Abstract
Ansuvimab (ansuvimab-zykl; EBANGA™) is a human monoclonal antibody developed by Ridgeback Biotherapeutics, which binds to the glycoprotein on Zaire ebolavirus (Ebola virus) to block its entry into host cells. Ansuvimab has been recently approved in the USA for the treatment of infection caused by Z. ebolavirus in adult and paediatric patients, including in neonates born to a mother who is RT-PCR positive for Z. ebolavirus infection, following the results of the PALM phase II/III trial. This article summarizes the milestones in the development of ansuvimab leading to this first approval for the treatment of infections caused by Ebola virus in adults and paediatric patients.
| Digital Features for this article can be found at 10.6084/m9.figshare.14036468. |
Ansuvimab (EBANGA™): Key points
| A monoclonal antibody was developed by Ridgeback Biotherapeutics for the treatment of Ebola virus infections. |
| Received its first approval on 21 Dec 2020 in the USA. |
| Approved for use in the treatment of infection caused by Z. ebolavirus in adult and paediatric patients, including in neonates born to a mother who is positive for Z. ebolavirus infection. |
Introduction
Ebola virus disease is a potentially fatal disease that occurs in patients who are infected by Zaire ebolavirus (Ebola virus), which may be transmitted via bodily fluids, zoonotic transmission or contact with contaminated surfaces [1]. Ansuvimab (ansuvimab-zykl; EBANGA™) is a human monoclonal IgG1 antibody developed by Ridgeback Biotherapeutics for the treatment of infections caused by Ebola virus in adult and paediatric patients, including neonates born to a mother who is RT-PCR positive for Ebola virus infection [2]. Ansuvimab was initially isolated from the blood of a survivor of the 1995 Kikwit Ebola outbreak, which demonstrated potent neutralisation of Ebola virus [3]. The recommended dosage of ansuvimab is 50 mg/kg via intravenous (IV) infusion over 60 min [2]. Ansuvimab received its first approval on 21 Dec 2020 in the USA for the treatment of infection caused by Z. ebolavirus in adult and paediatric patients, including in neonates born to a mother who is RT-PCR positive for Z. ebolavirus infection [1, 4].
Company Agreements
In December 2018, Ridgeback Biotherapeutics entered into a patent license agreement for intellectual property related to ansuvimab for the treatment of Ebola virus infection with the National Institute of Allergy and Infectious Diseases [5]. In September 2019 and April 2020, Ridgeback Biotherapeutics received contracts from the Biomedical Advanced Research and Development Authority in the Office of the Assistant Secretary for Preparedness and Response in the US Department of Health and Human Services to manufacture ansuvimab and to support its development [6, 7].
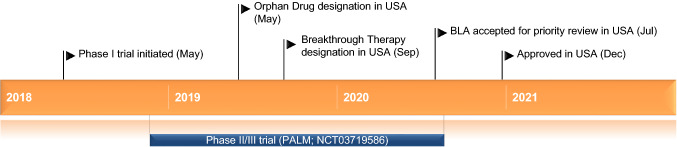
Key milestones in the development of ansuvimab for the treatment of Ebola virus infection. BLA Biologics License Application
Scientific Summary
Pharmacodynamics
Ansuvimab blocks binding between the Ebola virus glycoprotein (GP) and the Niemann-Pick C1 (NPC1) receptor by binding to the LEIKKPDGS epitope located in the receptor binding site of the GP1 subunit of GP [2]. NPC1 binding is an important step for Ebola virus infections, as this facilitates membrane fusion during viral entry [8]. Cryo-electron microscopy showed ansuvimab binding to the glycan cap and GP core domains in a near-perpendicular angle to the viral membrane [8]. Using biolayer interferometry, ansuvimab demonstrated a high affinity for GP1 without the mucin domain at pH 7.4 (KD 0.2 nM) and pH 5.3 (KD 0.6 nM) [8], and GP1 binding to NPC1 was inhibited by ansuvimab (IC50 0.09 µg/mL) [2]. EC50 values with ansuvimab were 0.06 µg/mL in a plaque-reduction neutralisation assay with Z. ebolavirus Mayinga, and 0.09 and 0.15 µg/mL in a lentivirus infectivity assay with Z. ebolavirus Mayinga and Z. ebolavirus Makona [2].
Antibody-dependent cellular cytotoxicity (ADCC) against GP-transfected target cells was observed with ansuvimab using flow cytometry, maximal ADCC activity occurred at an ansuvimab concentration of 0.03 µg/mL [3]. Ansuvimab 50 mg/kg administered to rhesus macaques on days 1–3 resulted in all macaques (n = 3) surviving after exposure to a lethal dose of Ebola virus in on day 0. Additionally, all treated macaques survived (n = 3) when treatment was delayed to 5 days’ post-exposure to a lethal dose of Ebola virus [3].
Pharmacodynamic interaction between ansuvimab and Ebola virus vaccines is unknown; concomitant treatment with ansuvimab and a live Ebola virus vaccine is not recommended, as ansuvimab may diminish the efficacy of the vaccine. Furthermore, resistance to ansuvimab has not been studied, the possibility of resistance to ansuvimab should be considered in patients who fail to respond to therapy, or relapse after an initial response [2].
Pharmacokinetics
No pharmacokinetic data are available for ansuvimab in Z. ebolavirus infected patients. In 18 healthy subjects, the pharmacokinetic profile of ansuvimab was consistent with other IgG1 monoclonal antibodies [2]. Following IV administration of ansuvimab 5 mg/kg (n = 3), ansuvimab 25 mg/kg (n = 5) and ansuvimab 50 mg/kg (n = 5) in healthy volunteers during a phase I pharmacokinetic trial, peak serum concentrations were 198.5, 829.4 and 1961.2 µg/mL, respectively, times to peak concentration were 3.2, 3.0 and 2.8 h, respectively, areas under the serum-time curve to day 28 were 1480, 8586 and 18588 µg∙day/mL, respectively and mean serum concentrations on days 0–28 were 52.9, 306.7, 663.9 µg/mL, respectively. β-phase half-lives were 20.1, 26.7 and 23.6 days in 3, 5 and 1 patient(s), respectively [9]. No data are available on the effect of age, kidney disease or hepatic impairment on the pharmacokinetics of ansuvimab [2].
| Alternative names | Ansuvimab-zykl; Ebanga; EboV mAb114; EVB114; mAb114; VRC EBOMAB092 00 AB |
| Class | Antivirals; monoclonal antibodies |
| Mechanism of Action | Glycoprotein inhibitors; virus internalisation inhibitors |
| Route of Administration | Intravenous |
| Pharmacodynamics and microbiology | Binds to the LEIKKPDGS epitope of the GP1 subunit located in the receptor binding site (KD 0.2 nM at pH 7.4, KD 0.6 nM at pH 5.3); inhibits GP1 and NPC1 binding (IC50 0.09 µg/mL); neutralises Ebola virus (EC50 0.06–0.15 µg/mL); maximal ADCC activity with an ansuvimab concentration of 0.03 µg/mL |
| Pharmacokinetics | In healthy volunteers receiving ansuvimab 50 mg/kg: Cmax 1961.21 µg/mL; tmax 2.75 h; AUC0–28d 18588 µg∙day/mL; beta phase t½ 23.6 days |
| Infusion-related adverse events | |
| Most frequent | Pyrexia |
| Occasional | Tachycardia, diarrhoea, vomiting, hypotension, tachypnoea |
| Rare | Chills, hypoxia |
| ATC codes | |
| WHO ATC code | J05A-X |
| EphMRA ATC code | J5B9 |
| Chemical Name | Immunoglobulin G1, anti-(Zaire ebolavirus glycoprotein glycan cap and GP1 domain) (human monoclonal mAb114 gamma1-chain), disulfide with human monoclonal mAb114 kappa-chain, dimer |
Therapeutic Trial
Treatment with ansuvimab resulted in a − 14.6% difference in the incidence of death (p < 0.035) in the ansuvimab group (35.1% of patients) versus the porgaviximab control group (49.7%) at 28 days (primary endpoint) during the PALM phase II/III clinical trial (NCT03719586) [10]. Additionally, a − 14.6% difference in the incidence of death was reported with ansuvimab versus porgaviximab in patients with a high initial viral load (initial Ebola virus nucleoprotein Ct ≤ 22; 69.9% vs 84.5% of patients), as well as in patients with a low viral load (initial Ebola virus nucleoprotein Ct > 22; 9.9% vs 24.5% of patients). In this open label trial, patients of any age with a confirmed Ebola virus infection, or infants with a mother who has a confirmed Ebola infection were treated with standard care and an investigational treatment; ansuvimab 50 mg/kg on day 1 (n = 174), porgaviximab 50 mg/kg on days 1, 3 and 7 as an active control (n = 169) or with two other investigational therapies (which are omitted here for brevity). The median time to the first negative PCR result for Ebola virus occurred at 16 days with ansuvimab and 27 days with porgaviximab (secondary outcome) [10].
Key clinical trials of ansuvimab in Ebola virus infections (National Institute of Allergy and Infectious Diseases)
| Drug(s) | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|
| Ansuvimab, REGN-EB3, remdesivir, porgaviximab | II/III | Completed | Congo, USA | PALM, NCT03719586 |
| Ansuvimab | I | Completed | USA | NCT03478891 |
Adverse Events
Ansuvimab had an acceptable tolerability profile in patients enrolled in the phase II/III PALM trial (n = 173); however, the assessment of adverse events (AEs) or reactions may have been confounded by symptoms of the Ebola virus infection [2]. Prespecified symptoms which occurred at an incidence ≥ 40% of patients were diarrhoea, pyrexia, abdominal pain and vomiting. 29% of ansuvimab-treated patients experienced a prespecified infusion-related AE, and ≈ 99% of patients receiving ansuvimab were administered the complete dose within 1 h; two patients (1%) did not receive the complete dose, and the infusion rate was decreased in 8 patients (5%) due to an AE. The most commonly occurring AEs during infusions in the ansuvimab (n = 173) or porgaviximab active control (n = 168) groups were pyrexia (17% of patients with ansuvimab group and 58% with porgaviximab), tachycardia (9% and 32%), diarrhoea (9% and 18%), vomiting (8% and 23%), hypotension (8% and 31%), tachypnoea (6% and 28%), chills (5% and 33%) and hypoxia (3% and 11%) [2].
Selected Grade 3 or 4 abnormalities in laboratory parameters which worsened from baseline in the ansuvimab and porgaviximab active control groups were creatinine > 1.8 × ULN or 1.5 × baseline (27% and 23%), potassium ≥ 6.5 mmol/L (15% and 12%), aspartate aminotransferase 5 × ULN (13% and 18%), alanine aminotransferase 5 × ULN (12% and 14%), sodium < 125 mmol/L (7% and 11%), potassium < 2.5 mmol/L (6% and 8%), sodium ≥ 154 mmol/L (5% and 4%) [2].
Monitoring patients for symptoms of hypersensitivity reactions (e.g. hypotension, chills or pyrexia) is recommended; discontinue ansuvimab and administer appropriate therapy in patients with severe or life-threatening hypersensitivity reactions. The rate of infusion may be decreased in patients experiencing an infusion-related or other AE [2].
Current Status
Ansuvimab received its first approval in the USA on 21 Dec 2020 for the treatment of infection caused by Z. ebolavirus in adult and paediatric patients, including in neonates born to a mother who is RT-PCR positive for Z. ebolavirus infection [1].
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Lee is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Footnotes
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
References
- 1.US Food & Drug Administration. FDA approves treatment for Ebola virus [media release]. 21 Dec 2020. https://www.fda.gov/.
- 2.Ridgeback Biotherapeutics. EBANGA (ansuvimab-zykl): US prescribing information. 2021. https://dailymed.nlm.nih.gov/. Accessed 28 Jan 2021.
- 3.Corti D, Misasi J, Mulangu S, et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351(6279):1339–1342. doi: 10.1126/science.aad5224. [DOI] [PubMed] [Google Scholar]
- 4.Ridgeback Biotherapeutics. Ridgeback Biotherapeutics LP announces the approval of Ebanga™ for Ebola [media release]. 22 Dec 2020. https://ridgebackbio.com/.
- 5.Ridgeback Biotherapeutics. Ridgeback Biotherapeutics LP announces licensing of mAb114, an experimental Ebola treatment, from the National Institute of Allergy and Infectious Diseases [media release]. 13 Dec 2018. https://ridgebackbio.com/.
- 6.Ridgeback Biotherapeutics. Breakthrough Ebola treatment receives contract from U.S. Government [media release]. 2 Apr 2020. https://ridgebackbio.com/.
- 7.U.S. Department of Health & Human Services. BARDA announces the 57th FDA approval/licensure/clearance for medical countermeasures supported by BARDA under novel public private partnerships [media release]. 21 Dec 2020. https://www.medicalcountermeasures.gov/.
- 8.Misasi J, Gilman MS, Kanekiyo M, et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science. 2016;351(6279):1343–1346. doi: 10.1126/science.aad6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudinski MR, Coates EE, Novik L, et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet. 2019;393(10174):889–898. doi: 10.1016/S0140-6736(19)30036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulangu S, Dodd LE, Davey RT, Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]