org/children/publications/child-welfare/child-poverty-and-vulnerability/research-study-on-children-with-disabilities-living-in-uganda
- UNICEF. (2018). Neonatal mortality. Retrieved from https://data.unicef.org/topic/child-survival/neonatal-mortality/
- United Nations. (2015). The Global strategy for women's, children's and adolescents' health (2016–2030). New York, NY: Every Woman Every Child. Retrieved from https://www.who.int/life-course/partners/global-strategy/globalstrategyreport2016-2030-lowres.pdf [Google Scholar]
- United Nations. (2016). Transforming our world: The 2030 agenda for sustainable development. Retrieved from https://sustainabledevelopment.un.org/post2015/transformingourworld
- Varni J. W., Burwinkle T. M., Seid M., Skarr D. (2003). The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambulatory Pediatrics, 3(6), 329–341. doi:10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Wallander J. L., Bann C. M., Biasini F. J., Goudar S. S., Pasha O., Chomba E., Carlo W. A. (2014). Development of children at risk for adverse outcomes participating in early intervention in developing countries: A randomized controlled trial. Journal of Child Psychology and Psychiatry and Allied Disciplines, 55(11), 1251–1259. doi:10.1111/jcpp.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallander J. L., McClure E., Biasini F., Goudar S. S., Pasha O., Chomba E., ... BRAIN-HIT Investigators. (2010). Brain research to ameliorate impaired neurodevelopment–home-based intervention trial (BRAIN-HIT). BMC Pediatrics, 10, 27. doi:10.1186/1471-2431-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2012). Early childhood development and disability: A discussion paper. Retrieved from https://apps.who.int/iris/handle/10665/75355
- World Health Organization. (2018a). Survive and thrive: Transforming care for every small and sick newborn. Retrieved from https://www.unicef.org/media/58076/file
- World Health Organization. (2018b). Nurturing care for early childhood development: A framework for helping children survive and thrive to transform health and human potential. Retrieved from https://www.who.int/maternal_child_adolescent/child/nurturing-care-framework/en/
- Zuurmond M., O'Banion D., Gladstone M., Carsamar S., Kerac M., Baltussen M., Polack S. (2018). Evaluating the impact of a community-based parent training programme for children with cerebral palsy in Ghana. PLoS One, 13(9), e0202096. doi:10.1371/journal.pone.0202096 [DOI] [PMC free article] [PubMed] [Google Scholar]