Abstract
The memories that we retain can serve many functions. They guide our future actions, form a scaffold for constructing the self, and continue to shape both the self and the way we perceive the world. Although most memories we acquire each day are forgotten, those integrated within the structure of multiple prior memories tend to endure. A rapidly growing body of research is steadily elucidating how the consolidation of memories depends on their reactivation during sleep. Processing memories during sleep not only helps counteract their weakening but also supports problem solving, creativity, and emotional regulation. Yet, sleep-based processing might become maladaptive, such as when worries are excessively revisited. Advances in research on memory and sleep can thus shed light on how this processing influences our waking life, which can further inspire the development of novel strategies for decreasing detrimental rumination-like activity during sleep and for promoting beneficial sleep cognition.
Keywords: learning, consolidation, targeted memory reactivation, sleep, well-being
MEMORY AND THE MAGIC TOOLBOX
People acquire and maintain an immense amount of information during their lifetimes, but how that happens is hard to fathom. Whereas memory research has focused heavily on acquisition and retrieval as the two most essential steps for memories to be of use, in this article we emphasize the additional processing that memories undergo during offline periods.
New knowledge is not assimilated instantaneously; rather, memory storage changes over time. Forgetting may be the usual fate of newly acquired information, unless memory consolidation can counter forgetting, producing enduring memories that are less susceptible to decay or interference (Paller 2009). Although consolidation may include many mechanisms operative over long periods of time (Dudai 2012), a crucial part of the consolidation process may occur over periods of sleep. In this article, we elaborate on how memory reactivation during sleep contributes to memory stability and memory use. We also consider how sleep-based processing can contribute to creative insights and psychological well-being.
As a nightly ritual, some people like to document each day’s highlights by writing a diary entry. Indeed, before going to sleep at night, each of us (diary writer or not) is capable of remembering a great deal about the day’s happenings, including activities, thoughts, emotions, and interactions with others. These are fully viable memories; however, most do not remain so easy to remember. On subsequent days, forgetting and interference take their toll. It would be much more challenging to retrospectively produce a complete diary entry after a few days have passed, and extremely difficult to remember the experiences of a random and not especially eventful day from the past.1
How is it that some memories can be recalled in detail years later? Forgetting and interference can apparently be avoided when memory storage is modified through consolidation mechanisms, considered in detail below. Still, memory storage falls short of providing us with a verbatim readout or a complete diary entry of the various events, thoughts, and emotions we experience each day. The information that we do maintain serves an arguably more important role than mere documentation: These memories shape our personalities, our perspectives, and our decision making. The human brain controls a remarkable capacity to adaptively learn from our experiences, thereby improving the way we cope with life’s challenges.
As a fanciful analogy, imagine a handyman’s magic toolbox, replete with tools that gradually adjust their functionality in accordance with the jobs they are given. The tools thus evolve to become optimized for jobs they are likely to encounter in the future. Likewise, the brain’s plasticity enables it to change its functionality in accordance with how it is used. Thanks to continual changes in memory storage, we not only have new knowledge that we can use, but we also have the ability to use our brains in different ways.
We postulate here that consolidation largely transpires without us knowing about it—because we are asleep at the time. Moreover, understanding the neurophysiology of memory processing during sleep may be the key to understanding how the memories formed while we are awake are preserved and transformed, and how they transform us. Ultimately, changes in memory storage during sleep may shape not only what we can remember but also who we are.
MEMORY CONSOLIDATION AT A SYSTEMS LEVEL
Declarative memories encompass many things that “we know that we know.” This knowledge is used in the decisions we make and forms the basis of a life story that we can tell ourselves. We also acquire nondeclarative memories, and therefore we have knowledge that “we don’t know that we know.” Nondeclarative knowledge is often used without concomitant remembering of where and when it was acquired. Memories in both categories, declarative and nondeclarative, do not necessarily function in isolation; they can interact with each other and with working memory (which functions to maintain and manipulate information online). Importantly, multiple types of memory commonly affect the way we think and the decisions we make.
A guiding principle in contemporary memory research is that we cannot rely on a single mechanism to explain all human memory phenomena. The challenge of understanding a given type of memory thus includes detailing how a specified brain system operates to support memory function and how that operation is or is not different from that of other brain systems. Despite this guiding principle, some concepts may apply in basically the same way for many types of memory. For example, sleep could be universally relevant—for perceptual learning (Karni et al. 1994), skill learning (Walker et al. 2002), paired-associate learning (Plihal & Born 1997), and maybe all types of learning. The jury is still out on this idea, but it seems clear at this point that many types of memory are subject to change in one way or another during sleep (Rasch & Born 2013).
Declarative memories depend on specific parts of the neocortex, each specializing in certain types of information processing. Memories for facts and events invariably depend on links among these cortically based pieces of a memory, such as different sensory qualities or conceptual attributes. Accordingly, declarative memories are characterized by their reliance on novel cross-cortical connections (Paller 2002). Plasticity in connections across brain regions, including multiple cortical regions and other brain structures, can be key to consolidation—or, more specifically, to systems consolidation, in contrast to synaptic consolidation, which concerns cellular and molecular facets of consolidation. Here we focus on the former type of consolidation.
The neocortex, on its own, is not well-equipped for transforming entire experiences into lasting memory traces. The apparatus of the medial temporal lobe, centrally including the hippocampus, provides the neocortex with the capacity for storing declarative memories in an enduring way. In keeping with this idea, damage to the medial temporal lobe can lead to a profound impairment in learning, or anterograde amnesia. The hippocampus presumably helps fulfill the need to rapidly acquire declarative memories (Marr 1971, McClelland et al. 1995, Norman et al. 2005), which is not the forte of the cortex working by itself (perhaps with some exceptions; see Hebscher et al. 2019). Declarative memories can be gradually stabilized via a slower process whereby cortical networks are altered under hippocampal guidance. In this way, some declarative memories can become independent of the hippocampus; memories may become schematic, may lose details including various contextual features concerning the circumstances of their acquisition, and may be transformed to only hold the bare facts, general outlines, or a portion of the information acquired. In addition, the hippocampus may continue to be involved in retrieval for some memories with regard to specific episodic details (Nadel & Moscovitch 1997, Miller et al. 2020). Although this scenario provides a sketch of the gradual memory changes of consolidation, the contributions of hippocampal–neocortical interactions to memory change are not fully understood.
The idea that memory stabilization is a gradual process that takes place after an initial encoding stage has its roots in the work of Müller & Pilzecker (1900). On the basis of their studies of learning nonsense syllables, they inferred that there are “certain physiological processes, which serve to strengthen the associations … [that] continue with increasing intensity for a period of time” (cited in Lechner et al. 1999, p. 81). Decades later, retrograde amnesia was observed in rodents given an electroconvulsive shock 15 minutes after learning (Duncan 1949). In contrast, memory remained intact when the shocks were delivered 1 hour after learning. This pattern of experimentally induced retrograde amnesia substantiates the notion of post-learning consolidation. For different types of memory and different animal species, the amount of time required for a memory to become immune to an amnestic insult may differ. The proposal that human declarative memories undergo consolidation for many months after learning, initially regarded by researchers as surprising, is now widely accepted (Squire 1992, Squire & Wixted 2011). There is certainly still room for alternative views and for debate on many aspects of consolidation (e.g., Yonelinas et al. 2019), including how to characterize the hippocampal contribution (e.g., Murray et al. 2007, Aly & Ranganath 2018). The path to ascribing a major role to the hippocampus in memory consolidation followed from reports of severe memory impairments in patient H.M., who received a medial temporal lobectomy as treatment for intractable epilepsy (Scoville & Milner 1957). Damage to this brain region generally produces not only anterograde amnesia, but also temporally graded retrograde amnesia, which spares remote but not recent declarative memories. Evidence from retrograde memory deficits thus fits with the notion that there can be a protracted time period following initial acquisition, as consolidation progresses, when declarative memories depend on a hippocampal–neocortical dialogue. What does this dialogue entail?
A putative mechanism for declarative memory consolidation involves the offline engagement of the same neural circuits involved in learning, thereby reinstating the learning-related activity in the service of memory reactivation. This reactivation may support consolidation whether or not there is a concurrent experience of conscious retrieval. A relevant sort of physiological reactivation was first observed in the activity of rodent hippocampal place neurons, which fire selectively when the animal occupies a specific patch of space. Rodent hippocampal place cells with fields used in recent exploration fired more frequently during sleep compared to cells with fields not encountered during pre-sleep exploration (Pavlides & Winson 1989). In a seminal study on memory consolidation, Wilson & McNaughton (1994) found that coordinated place cell activity in the hippocampus was likely to reoccur again during sleep. This finding was later extended to show that the temporal patterns of action potentials exhibited during wake are repeated during sleep, constituting memory replay (Skaggs & McNaughton 1996). These results brought forward the hypothesis that memory is strengthened via replay within the hippocampus. Additional studies in rodents showing coordinated replay between the hippocampus and the neocortex during sleep suggest that replay is an essential aspect of the hippocampal–neocortical dialogue that can stabilize cortical representations (Foster 2017). Many findings now support the idea that memory reactivation during both waking rest periods and sleep is relevant for the modification, integration, and stabilization of memories.
Memory processing may be particularly effective during sleep due to its unique physiological and neurochemical properties (see the sidebar titled Sleep Physiology). Of course, memories can readily be modified or updated during wake in light of new experiences (for a review of wake reactivation and consolidation, see Tambini & Davachi 2019). During sleep, as a consequence of increased sensory gating and environmental factors—when sleeping in a secluded space that is quiet and dark—there is usually far less incoming sensory information (nevertheless, sensory processing continues; see below). Consolidation may indeed be more effective, and less subject to interference or contamination, when memories are reactivated during sleep.
PHYSIOLOGY OF MEMORY CONSOLIDATION DURING SLEEP
Behavioral studies of memory consolidation during sleep have produced ample evidence of superior retrieval of various types of information after a period of sleep compared to a period of wake (e.g., Karni et al. 1994, Plihal & Born 1997, Walker et al. 2002, Tucker et al. 2006). In rodents, consistent evidence has suggested a causal role for rapid eye movement (REM) sleep in memory consolidation (Smith 1995, Rasch & Born 2013, Klinzing et al. 2019). Historically, there have been many attempts to tie human REM sleep to memory (e.g., Crick & Mitchison 1983, Winson 1985, Paller & Voss 2004), and diverse ideas about its role in consolidation are still debated. Here we emphasize the better understood role of non-REM (NREM) sleep.
A prominent account of sleep-based consolidation, sometimes termed the active systems consolidation hypothesis, suggests that memory reactivation in the hippocampus during NREM sleep dictates changes in cortical networks (Buzsáki 1998, Born et al. 2006). This proposed mechanism embraces selectivity, as some memories are reactivated and others not. An alternative account, the synaptic homeostasis hypothesis, instead attributes the memory benefits of sleep to widespread downscaling of synaptic strength, thereby increasing the signal-to-noise ratio for memory retrieval (Tononi & Cirelli 2014). Importantly, synaptic downscaling and active consolidation are not mutually exclusive. Recent accounts emphasize the critical contributions of both reactivation and synaptic downscaling for retaining old memories and for encoding new ones (Klinzing et al. 2019).
The implementation of sleep-related consolidation through reactivation appears to be intimately related to certain physiological features of sleep characterized as field-potential oscillations, particularly during slow-wave sleep (SWS). These oscillations appear to be orchestrated together in the form of nested oscillations (e.g., Staresina et al. 2015), as described below and depicted in Figure 1. Sharp-wave/ripple complexes (SWRs), found locally in the hippocampus, are nested in the troughs of thalamo-cortical sleep spindles, which, in turn, ride on the peaks (or up-states) of cortical slow oscillations (SOs). Accordingly, consolidation is enabled by a synchronized temporal frame for communication among brain areas (Diekelmann & Born 2010).
Figure 1.
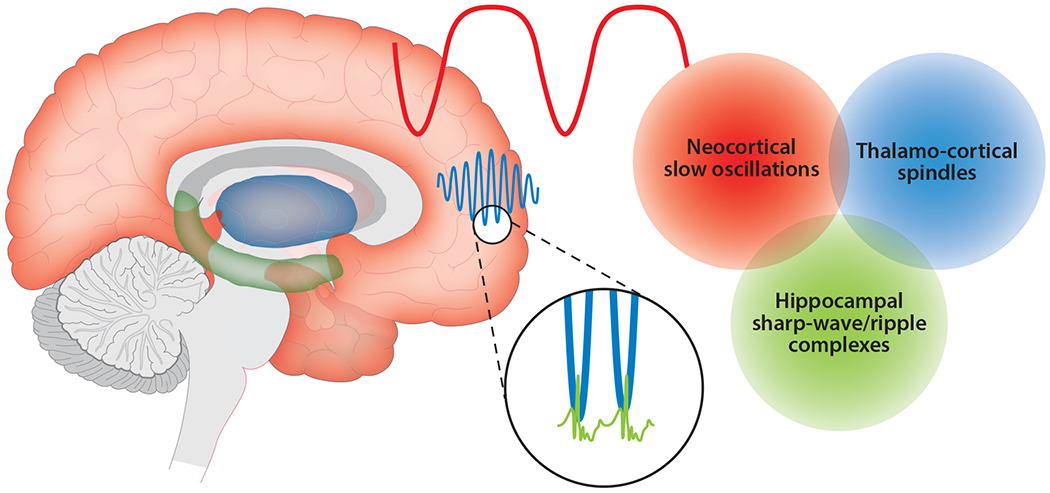
The consolidation of declarative memories during slow-wave sleep is thought to involve multiple brain regions and neuronal interactions, reflected in the set of brain oscillations pictured here. The slowest of these are neocortical slow oscillations (red). The so-called up-states of these oscillations coincide with high levels of neuronal activity across many regions of the cortex, which makes the up-states conducive to cross-cortical interactions. The synchronization of thalamo-cortical spindles (blue) with slow-oscillation up-states facilitates memory processing. Sharp-wave/ripple complexes (green, shown at a larger scale) can be generated in the hippocampus and are also synchronized with spindles. Ripples coincide with hippocampal replay and are causally associated with memory processing. Figure adapted from Born & Wilhelm (2012).
Sharp-Wave/Ripple Complexes
SWRs are commonly observed in recordings from area CA1 of the rodent hippocampus. Somewhat similar observations have been made in human intracranial recordings. The high-frequency ripples (70–110 Hz in humans, 150–200 Hz in rodents) coincide with a characteristic high-amplitude wave (i.e., a sharp wave). Hippocampal-place-cell replay patterns are predominantly observed contemporaneously with SWRs, during both sleep (Wilson & McNaughton 1994) and wake (Nádasdy et al. 1999). Furthermore, disruption of SWRs during sleep negatively affects memory (Girardeau et al. 2009), whereas their artificial extension benefits memory during wake (Fernández-Ruiz et al. 2019). In fact, the causal role of SWRs in memory consolidation has received more support than that of hippocampal replay (Laventure & Benchenane 2020).
SWRs likely contribute to memory consolidation in conjunction with other physiological events. Rodent SWRs take place predominantly within the time frame of thalamo-cortical spindles, and their propagation from the hippocampus may add to the excitation of spindles (Sirota et al. 2003). Human findings are also consistent with the idea that SWRs are nested within SOs and spindles (Helfrich et al. 2018, Staresina et al. 2015). Indeed, SWRs have been considered to coincide with hippocampal output that can eventually reach various cortical zones. In support of this idea, SWRs in human hippocampal recordings precede activity in adjacent cortex (Axmacher et al. 2008, Nir et al. 2011). Notably, coupling between SWRs in the hippocampus and ripple-like activity in the neocortex was strengthened by sleep that followed learning (Khodagholy et al. 2017). Additional evidence linking wake SWRs to memory includes their timing, anticipating recollection in both free-recall tests (Norman et al. 2019) and cued-recall tests (Vaz et al. 2019).
SWRs and simultaneous neocortical ripple-like activity could reflect the aforementioned hippocampal–neocortical dialogue. Sleep spindles could play a synchronizing role for this mechanism (Ngo et al. 2020). Complicating this story, however, Axmacher et al. (2008) reported that memory performance correlated with ripples in the human rhinal cortex but not the hippocampus, and that more ripples occurred during quiet wake than sleep in both the hippocampus and the rhinal cortex. How exactly is memory processing different during wake and during sleep? Differences between SWRs may provide clues. In rodents, wake SWRs coincide with high-fidelity replay and remain local, whereas sleep SWRs coincide with noisier replay and, as described below, are coordinated with widespread cortical SOs (Roumis & Frank 2015). Accordingly, high-fidelity reactivation may support memory-guided planning during wake, whereas noisier, distributed reactivations may support generalization during sleep.
Hippocampal SWRs seem to be triggered by SOs and spindles from the neocortex (Sirota et al. 2003, Nir et al. 2011). A cortical-to-hippocampal trajectory is also consonant with replay in the visual cortex preceding replay in the hippocampus (Ji & Wilson 2007). Moreover, replay in these two brain regions tends to occur during distinct temporal frames, such that both replay and SWRs may be synchronized by SO timing.
Slow Oscillations
SOs (≤1 Hz), observed in the scalp electroencephalogram (EEG) or in local field potentials, reflect coordinated neural activity of large areas of the cortex and typically serve to coordinate higher-frequency local activity (Varela et al. 2001). During the SO trough (or down-state), pyramidal neurons and interneurons are silenced, whereas during the SO peak (or up-state), neuronal burst-like activity predominates (Volgushev et al. 2006). SOs during sleep do not occur simultaneously throughout cortex; they typically appear frontally first and travel posteriorly (Massimini et al. 2004). Intracranial recordings of SO propagation documented various paths, such as from neocortex to parahippocampal gyrus to entorhinal cortex to hippocampus (Nir et al. 2011). Whereas many SOs follow such cortical trajectories, many other SOs occur locally. Different types of information processing may thus correspond to different types of SO patterns, particularly in relation to cortical origin and trajectory. Indeed, SO activity over a specific cortical area can reflect previous learning dependent on that area (Huber et al. 2004).
SOs are the hallmark of SWS. Both low-frequency EEG power and SWS duration have repeatedly been found to be correlated with memory improvement over sleep, primarily for declarative memory (e.g., Backhaus et al. 2007, Westerberg et al. 2012). More directly, SOs have been linked with consolidation in studies using transcranial direct current stimulation or auditory stimulation to entrain SOs. Many such studies have shown enhanced SOs and a concurrent improvement in retention over sleep (Marshall et al. 2006, Ngo et al. 2013, Westerberg et al. 2015, Papalambros et al. 2017). However, SO enhancement has also occurred in the absence of observed memory improvements (Cox et al. 2014, Weigenand et al. 2016, Henin et al. 2019, Papalambros et al. 2019). One possible explanation for the mixed memory results in these studies is that the word-pair recall measures used often had low test-retest reliability, which would not be conducive to producing consistent results in repeated-measure designs comparing stimulation and sham stimulation. Studies with improved memory measures or more powerful experimental designs are needed to clarify this discrepancy in the literature. Nevertheless, the existing results offer considerable support for the notion that SOs function as a driving mechanism for strengthening memories during sleep.
Sleep Spindles
A sleep spindle is defined as a waxing-and-waning oscillation at 11–16 Hz with a duration between 0.5 s and 3 s. Spindles originate in the thalamus and propagate to different neocortical sites (Fernandez & Luthi 2020). Although models explaining spindles’ contribution to memory focus on their interaction with SOs, they are most frequent in N2, when SOs are less common than during SWS (Purcell et al. 2017). However, given findings that memory changes after a nap correlated with SWS spindle activity but not with N2 spindle activity (Antony et al. 2012, Cox et al. 2012), spindles during SWS may be particularly important for consolidation.
The importance of the link between SOs and spindles is underscored by the finding that their coupling predicts the degree of memory strengthening over sleep (e.g., Latchoumane et al. 2017). Furthermore, manipulating SOs during sleep also increases sleep spindles (e.g., Marshall et al. 2006). Phase-amplitude coupling between SOs and spindles is often observed, as spindles are prevalent during the SO up-state (e.g., Steriade et al. 1993,Mölle et al. 2002). Yet, spindles and SOs in human intracranial recordings often do not coincide (Nir et al. 2011). Analyses of spindle-SO coupling patterns must take into account (a) that spindles are highly variable across individuals (Cox et al. 2019) and (b) that there may be multiple spindle subtypes. Spindles have been distinguished based on their spatial distribution in the brain, their frequency, and their behavioral relevance. For example, slow spindles (≤13 Hz) tend to be nested in the down-state of the SO, whereas fast spindles (>13 Hz) are nested in the up-state (Mölle et al. 2011, Cox et al. 2014).
Increasing empirical support has convincingly linked spindles with memory consolidation (Antony et al. 2018, Cairney et al. 2018), though the literature on spindles historically emphasized many other functions, such as sensory processing and intelligence (Fernandez & Luthi 2020). Higher spindle density has been associated with better memory in word-pair learning (Gais et al. 2002), visuospatial learning (Clemens et al. 2006), episodic learning (Cox et al. 2012), and procedural learning (Milner et al. 2006, Nishida & Walker 2007). In a pharmacological study, the GABAA agonist zolpidem (Ambien®) was found to increase sleep spindles and to improve word-pair recall but not motor-sequence learning (Mednick et al. 2013).
Further insights about spindles and their role in memory consolidation may be obtained using methods to manipulate spindles. For example, auditory entrainment produced an increase in spindles with characteristics resembling those of endogenous spindles (Antony & Paller 2017). Similarly, electrical stimulation with transcranial alternating current was used to boost spindle activity, also benefiting motor memory consolidation (Lustenberger et al. 2016). Future studies should investigate the functional relevance of cross-frequency coupling via conjoint manipulations, such as manipulations of spindles and SOs, combined with extensive memory testing.
Nested Oscillations and Hippocampal-Cortical Communication
Given that systems consolidation of a declarative memory entails coordinated memory processing across cortex and hippocampus, cross-frequency coupling across regions may be a critical ingredient. Because thalamo-cortical SOs can propagate throughout large parts of the cortex, a plurality of cortical regions participating in a declarative memory can be primed to interact. Hippocampal projections can then precisely engage the relevant cortical circuits, as coordination between spindles and ripples could allow SWRs to dictate replay in those specific circuits (Geva-Sagiv & Nir 2019).
A rapid flow of information engaging a cortical-hippocampal-cortical loop can thus be described (Rothschild et al. 2017, Lewis & Bendor 2019, Rothschild 2019). The initial cortical activity, corresponding to an incomplete memory trace, determines which memory will be reactivated. For example, the first step could be that one or more pieces of a recent experience might come to be reactivated cortically. Second, this information would be projected to the hippocampal networks that were also activated during the original experience, via the same anatomical pathways from cortex to hippocampus. Then, wider cortical involvement would unfold in the third step, based on hippocampal replay accompanied by SWRs, instantiating pattern completion to recruit other pieces of that recent experience. Consequently, the multimodal and multidimensional components initially encoded across the cortex could be fully reactivated.
In this way, an interaction between hippocampal networks and cortical networks could enhance cortico-cortical connections to solidify a complete declarative memory trace. While this cortical-hippocampal-cortical loop is steadily being characterized in more anatomical detail, specific functional consequences can also be investigated by manipulating the content of the memory reactivation, as described below.
Toward a Causal Link Between Reactivation and Consolidation
Neural evidence that could causally link reactivation with consolidation faces several interpretive challenges. When a person reactivates a memory in the normal course of remembering, it can be difficult to disentangle neural signatures of reactivation from new learning. Hippocampal activation observed using neuroimaging during recall, for example, could signify that the hippocampus is enabling reactivation or, alternatively, that the hippocampus is contributing to learning the novel experience of the recall episode itself (which could then be remembered later). Both types of processing are likely to occur concurrently. Similarly, we cannot easily dissociate acquisition from retrieval: If a certain oscillation is observed in the hippocampus during acquisition, it could be because old semantic information must be retrieved to make sense of the new information in context.
During sleep, some of these same interpretive issues may be in play but to a lesser extent, as new episodic memories are not readily formed as they are during wake. Importantly, plasticity can still be operative in neural circuits, though various views on the cellular mechanisms of plasticity during sleep have been controversial. Although this literature is beyond the scope of this review, we note that some views emphasize a facilitation of cortical plasticity during SWS (e.g., Timofeev & Chauvette 2017).
How can mechanisms of reactivation, and the function of reactivation in the service of consolidation, be studied in humans? Here we advocate for using sensory stimulation methods. Although the sleeping brain suppresses sensory input during sleep, some sensory processing nevertheless persists, perhaps in order to monitor the surroundings (Andrillon & Kouider 2019). We can leverage this standby mode of stimulus processing in two different ways. First, as described above, we can use stimuli to manipulate brain physiology, as in entraining SOs and spindles. Second, as described below, we can use learning-related stimulation to systematically manipulate memory processing.
TARGETED MEMORY REACTIVATION
Targeted memory reactivation (TMR) is a technique for probing memory reactivation during sleep (Oudiette & Paller 2013, Schouten et al. 2017). TMR can also be used during wake, although the term has most often been used in the context of sleep studies. First, stimuli such as sounds or scents are associated with specific newly learned information (Figure 2a). Then, the same sensory stimuli are unobtrusively presented during sleep. To avoid awakenings, sounds are often presented at a low intensity. The chief finding from recent TMR studies was confirmed by a meta-analysis that included results from over 90 experiments (Hu et al. 2020): When TMR was applied during SWS or N2, memory was selectively improved for the associated information compared to comparable information for which TMR was not applied.
Figure 2.
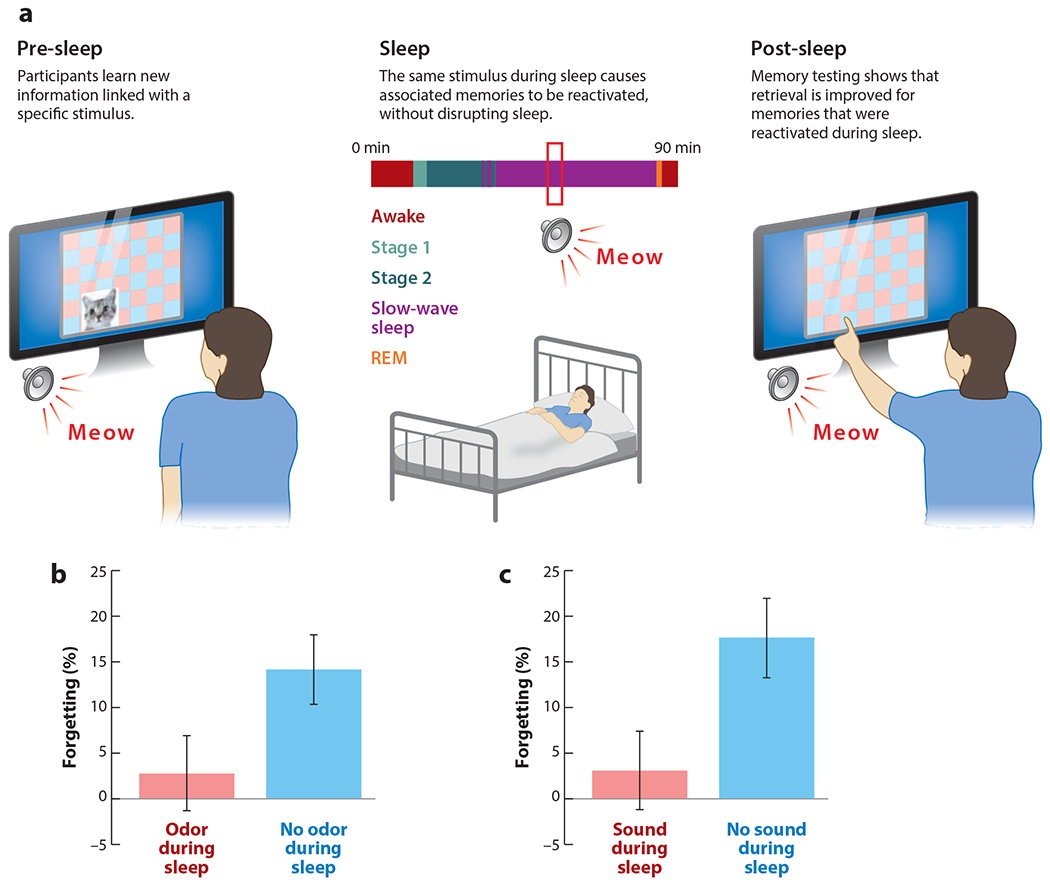
(a) Targeted memory reactivation experiments generally include three phases. In the first phase (pre-sleep), participants acquire some new information. This information is coupled with stimuli that are included either as background context or as part of the learning (e.g., a meow sound paired with the spatial location of a cat image). In the second phase (sleep), these stimuli are unobtrusively presented during sleep. In the third phase (post-sleep), memory is tested after sleep. (b) In the experiment of Rasch et al. (2007), spatial learning of 15 objects, each shown in two locations on a 5 × 6 grid, was accomplished while a rose odor was present. Next, during an overnight sleep session with polysomnographic monitoring, the odor or an odorless vehicle was presented during sleep. Finally, spatial memory was assessed the next morning. The results showed relatively better recall when the odor had been presented during slow-wave sleep compared to when it had not. This memory effect was not observed when odors were presented during rapid eye movement (REM) sleep or when the memory test was one of motor learning instead. (c) In the experiment of Rudoy et al. (2009), spatial learning of 50 objects shown in random locations on a grid was accomplished while sounds were presented. For each object, a matching sound was used. Next, during an afternoon nap with polysomnographic monitoring, half of the sounds were presented at a low intensity. Finally, spatial memory was assessed when participants attempted to place each object at precisely the correct screen location. The results showed relatively better memory performance for objects if corresponding sounds had been presented during slow-wave sleep than if not. Error bars represent standard error of the mean.
Many older studies paved the way for the recent proliferation of TMR experiments. The earliest studies seldom used polysomnographic methods [i.e., continuous EEG, electrooculogram (EOG), and electromyogram (EMG) recordings] to ensure that individuals were asleep at the time of stimulation and to determine sleep stage. TMR experiments lacking polysomnography leave room for doubt about what happened during sleep. Also, some studies were ignored because they did not fit with the Zeitgeist on memory and sleep. For example, a study by Tilley (1979) did not have a large impact because sounds played during REM did not enhance memory performance, and at the time REM was the central focus of questions about consolidation during sleep. However, Tilley also reported a TMR effect for sounds presented during N2.
This research approach began to gain widespread interest following a study by Rasch et al. (2007), wherein a spatial-learning improvement was produced using a rose odor during learning and again during SWS (Figure 2b). In a subsequent TMR study by Rudoy et al. (2009), spatial learning was similarly improved but using sounds (Figure 2c): The results showed less forgetting for object locations that were cued by a sound during sleep compared to those that were not cued. In this and many subsequent experiments, half of the sounds were selected for presentation during sleep using a form of stratified sampling (i.e., matching the two sets on pre-sleep recall accuracy). Within-subject comparisons then allowed nonspecific effects of sleep, such as alertness and interference, to be ruled out. The relative benefit in recall accuracy for cued compared to uncued conditions, as now observed in many TMR studies (Hu et al. 2020), demonstrates that stimuli presented during sleep can function to reactivate specific memories.
Memory changes observed in studies comparing post-sleep to pre-sleep performance are commonly used to support the inference that memories are indeed reactivated by TMR during sleep. This estimate of memory change is often subject to the proviso that the act itself of testing memory before sleep changes storage, as predicted by the testing effect (Roediger & Karpicke 2006). Yet, sound experimental designs in typical TMR studies still allow for valid comparisons between two conditions, one with and one without TMR cues presented during sleep, using either between-subjects or within-subjects comparisons. A further advantage of this type of design is that participants commonly exhibit no knowledge of whether cues were presented or which cues were presented, so no demand characteristics or strategic confounds are operative during post-sleep memory testing. Although factors such as testing effects, forgetting, interference, and consolidation may all affect retrieval after a delay, and potentially confound memory comparisons between a retention period with sleep and one without sleep, differential memory changes due to a TMR manipulation can be firmly attributed to differential consolidation for cued relative to uncued memories.
These methods for manipulating reactivation of specific memories complement other methods of studying sleep-related memory consolidation and provide a valuable tool for deciphering the neural mechanisms whereby reactivation engages memory consolidation. Sound delivery, in particular, provides a marker for specifying a time frame during which reactivation may be more likely.
To establish TMR as an effective tool for investigating consolidation, and to better understand its boundary conditions, it is essential to determine what types of learned material can be reactivated, what methods produce reactivation, and which brain mechanisms are engaged. TMR can improve many other types of learning in addition to spatial learning, including many examples of both declarative and nondeclarative memory [Hu et al. (2020) provide a comprehensive list as of mid-2019]. For example, TMR studies have shown a benefit for skill learning (Antony et al. 2012), vocabulary learning (Schreiner & Rasch 2015), and word recall (Fuentemilla et al. 2013). TMR studies have used multiple types of auditory stimulation during sleep, including pure tones, frequency-modulated tones, segments of popular music, environmental sounds, and spoken words.
In rodents, TMR was used to directly link memory reactivation with hippocampal replay: When tones associated with spatial learning on a two-arm maze were played during NREM sleep, corresponding hippocampal place cells were preferentially reactivated over the next few seconds (Bendor & Wilson 2012). Two tones were used in this experiment, one associated with a leftward response and the other with a rightward response, and both were presented during sleep. Therefore, unlike in most human TMR studies, there was no opportunity to determine whether memory changed due to the TMR procedure. In a subsequent study in rodents, Rothschild et al. (2017) found that a sound cue associated with pre-sleep learning biased auditory cortex activity, which predicted hippocampal SWR activity. These results were thus used to support the cortical-hippocampal-cortical loop theory of reactivation-dependent memory consolidation outlined above.
Neuroimaging has provided additional anatomical perspectives. For example, Cousins et al. (2016) used functional magnetic resonance imaging (fMRI) to reveal post-sleep changes due to TMR in brain activity associated with motor learning. Time in SWS correlated with greater hippocampal and caudate activity for cued versus uncued sequences in subsequent wake. Also, time in REM correlated with an increase in cerebellar and cortical motor activity for cued versus uncued sequences, suggesting that the effects of TMR during NREM sleep may depend on subsequent REM sleep as well. In an fMRI study of spatial learning using an auditory TMR procedure, the degree of memory benefit was found to be correlated with activity in the medial temporal lobe and the cerebellum, as well as with the degree of parahippocampal-precuneus connectivity, thus identifying several candidate measures of brain activity associated with sound-cued memory reactivation (van Dongen et al. 2012; see also Berkers et al. 2018). An fMRI study using an olfactory TMR procedure found that scents associated with prior learning elicited learning-related patterns of brain activity during sleep, and this activity was correlated with post-sleep spatial memory improvement (Shanahan et al. 2018). Complementing these neuroimaging findings, results from a study in epileptic patients suggested that one requirement for TMR is a relatively intact medial temporal region (Fuentemilla et al. 2013). Word recall benefits from TMR were found for individuals in a healthy control group and for patients who had brain damage due to hippocampal sclerosis, but not if there was bilateral damage. Furthermore, across all patients the degree of benefit correlated with the volume of spared hippocampus.
The efficacy of TMR cues undoubtedly depends on many factors. Four such factors are as follows: (a) The cue must gain sufficient sensory processing but not produce awakening; (b) the association between the cue and the learned information must be sufficiently strong and specific, so that the cue preferentially reactivates the intended information; (c) memory measures must be highly sensitive and reliable; and (d) at the time of TMR, memories must be sufficiently strong for veridical memory reactivation to occur, but not so strong that there is no possibility for improvement (Creery et al. 2015, Cairney et al. 2016). Also, the improvement must be sufficient to produce superior performance compared to the uncued condition, which can be difficult to evaluate with a binary memory measure (correct/incorrect) that may be relatively insensitive to gradations of memory strength. The testing procedures, including the delay, must be selected with these requirements in mind, and in consideration of possible floor or ceiling effects.
Whereas TMR can selectively improve memory, we do not know whether the reactivation it produces is the same as spontaneous reactivation. To answer this question, neural signals could be compared for TMR-induced and spontaneous reactivation during sleep. Encouragingly, recent TMR studies have made progress in monitoring reactivation. Reinstatement of memory-specific neural signatures following cues has been observed using both EEG and fMRI (Belal et al. 2018, Cairney et al. 2018, Schreiner et al. 2018, Shanahan et al. 2018, Wang et al. 2018). Two of these studies showed that this reactivation coincided with spindle activity (Cairney et al. 2018, Wang et al. 2018), converging with the aforementioned evidence implicating spindles. These results from TMR studies also converge with findings from studies of spontaneous reactivation that have also implicated spindles as a crucial component in decoding reactivated content (Bergmann et al. 2012, Schönauer et al. 2017).
Antony et al. (2018) used TMR to directly investigate the relevance of spindles. Their results showed that memory reactivation was most effective when a spindle occurred shortly after a TMR cue. Spindles are unlikely to occur in close succession and are typically separated by 3–6 s. Accordingly, an optimal time for cue presentation is at the end of this refractory period. Timing cue presentations accordingly was indeed found to favor memory benefits. Speculatively, the gap between sleep spindles may be helpful for segregating reactivation events during sleep (Antony et al. 2018).
Going beyond research strategies that focus on solidifying prior learning during sleep, a different approach considers the potential to learn new things during sleep. Such efforts have achieved limited success in the circumscribed territory of associative learning (Arzi et al. 2012) and perceptual learning (Andrillon et al. 2017). Also, Arzi et al. (2014) showed that sleep conditioning changed waking behavior in participants who wished to quit smoking. Conditioned pairing of a noxious odor with the odor of smoking during stage N2 and REM reduced the number of cigarettes smoked compared to various control conditions. New learning during sleep may be limited to simple types of memory. On the other hand, new procedures derived from TMR studies may open up other opportunities. For example, new learning may be possible during lucid dreaming (Konkoly et al. 2020). In another study, TMR was accomplished using a sound associated with learning to suppress a memory (Simon et al. 2018). The results showed that new associations were formed during sleep when this suppression-related sound was presented along with sounds related to other memories. Because this TMR procedure apparently weakened those memories, we can infer that those memories became associated with the suppression action. Accordingly, a wide range of strategies should be pursued to map out the potential for TMR to change memories.
In summary, TMR methods provide a powerful tool for probing sleep-related memory consolidation and its consequences. Future studies using novel variations of these methods hold promise for providing additional insights into the relevant neurocognitive mechanisms. Furthermore, applying TMR methods outside the laboratory has potential for shaping sleep consolidation to improve memory and various other aspects of life.
SLEEP AS A CONTRIBUTOR TO CREATIVE INSIGHT AND EMOTIONAL REGULATION
Processing memories during sleep can lead not only to the strengthening of particular memories but also to other sorts of memory modification. For example, two distinct memories may become associated, such as when a connection emerges between a recent event and a remote event. Such connections can also be the basis for creativity and problem solving. The time-honored belief that a difficult decision can be dealt with more effectively after sleeping on it calls attention to the role of sleep in creativity and problem solving. Historically, creative insight has routinely been linked with dreaming, as in the anecdotes of August Kekulé’s discovery of the structure of benzene, Otto Loewi’s discovery of chemical transmission, Dmitry Mendeleev’s discovery of the periodic table of elements, and Paul McCartney’s creation of the melody for Yesterday.
Problem Solving and Creativity
A TMR study by Sanders et al. (2019) strongly corroborates the relevance of sleep for problem solving. In this study, participants were stumped by a set of puzzles, each of which was accompanied by a repeating musical theme (Figure 3). Overnight, half of the themes were presented during SWS, which functioned to selectively increase the likelihood of producing solutions for those puzzles compared to the remaining puzzles.
Figure 3.
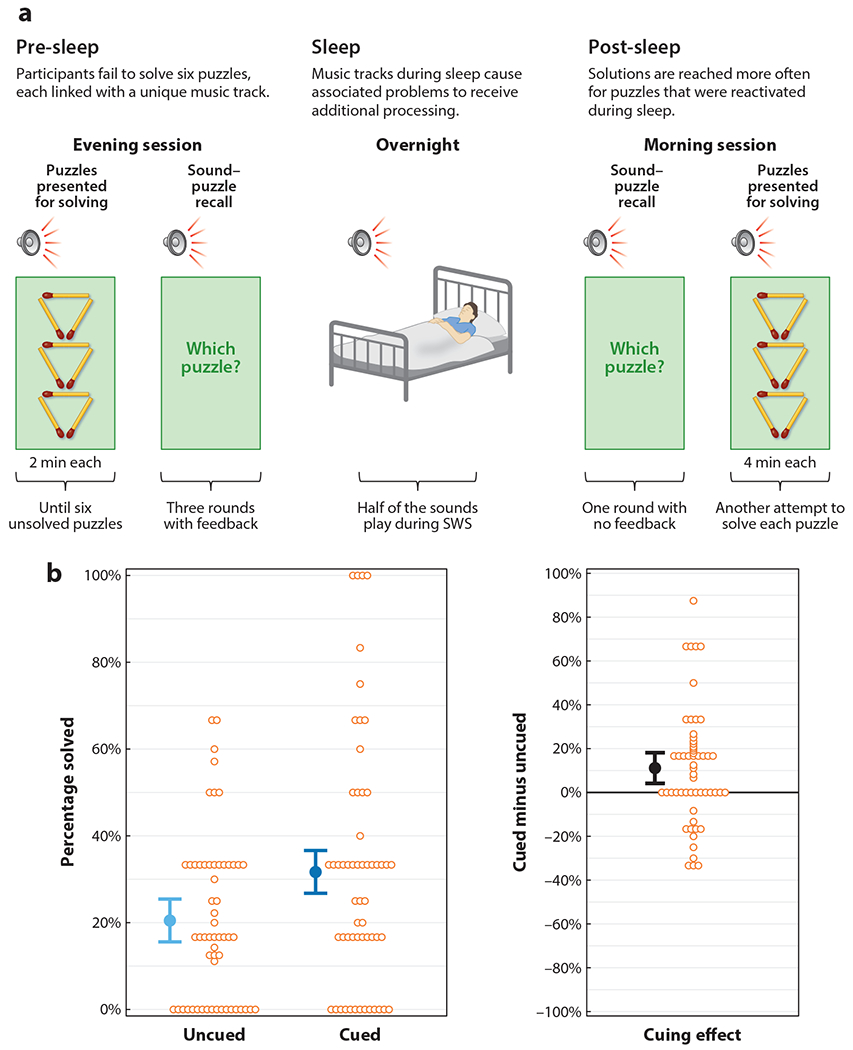
Experimental design and results from Sanders et al. (2019). (a) The participants attempted to solve a set of challenging puzzles (including matchstick, spatial, verbal, and rebus puzzles). Each puzzle was presented together with an arbitrary musical theme or sound, and participants were required to master these associations. The pre-sleep learning phase of the experiment ended when there were six puzzles that could not be solved in the 2 minutes allotted for working on each one. Participants slept in their own homes using a wireless sleep-monitoring device linked to a computer, which covertly presented sounds when slow-wave sleep (SWS) was detected. In this way, three of the six unsolved puzzles were reactivated using the corresponding sounds. The matchstick puzzle shown as an example came with instructions to move three matchsticks to create four equilateral triangles. (b) The results obtained the next day confirmed that memory reactivation had the predicted effect: When participants attempted to solve the same six puzzles, solving rates were higher for cued puzzles than for uncued puzzles. In the left panel, each orange circle represents a single participant’s success rate for one type of puzzle. The blue circles represent the average for each condition. Error bars represent 95% confidence intervals. The right panel shows the difference between the two conditions similarly. Figure adapted from Sanders et al. (2019).
Even though puzzle memories were presumably reactivated during SWS in this experiment, the investigators could not exclude the possibility that REM was also important for the observed effect on problem solving. Other evidence supports the idea that REM sleep may be an optimal time for broad semantic associations that are perhaps indicative of creativity (Stickgold et al. 1999). REM has also been empirically associated with solving word problems using solution hints from an ostensibly unrelated task (Cai et al. 2009). Yet, some studies failed to find sleep benefits for problem solving (Brodt et al. 2018, Schönauer et al. 2018). Another example of problem solving was examined using a tedious numerical task that could instead be completed on the basis of a hidden shortcut. Following an 8-hour break including either sleep or wake, only 35% of the participants made this discovery. Participants who slept, compared to those who did not, were more likely to discover the shortcut, presumably by restructuring their memories of the task (Wagner et al. 2004). A follow-up study showed that EEG activity during SWS in the form of power in the beta band (17–25 Hz) and perhaps part of the spindle band (10–11 Hz) predicted which participants would discover the shortcut (Verleger et al. 2013).
Although creativity is inherently connected with problem solving, very few studies have directly addressed the relevance of sleep for creativity. Only one study adopted the TMR methodology in this context. This study used a creativity task that required participants to generate ways to motivate other people to do volunteer work (Ritter et al. 2012). Instructions were given at night and the test was taken the next morning. Impartial raters scored the degree of creativity in the responses. Higher creativity was found when task-related odors were presented overnight compared to different-odor and no-odor control conditions in other participants. Additionally, participants in the task-related odor condition performed better than controls in selecting their most creative idea. However, the design did not allow the investigators to connect any specific sleep stage to this benefit. Odors presumably reactivated the task instructions and prompted ideas for creative solutions, just as musical sounds helped participants in the study of Sanders et al. (2019) reach solutions.
The available evidence is broadly consistent with the supposition that memory processing during sleep can be useful for various cognitive challenges that arguably share some properties with creativity. For example, reprocessing of recent episodic memories during sleep can support gist abstraction and generalization, which are useful modes of memory transformation (Lewis & Durrant 2011, Lutz et al. 2017, Schapiro et al. 2017, Tamminen et al. 2017). Ideally, sleep could support creative ways to relate recent experiences to current goals (Paller & Voss 2004). Indeed, Winson’s (1985, 2004) prescient proposal emphasized that offline memory processing engaged in the sleeping brain would help in dealing with ongoing issues encountered during the waking state. Cartwright (2010) conducted many studies of dreaming that led her to also emphasize this view. Earlier dreams in the night are generally based on recent memory fragments, whereas later dreams incorporate memory fragments from an increasingly farther past (Roffwarg et al. 1978). Cartwright (1990) found that dreams during a single night sometimes all relate to a common theme, drawing on relevant knowledge from progressively further back in time. She identified systematic relationships between the dreams of recently divorced individuals and their postdivorce coping strategies, and critically, she found better emotional adjustment the more dreaming was used in this fashion.
A reasonable speculation, then, is that in dreams, and perhaps in sleep more generally, emotional issues can be worked through and behavioral strategies can be adjusted with reference to very recent experiences, older experiences, and their relationships. Strikingly, this use of memory processing during sleep is in keeping with the conception of consolidation described above, whereby recent memories are integrated with older ones to facilitate storage. Two benefits of sleep can thus be described as (a) reactivating and reorganizing memories and (b) creatively fine-tuning strategies in the service of solving current problems. These two benefits correspond roughly to modifying declarative memories and nondeclarative memories, respectively.
Emotional Memories and Emotional Regulation
How might sleep zero in on the specific memory processing that would be optimal for guiding problem solving? Ideally, the specific memories reactivated should be those relevant in some way for important upcoming challenges. It is thus sensible that emotional factors should enter into this computation.
Various findings support the claim that emotional memories are preferentially consolidated during sleep. Emotional memories likely benefit from arousal-related tagging during encoding (Payne & Kensinger 2018). TMR was used in one study that compared results for negative emotional pictures and neutral pictures (Cairney et al. 2014). The participants first learned picture-location associations as in many other TMR experiments, in this case with just six locations. Half of the pictures were cued during SWS using semantically related sounds. After sleep, an interesting pattern of results was revealed: Total time spent in SWS predicted faster spatial recall responses for cued pictures, but only in the negative emotion condition. Cuing did not influence spatial recall accuracy. The number of spindles during SWS also predicted the speed of recall responses, suggesting that sleep spindles mediate a selective enhancement of reactivated emotional memories.
REM may be particularly important for processing emotional memories, but the evidence is mixed. Although amygdala activity is increased during REM sleep (Maquet et al. 1996), TMR cues improved emotional memories not when presented during REM but rather when presented during NREM (Lehmann et al. 2016). Of course, both NREM and REM may be relevant. The evidence on whether memory processing during sleep increases or decreases arousal regulation for emotional memories in also unclear (Tempesta et al. 2018). In one study, women exposed to a traumatic movie followed by a period of sleep experienced fewer traumatic memories when compared with women exposed to the traumatic movie followed by a neutral movie (Kleim et al. 2016). In another study, sleep preserved the autonomic response to emotional stimuli after sleep, but it reduced the autonomic response as well as valence ratings to emotional stimuli one week later (Bolinger et al. 2019). That is, sleep may be helpful for arousal regulation not on the next day but after some number of days. Although more research is certainly needed in this area, multiple investigators have championed the view that sleep aids the overnight resolution of emotional distress (e.g., Walker & van der Helm 2009). Notably, emotional benefits from sleep may be secondary to memory reorganization (Vanderheyden et al. 2015).
Lack of sleep can certainly contribute, perhaps indirectly, to emotional dysregulation (as may be particularly evident, anecdotally, in cranky children in need of sleep). In an fMRI study, reduced top-down cognitive control was evident in sleep-deprived compared to control participants (Yoo et al. 2007). Decreased connectivity between the medial prefrontal cortex and the amygdala was presumed to have caused greater amygdala activation and thus to have increased emotional reactivity.
Going beyond the commonsense idea that an insufficient quantity of sleep can make someone grumpy, we should also consider both the quality of sleep and the quality of the memory processing that transpires during sleep. How memories are processed during sleep could determine whether there are negative or positive consequences for psychological well-being. In the next section we explore the negative consequences more broadly.
SLEEP AND PSYCHOLOGICAL WELL-BEING
Bringing together the critical role of sleep in memory consolidation with the fact that memory is used for many cognitive functions leads to the following further inference: Sleep disturbances may have far-reaching cognitive consequences. Certainly drowsiness can affect cognition in many ways, but there’s more to it than simply the quantity of sleep. Memory processing during sleep might not be working properly. Even if there is sufficient sleep and plenty of time for overnight memory processing, that processing may become dysfunctional. A flaw could develop in the way memories are selected for reactivation or in the way they are processed, with the consequence that the normal progress of nightly consolidation could go awry. If so, the outcome may extend to pervasive problems for one’s well-being and mental health. Speculatively, some affective disorders may have at their core a dysfunction in memory processing during sleep.
Major depressive disorder (MDD), for example, is characterized by impaired sleep continuity, lower-than-normal density of NREM sleep, and higher-than-normal density of REM sleep (Steiger & Pawlowski 2019). Additionally, slow-wave activity (conventionally defined as EEG power at 0.5–4 Hz), which includes the SOs described above, is abnormal in two ways. First, patients suffering from MDD exhibit reduced slow-wave activity relative to healthy controls (Borbély et al. 1984). Second, the dynamics of this activity is altered. A decline in slow-wave activity over the course of a night of sleep is the typical pattern, thought to be a sign of normal sleep physiology. This decline is disrupted in depression (Kupfer et al. 1986) and restored upon effective treatment (Jindal et al. 2003). Moreover, insomnia is common in patients with depression (Buysse et al. 2008, Manber et al. 2008). Relatedly, bright-light therapy can be helpful in depression, in conjunction with its effects on circadian rhythms and sleep (Pail et al. 2011). Memory symptoms noted in depression, particularly overgeneral autobiographical retrieval (Williams et al. 2007), may also fit with the dependence of memory function on sleep and the alteration of sleep in depression.
A persistent controversy in depression research has concerned whether sleep abnormalities are a result of depressive illness or a contributing factor to it. The latter direction of causality has received considerable support, including evidence that sleep abnormalities often precede depressive episodes (Ohayon & Roth 2003). Importantly, however, these do not need to be mutually exclusive alternatives. A bidirectional relationship is likely, as sleep and affective symptoms may reinforce each other throughout the progression of MDD (Bao et al. 2017).
To further understand the mechanisms whereby sleep abnormalities may be operative in producing or exacerbating mood disorders such as MDD, research is needed to relate measures of sleep physiology in patients to cognition and mood. In particular, we concern ourselves here with the memory functions of sleep and the contribution of memory to MDD progression. Some of the most debilitating symptoms of MDD are tied to memory. By one account, patients suffering from MDD hold and empower negative representations about the self that serve to bias memory processing toward negative experiences (Everaert et al. 2012). Consonant with this idea, depressed patients tend to recall negative memories better than healthy individuals do (Fattahi Asl et al. 2015). It is therefore tempting to hypothesize that sleep disturbance and MDD are mediated by biasing effects of memory consolidation. Multiple investigators thinking along these lines have emphasized the role of REM sleep, suggesting that its prominence in MDD serves to overemphasize the consolidation of negative memories (Walker & van der Helm 2009, Harrington et al. 2018). Correspondingly, the three major classes of antidepressant drugs all profoundly suppress REM sleep (Vertes & Eastman 2000). SWS has garnered far less attention in research on depression and related disorders, but given the wealth of evidence supporting the role of SWS in memory consolidation, it may be more relevant in this context than commonly assumed.
Insights into other psychiatric disorders beyond depression may also emerge through investigations of the relevance of memory consolidation during SWS. For example, other mood disorders such as social anxiety disorder can involve a negative memory bias as in depression (Glazier & Alden 2019), and the bias may be operative during sleep. Supporting sleep’s suggested role in overemphasizing the consolidation of negative memories, one TMR study found that cueing negative memories resulted in more negative ratings one week later in socially anxious adolescents but not in healthy ones (Groch et al. 2017).
Sleep disturbances are reported in 50–80% of people with psychiatric disorders (Franzen & Buysse 2017). In posttraumatic stress disorder (PTSD), memory issues are particularly prominent, as are sleep-related symptoms, and some PTSD symptoms have been attributed to an excessive consolidation of negative memories (Pitman et al. 2000). Sleep disturbances immediately following traumatic experiences may serve as a protective mechanism to prevent the consolidation of traumatic memories, though consolidation of emotional memories may span many nights (Bolinger et al. 2019).
An excess of arousals during REM sleep may be one sign of maladaptive sleep in relation to emotional distress. This type of unstable or disrupted REM sleep has been observed in PTSD (Germain et al. 2008) and in insomnia (Riemann et al. 2012). To examine the association between this sign of low-quality sleep and emotional coping, Wassing and colleagues (2019) subjected healthy individuals to an episode of self-focused distress, and they found that sleep with abundant spindles followed by uninterrupted REM sleep predicted a healthier orientation the next day, as reflected by an adaptation in amygdala fMRI activity (Figure 4). If we extend ideas from this experiment to patients, it could be that individuals who suffer the most persistent distress following trauma or emotional insult are those plagued by maladaptive memory processing during sleep; REM sleep punctuated by signs of arousal, presumably mediated by ascending noradrenergic systems, could be a sign of this specific type of maladaptive sleep-thinking. A fruitful direction for future research would be to flesh out the connections among REM stability, spindles prior to REM, and memory processing during sleep in relation to distressing memories.
Figure 4.
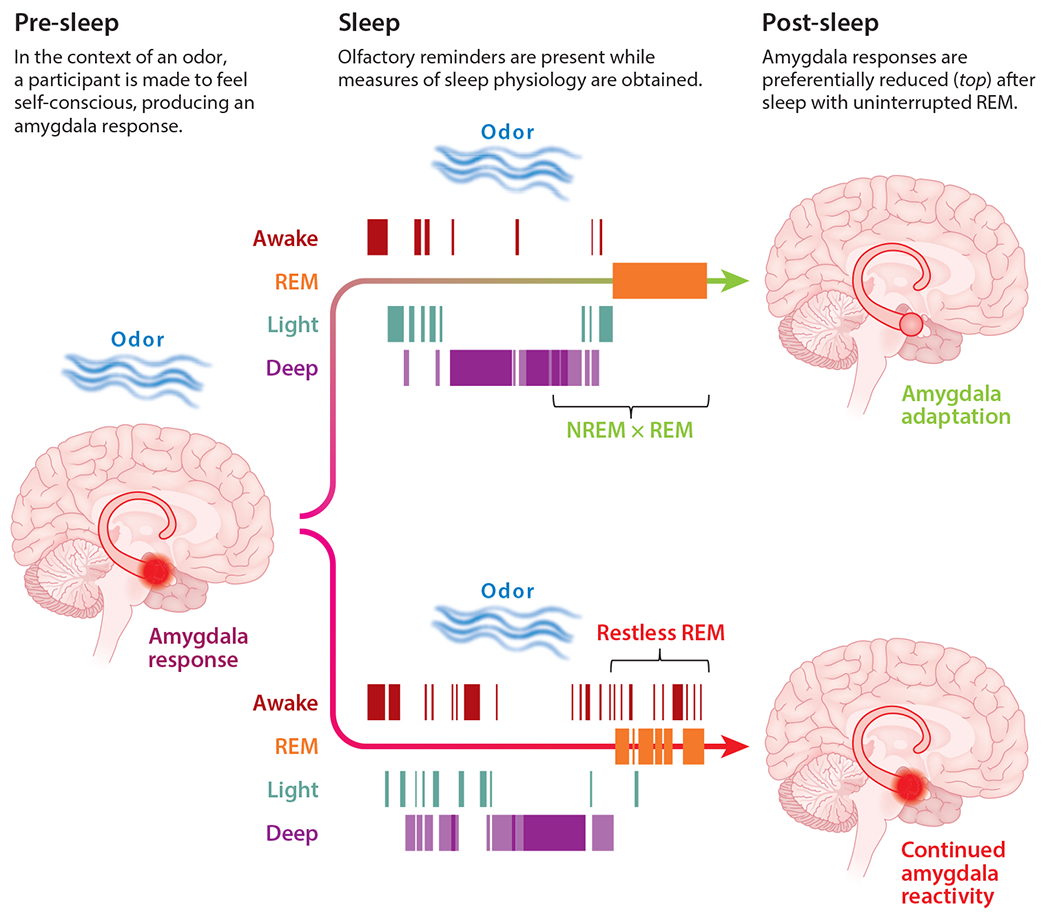
Wassing et al. (2019) used a variant of the targeted memory reactivation (TMR) procedure to examine how participants responded to a distressing episode. Before sleep, participants listened to a recording of their own off-key singing, which elicited a strong amygdala response. During sleep, odors linked with the stressful episode (and a control odor) were presented over multiple stages of sleep. Regardless of the TMR manipulation, two patterns of sleep were observed: sleep with undisturbed rapid eye movement (REM) periods (top) or REM interrupted by brief arousals (bottom). Amygdala responses the next day were reduced to the extent that REM was uninterrupted and also to the extent that spindles were frequent in the non-REM (NREM) period prior to REM. TMR with the stress-associated odor was found to enhance these effects. These findings demonstrate a specific connection between sleep after a stressful experience and future emotional responses. Figure adapted with permission from Wassing et al. (2019).
Overall, this conceptualization of the interactions among maladaptive consolidation, sleep problems, and memory-related emotional problems may prove to be fruitful for understanding mental disorders. The memory work accomplished during sleep ideally supports adaptive memory function, but it can become maladaptive instead. In depression and anxiety, a patient’s day may be filled with excessive rumination and worry. Negative thoughts may be recirculated to the detriment of the person’s well-being. Harmful thinking may pervade sleep as well, producing further negative consequences. In this sense, optimal memory processing during sleep may elude psychiatric patients—and by extension, perhaps the same applies to those who experience less extreme psychological difficulties. Further investigation of sleep-related memory consolidation mechanisms could thus lead to new insights into sleep that apply whether an individual has clinical symptoms or not.
HARNESSING SLEEP TO IMPROVE PSYCHOLOGICAL WELL-BEING
The cumulative research using TMR shows that sleep-based consolidation is modifiable. Although much remains to be understood about this memory processing—including its neural substrates, its consequences, and the factors that dictate which memories are reactivated during sleep—at this point we can suggest some possible strategies for promoting beneficial sleep cognition. Efforts to optimize memory processing during sleep could have ramifications not only for memory but also for improving quality of life.
The importance of one’s quality of sleep is widely recognized. Standard ideas about sleep hygiene are now promoted widely (e.g., Walker 2017). Good sleep habits include endeavoring to obtain a sufficient quantity of sleep every night; arranging a dark, quiet, and cool sleep environment; avoiding alcohol, caffeine, and late-night screen use; and adopting a consistent bedtime and wake-up time seven days a week.
But what constitutes good-quality sleep? Our view is that the definition of sleep quality should be widened. It should not be limited to standard sleep physiology metrics such as the total time asleep, the continuity of sleep, or the density of slow waves and spindles. Such measures might be insensitive to subtle memory transactions. The importance of how memories are processed during sleep, and of the specific sorts of memories that are reactivated, must also be considered. Ultimately, functional measures should be developed to assess sleep’s contribution to memory, which means taking into account the degree of beneficial or detrimental memory reactivation.
A major factor that may influence sleep quality is the reemergence of negative memories. Much as waking rumination can be harmful, a proliferation of negative memories may also occur during sleep and may likewise have negative psychological consequences. Sleep-based rumination might be quite prevalent, regardless of clinical diagnosis. If rumination while awake can take a toll on well-being and reinforce a preoccupation with negative concerns, then the same may be true for sleep—harmful thoughts may pervade one’s sleep cognition, with harmful consequences. One’s remembered dream content might occasionally reflect these negative thoughts. Indeed, recurring nightmares or recurring stressful dreams could be a symptom of more pervasive suboptimal sleep. With reference to the literature reviewed above, memory processing during SWS could be very important, whether or not negative dreams are recalled after awakening.
Broadening this idea, rumination may be just one variant of a larger category of maladaptive memory processing during sleep. To take an extreme example, if the mind is excessively agitated during sleep, incessantly revisiting negative thoughts and memories, one consequence could be objectively poor sleep. This poor sleep could include a difficulty staying asleep or maintaining certain sleep stages, as in the arousal-filled REM periods noted above. Alternatively, sleep could look fairly normal electrophysiologically, but the nature of the memory processing could still have unwanted consequences for the individual after sleep. Intuitively, many individuals experience waking up “on the wrong side of the bed” after a full night of sleep, feeling unrested and ill-tempered. In both cases—maladaptive sleep with or without obvious signs of sleep disruption—there could be harmful ramifications for the waking mind.
A key empirical question is whether cultivating calm sleep can produce benefits for the waking mind. A worthy future goal for TMR research would be to test various methods to calm the mind during sleep. Exploring new strategies in this direction requires departing from the orthodox assumption that nothing can be done about the paths our minds take while we are asleep.
Such exploration must proceed together with research aimed at advancing our understanding of sleep, given that sleep may help with adaptive processing of traumatic memories. It could be that overnight emotional turmoil is the price we pay for subsequent waking benefits. If so, this sort of sleep-work should be perpetuated, not eliminated. This possibility deserves to be investigated further, but here we present an opposing line of thought: We suggest that sleep with maladaptive memory processing can be detrimental, in which case it could be helpful to change it, for instance through the counter-reactivation or inception of positive memories, feelings, or concepts.
Given that memory processing during sleep is modifiable, sleep-based consolidation affords the opportunity not just for improving memory function but also for restructuring the self. It may be possible to adjust the memories that we emphasize and maintain at the forefront of our psyche in an intentional and strategic way, in order to reach one’s goals of self-improvement. For example, if one values a reduction in self-centeredness and an increase in compassion for others, this intention can lead one to prioritize certain memories for reactivation and subsequent consolidation. Such an approach does not require something as unrealistic as drastically altering one’s set of autobiographical memories or starting all over by forgetting them, as in psychogenic amnesia. Rather, one might seek to gradually adjust both one’s declarative knowledge and one’s habits in a prosocial direction. In this sense, changing for the better concerns both declarative and nondeclarative memories. Change is possible based on what information we emphasize and recapitulate while awake, which is subject to further memory processing during sleep.
The natural, daily course of intentional wake memory processing followed by sleep memory processing may be sufficient to put into play this scenario of change for the better. A TMR protocol could also be adapted for use in conjunction with wake training to encourage positive changes. One example of such an application is in the context of the social biases that can implicitly affect our decisions and behavior toward others. Counter-stereotype training is one method to attempt to adjust such biases. In one study, TMR during sleep following such training was found to enhance bias changes, as indexed by performance measures from the Implicit Association Test (Hu et al. 2015). These measures, however, are not high in test-retest reliability and are poor predictors of discriminatory behavior (Oswald et al. 2013). Also, one attempt to closely replicate these TMR results failed to do so (Humiston & Wamsley 2019). Ingrained attitudes may be difficult to change and also difficult to measure. Yet, various sorts of efforts along related lines might be worth pursuing in the interest of developing stronger cognitive control—for example, to increase prosocial tendencies.
Aside from TMR, there may be other ways to change the specific processing engaged by the sleeping brain. One tactic would be to control pre-sleep mental activity, in that positive waking thoughts could produce positive sleeping thoughts. Mental content during the last few minutes before falling asleep might be particularly influential. Indeed, a pre-sleep hypnosis procedure has been shown in several studies to increase sleep physiology measures of SWS in highly hypnotizable young subjects (e.g., Cordi et al. 2020). Adding TMR might extend these benefits further. More research should examine the consequences of tactics such as holding the pre-sleep intention to fill one’s sleep with positive thoughts. Dream control provides a further demonstration that we are not helplessly at the mercy of whatever happens during sleep. In the context of a lucid dream, for example, an individual can engage the intention to change what happens next. Changing a dream can be accomplished by using pre-sleep intentions and by using a variant of the TMR procedure to prompt particular dream content (Konkoly et al. 2020).
The TMR research summarized above could be extended in many ways to attempt to instill particular thoughts during sleep. TMR procedures begin with pre-sleep learning, which in this case could involve a positive cognitive-affective orientation. Various tasks can be used to increase calm and peaceful states. Another option would be to decrease negative thinking, engaging strategies to dampen or reframe anxious, stressful, or otherwise maladaptive thoughts. Innovative TMR strategies could take aim at both of these goals to determine whether such changes are possible. Whereas Wassing and colleagues (2019) used TMR to reinstate the context of a particularly stressful and negative affect-laden experience, a variation on their design could instead reinstate positive experiences or invoke strategies that effectively reduce the unfortunate impact of negative experiences.
Finally, we acknowledge that the link between sleep and waking cognition is bidirectional. The quality of sleep reflects cognitive activity recently engaged while awake; the quality of wakefulness, in turn, reflects consolidation and other cognitive activity engaged while asleep; the quality of life reflects both. We may tend to ignore the portion of our lives occupied by sleep because it is predominantly out of view (like the dark side of the moon), but we do so at our peril. By understanding the mutual relationships between sleep and wake, and by embracing innovative ways to improve sleep, we can change the waking mind for the better.
SLEEP PHYSIOLOGY.
Sleep is classically divided into stages characterized by distinct neural and bodily functions. Although future advances in sleep research and neural decoding may lead to refinements in our thinking about sleep physiology, contemporary schemes distinguish rapid eye movement (REM) sleep from three stages of non-REM (NREM) sleep. Each stage has characteristic electrophysiological features evident in the electroencephalogram, electrooculogram, and electromyogram. NREM includes stage 1 (N1), stage 2 (N2), and stage 3 (N3). Sleep progresses from light sleep to deeper sleep across these three stages as the ease of arousability decreases. N3 (formerly divided into N3 and N4) is also termed slow-wave sleep due to the high-amplitude slow waves (0.5–4 Hz) observed in the EEG. Nocturnal sleep consists of multiple cycles, typically 90 minutes in duration, including light sleep, slow-wave sleep, REM, and then a return to NREM. The majority of sleep is usually spent in N2 and slow-wave sleep, the stages most strongly implicated in memory consolidation to date.
ACKNOWLEDGMENTS
We thank our colleagues for many fruitful discussions, including Jessica Payne, Marcia Grabowecky, Bjorn Rasch, Ken Norman, Phyllis Zee, John Wixted, and members of Ken Paller’s lab group. We are also grateful for funding from the Human Frontier Science Program (to E.S.) and from the Mind Science Foundation, the McKnight Foundation, DARPA, NIH (R01-NS112942, T32-NS047987, and T32-HL007909), and NSF (BCS-1921678 and BCS-1829414).
Glossary
- Memory consolidation
changes in memory storage following initial acquisition that stabilize new information and can include integration, transformation, and generalization
- Memory reactivation
reemergence of information stored in brain networks during a previous experience, with or without the conscious experience of retrieving the memory
- Declarative memories
the memories we rely on for the recall and recognition of factual and event information
- Nondeclarative memories
an umbrella term for skills, habits, conditioning, priming, and other types of implicit learning, all typically exhibited without conscious retrieval
- Anterograde amnesia
a memory disorder of impaired learning of new facts and events
- Retrograde amnesia
a memory disorder of impaired retrieval of facts and events acquired prior to the onset of the disorder
- Active systems consolidation hypothesis
the hypothesis that cortical traces of declarative memories are shaped during slow-wave sleep through hippocampal reactivation, facilitated by synchronized oscillations in different brain regions
- Slow-wave sleep (SWS)
the deepest stage of sleep, dominated by cortical slow waves (0.5–4 Hz) that are observed in the electroencephalogram
- Nested oscillations
oscillations at one frequency coinciding with features (e.g., peak or trough) of another, lower-frequency oscillation
- Hippocampal replay
neuronal activity recapitulating that at learning, thought to constitute a readout of stored information as a sequence of place-cell action potentials
- Targeted memory reactivation (TMR)
a method involving the unobtrusive presentation of learning-related stimuli during sleep, thereby biasing reactivation and impacting consolidation
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
1 A remarkable exception to this rule has been documented in people with so-called highly superior autobiographical memory. Fewer than 100 individuals have been reported to have this capability. Their superior abilities have yet to be explained, but they are limited to autobiographical events and certain facts that are personally meaningful (LePort et al. 2012). Instead of forgetting most days, they seem to remember nearly every day. They can recall events from long ago with the same ease that most of us recall what happened yesterday. Sleep may contribute to this capability. Our preliminary finding of a high density of sleep spindles in several of these individuals (Westerberg et al. 2020) suggests that their sleep may be particularly beneficial, but additional evidence is needed to confirm this possibility.
LITERATURE CITED
- Aly M, Ranganath C. 2018. New perspectives on the hippocampus and memory. Neurosci. Lett. 680:1–3 [DOI] [PubMed] [Google Scholar]
- Andrillon T, Kouider S. 2019. The vigilant sleeper: neural mechanisms of sensory (de)coupling during sleep. Curr. Opin. Physiol. 15:47–59 [Google Scholar]
- Andrillon T, Pressnitzer D, Léger D, Kouider S. 2017. Formation and suppression of acoustic memories during human sleep. Nat. Commun. 8(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, Gobel EW, O’Hare JK, Reber PJ, Paller KA. 2012. Cued memory reactivation during sleep influences skill learning. Nat. Neurosci. 15(8):1114–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, Paller KA. 2017. Using oscillating sounds to manipulate sleep spindles. Sleep 40(3):zsw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, Piloto L, Wang M, Pacheco P, Norman KA, Paller KA. 2018. Sleep spindle refractoriness segregates periods of memory reactivation. Curr. Biol. 28(11):1736–43.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, Holtzman Y, Samnon P, Eshel N, Harel E, Sobel N. 2014. Olfactory aversive conditioning during sleep reduces cigarette-smoking behavior. J. Neurosci. 34(46):15382–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzi A, Shedlesky L, Ben-Shaul M, Nasser K, Oksenberg A, et al. 2012. Humans can learn new information during sleep. Nat. Neurosci. 15(10):1460–65 [DOI] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. 2008. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131(7):1806–17 [DOI] [PubMed] [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F,Junghanns K. 2007. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn. Mem. 14(5):336–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y-P, Han Y, Ma J, Wang R-J, Shi L, et al. 2017. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neurosci. Biobehav. Rev. 75:257–73 [DOI] [PubMed] [Google Scholar]
- Belal S, Cousins J, El-Deredy W, Parkes L, Schneider J, et al. 2018. Identification of memory reactivation during sleep by EEG classification. NeuroImage 176:203–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, Wilson MA. 2012. Biasing the content of hippocampal replay during sleep. Nat. Neurosci. 15(10):1439–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. 2012. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59(3):2733–42 [DOI] [PubMed] [Google Scholar]
- Berkers RMWJ, Ekman M, van Dongen EV, Takashima A, Paller KA, Fernández G. 2018. Cued reactivation during slow-wave sleep induces connectivity changes related to memory stabilization. Sci. Rep. 8:16958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolinger E, Cunningham TJ, Payne JD, Bowman MA, Bulca E, et al. 2019. Sleep’s benefits to emotional processing emerge in the long term. Cortex 120:457–70 [DOI] [PubMed] [Google Scholar]
- Borbély AA, Tobler I, Loepfe M, Kupfer DJ, Ulrich RF, et al. 1984. All-night spectral analysis of the sleep EEG in untreated depressives and normal controls. Psychiatry Res. 12(1):27–33 [DOI] [PubMed] [Google Scholar]
- Born J, Rasch B, Gais S. 2006. Sleep to remember. Neuroscientist 12(5):410–24 [DOI] [PubMed] [Google Scholar]
- Born J, Wilhelm I. 2012. System consolidation of memory during sleep. Psychol. Res. 76(2):192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt S, Pöhlchen D, Táumer E, Gais S, Schönauer M. 2018. Incubation, not sleep, aids problem-solving. Sleep 41(10):155. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rössler W. 2008. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep 31(4):473–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G 1998. Memory consolidation during sleep: a neurophysiological perspective. J. Sleep Res. 7(S1):17–23 [DOI] [PubMed] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. 2009. REM, not incubation, improves creativity by priming associative networks. PNAS 106(25):10130–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney SA, Durrant SJ, Hulleman J, Lewis PA. 2014. Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep 37(4):701–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney SA, Guttesen AÁV, El Marj N, Staresina BP. 2018. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr. Biol. 28(6):948–954.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney SA, Lindsay S, Sobczak JM, Paller KA, Gaskell MG. 2016. The benefits of targeted memory reactivation for consolidation in sleep are contingent on memory accuracy and direct cue-memory associations. Sleep 39(5):1139–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright R 1990. A network model of dreams. In Sleep and Cognition, ed. Bootzin RR, Kihlstrom JF, Schacter DL, pp. 179–89. Washington, DC: Am. Psychol. Assoc. [Google Scholar]
- Cartwright RD. 2010. The Twenty-Four Hour Mind: The Role of Sleep and Dreaming in Our Emotional Lives. Oxford, UK: Oxford Univ. Press [Google Scholar]
- Clemens Z, Fabó D, Halász P. 2006. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci. Lett. 403(1):52–56 [DOI] [PubMed] [Google Scholar]
- Cordi MJ, Rossier L, Rasch B. 2020. Hypnotic suggestions given before nighttime sleep extend slow-wave sleep as compared to a control text in highly hypnotizable subjects. Int. J. Clin. Exp. Hypn. 68(1):105–29 [DOI] [PubMed] [Google Scholar]
- Cousins JN, El-Deredy W, Parkes LM, Hennies N, Lewis PA. 2016. Cued reactivation of motor learning during sleep leads to overnight changes in functional brain activity and connectivity. PLOS Biol. 14(5):e1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Hofman WF, Talamini LM. 2012. Involvement of spindles in memory consolidation is slow wave sleep-specific. Learn. Mem. 19(7):264–67 [DOI] [PubMed] [Google Scholar]
- Cox R, Rüber T, Staresina BP, Fell J. 2019. Heterogeneous profiles of coupled sleep oscillations in human hippocampus. Neuroimage 202:116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, van Driel J, de Boer M, Talamini LM. 2014. Slow oscillations during sleep coordinate interregional communication in cortical networks. J. Neurosci. 34(50):16890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creery JD, Oudiette D, Antony JW, Paller KA. 2015. Targeted memory reactivation during sleep depends on prior learning. Sleep 38(5):755–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F,Mitchison G. 1983. The function of dream sleep. Nature 304:111–14 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. 2010. The memory function of sleep. Nat. Rev. Neurosci. 11(2):114–26 [DOI] [PubMed] [Google Scholar]
- Dudai Y 2012. The restless engram: Consolidations never end. Annu. Rev. Neurosci. 35:227–47 [DOI] [PubMed] [Google Scholar]
- Duncan CP. 1949. The retroactive effect of electroshock on learning. J. Cotnp. Physiol. Psychol. 42(1):32–44 [DOI] [PubMed] [Google Scholar]
- Everaert J, Koster EHW, Derakshan N. 2012. The combined cognitive bias hypothesis in depression. Clin. Psychol. Rev. 32(5):413–24 [DOI] [PubMed] [Google Scholar]
- Fattahi Asl A, Ghanizadeh A, Mollazade J, Aflakseir A. 2015. Differences of biased recall memory for emotional information among children and adolescents of mothers with MDD, children and adolescents with MDD, and normal controls. Psychiatry Res. 228(2):223–27 [DOI] [PubMed] [Google Scholar]
- Fernandez LMJ, Luthi A. 2020. Sleep spindles: mechanisms and functions. Physiol. Rev. 100:805–68 [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz A, Oliva A, de Oliveira EF, Rocha-Almeida F, Tingley D, Buzsaki G. 2019. Long-duration hippocampal sharp wave ripples improve memory. Science 364(6445):1082–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ. 2017. Replay comes of age. Annu. Rev. Neurosci. 40:581–602 [DOI] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ. 2017. Sleep in psychiatric disorders. In Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects , ed. Chokroverty S, pp. 977–96. New York: Springer [Google Scholar]
- Fuentemilla L,Miró J, Ripollás P, Vilà-Balló A,Juncadella M, et al. 2013. Hippocampus-dependent strengthening of targeted memories via reactivation during sleep in humans. Curr. Biol. 23(18):1769–75 [DOI] [PubMed] [Google Scholar]
- Gais S, Mölle M, Helms K, Born J. 2002. Learning-dependent increases in sleep spindle density. J. Neurosci. 22(15):6830–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. 2008. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med. Rev. 12(3):185–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva-Sagiv M, Nir Y. 2019. Local sleep oscillations: implications for memory consolidation. Front. Neurosci. 13:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. 2009. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12(10):1222–23 [DOI] [PubMed] [Google Scholar]
- Glazier BL, Alden LE. 2019. Social anxiety disorder and memory for positive feedback. J. Abnorm. Psychol. 128(3):228–33 [DOI] [PubMed] [Google Scholar]
- Groch S, Preiss A, McMakin DL, Rasch B, Walitza S, et al. 2017. Targeted reactivation during sleep differentially affects negative memories in socially anxious and healthy children and adolescents. J. Neurosci. 37(9):2425–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington MO, Johnson JM, Croom HE, Pennington K, Durrant SJ. 2018. The influence of REM sleep and SWS on emotional memory consolidation in participants reporting depressive symptoms. Cortex 99:281–95 [DOI] [PubMed] [Google Scholar]
- Hebscher M, Wing E, Ryan J, Gilboa A. 2019. Rapid cortical plasticitysupports long-term memory formation. Trends Cogn. Sci. 23(12):989–1002 [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Mander BA,Jagust WJ, Knight RT, Walker MP. 2018. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron 97(1):221–30.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin S, Borges H, Shankar A, Sarac C, Melloni L, et al. 2019. Closed-loop acoustic stimulation enhances sleep oscillations but not memory performance. eNeuro 6(6):0306–19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Antony JW, Creery JD, Vargas IM, Bodenhausen GV, Paller KA. 2015. Unlearning implicit social biases during sleep. Science 348(6238):1013–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Cheng L, Chiu MH, Paller KA. 2020. Promoting memory consolidation during sleep: a meta-analysis of targeted memory reactivation. Psychol. Bull. 146:218–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Felice Ghilardi M, Massimini M, Tononi G. 2004. Local sleep and learning. Nature 430(6995):78–81 [DOI] [PubMed] [Google Scholar]
- Humiston GB, Wamsley EJ. 2019. Unlearning implicit social biases during sleep: a failure to replicate. PLOS ONE 14(1):e0211416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10(1):100–7 [DOI] [PubMed] [Google Scholar]
- Jindal RD, Friedman ES, Berman SR, Fasiczka AL, Howland RH, Thase ME. 2003. Effects of sertraline on sleep architecture in patients with depression. J. Clin. Psychopharmacol. 23(6):540–48 [DOI] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. 1994. Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265(5172):679–82 [DOI] [PubMed] [Google Scholar]
- Khodagholy D, Gelinas JN, Buzsáki G. 2017. Learning-enhanced coupling between ripple oscillations in as-sociation cortices and hippocampus. Science 358(6361):369–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim B, Wysokowsky J, Schmid N, Seifritz E, Rasch B. 2016. Effects of sleep after experimental trauma on intrusive emotional memories. Sleep 39(12):2125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing JG, Niethard N, Born J. 2019. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 22(10):1598–610 [DOI] [PubMed] [Google Scholar]
- Konkoly KR, Appel K, Chabani E, Mangiaruga A, Gott J, et al. 2020. Real-time dialogue between experimenters and dreamers during REM sleep. Work. Pap., Northwestern Univ., Evanston, IL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Reynolds CF, Ulrich RF, Grochocinski VJ. 1986. Comparison of automated REM and slow-wave sleep analysis in young and middle-aged depressed subjects. Biol. Psychiatry 21(2):189–200 [DOI] [PubMed] [Google Scholar]
- Latchoumane C-FV, Ngo H-VV, Born J, Shin H-S. 2017.Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95(2):424–35.e6 [DOI] [PubMed] [Google Scholar]
- Laventure S, Benchenane K. 2020. Validating the theoretical bases of sleep reactivation during sharp-wave ripples and their association with emotional valence. Hippocampus 30(1):19–27 [DOI] [PubMed] [Google Scholar]
- Lechner HA, Squire LR, Byrne JH. 1999. 100 years of consolidation—remembering Muller and Pilzecker. Learn. Mem. 6(2):77–87 [PubMed] [Google Scholar]
- Lehmann M, Schreiner T, Seifritz E, Rasch B. 2016. Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep. Sci. Rep. 6(1):39229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePort AKR, Mattfeld AT, Dickinson-Anson H, Fallon JH, Stark CEL, et al. 2012. Behavioral and neuroanatomical investigation of Highly Superior Autobiographical Memory (HSAM). Neurobiol. Learn. Mem. 98(1):78–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Bendor D.2019. How targeted memory reactivation promotes the selective strengthening of memories in sleep. Curr. Biol. 29(18):R906–12 [DOI] [PubMed] [Google Scholar]
- Lewis PA, Durrant SJ. 2011. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn. Sci. 15(8):343–51 [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F. 2016. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr. Biol. 26(16):2127–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz ND, Diekelmann S, Hinse-Stern P, Born J, Rauss K. 2017. Sleep supports the slow abstraction of gist from visual perceptual memories. Sci. Rep. 7:42950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, Pedro-Salcedo MGS, Kuo TF, Kalista T. 2008. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep 31(4):489–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Péters J-M, Aerts J, Delfiore G, Degueldre C, et al. 1996. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature 383(6596):163–66 [DOI] [PubMed] [Google Scholar]
- Marr D 1971. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. B 262(841):23–81 [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J. 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444(7119):610–13 [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. 2004. The sleep slow oscillation as a traveling wave. J. Neurosci. 24(31):6862–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL,McNaughton BL, O’Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102(3):419–57 [DOI] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, et al. 2013. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J. Neurosci. 33(10):4494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TD, Chong TT-J, Aimola Davies AM, Johnson MR, Irani SR, et al. 2020. Human hippocampal CA3 damage disrupts both recent and remote episodic memories. eLife 9:e41836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner CE, Fogel SM, Cote KA. 2006. Habitual napping moderates motor performance improvements following a short daytime nap. Biol. Psychol. 73(2):141–56 [DOI] [PubMed] [Google Scholar]
- Mölle M, Bergmann TO, Marshall L, Born J. 2011. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep 34(10):1411–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Gais S, Born J. 2002. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 22(24):10941–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G, Pilzecker A. 1900. Experimented Beiträge zur Lehre vom Gedächtnis [Experimental contributions to the theory of memory]. Leipzig, Ger: Verlag von Johann Ambrosius Barth [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. 2007. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci. 30:99–122 [DOI] [PubMed] [Google Scholar]
- Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19(21):9497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. 1997. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 7(2):217–27 [DOI] [PubMed] [Google Scholar]
- Ngo H-VV, Fell J, Staresina BP. 2020. Sleep spindles mediate hippocampal-neocortical coupling during sharp-wave ripples. eLife 9:e57011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo H-VV,Martinetz T, Born J, Mölle M. 2013. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78(3):545–53 [DOI] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, et al. 2011. Regional slow waves and spindles in human sleep. Neuron 70(1):153–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M, Walker MP 2007. Daytime naps, motor memory consolidation and regionally specific sleep spin-dles. PLOS ONE 2(4):e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA,Newman EL,Perotte AJ. 2005. Methods for reducing interference in the Complementary Learn-ing Systems model: oscillating inhibition and autonomous memory rehearsal. Neural Netw. 18(9):1212–28 [DOI] [PubMed] [Google Scholar]
- Norman Y, Yeagle EM, Khuvis S, Harel M, Mehta AD, Malach R. 2019. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science 365(6454):eaax1030. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Roth T. 2003. Place of chronic insomnia in the course of depressive and anxiety disorders. J. Psychiatr. Res. 37(1):9–15 [DOI] [PubMed] [Google Scholar]
- Oswald FL,Mitchell G, Blanton H,Jaccard J, Tetlock PE. 2013. Predicting ethnic and racial discrimination: a meta-analysis of IAT criterion studies. J. Pers. Soc. PsychoO. 105(2):171–92 [DOI] [PubMed] [Google Scholar]
- Oudiette D, Paller KA. 2013. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn. Sci. 17(3):142–49 [DOI] [PubMed] [Google Scholar]
- Pail G, Huf W, Pjrek E, Winkler D, Willeit M, et al. 2011. Bright-light therapy in the treatment of mood disorders. Neuro psychobiology 64(3):152–62 [DOI] [PubMed] [Google Scholar]
- Paller KA. 2002. Cross-cortical consolidation as the core defect in amnesia: prospects for hypothesis testing with neuropsychology and neuroimaging. In The Neuropsychology of Memory , ed. Squire LR, Schacter DL, pp. 73–87. New York: Guilford. 3rd ed. [Google Scholar]
- Paller KA. 2009. Memory consolidation: systems. In Encyclopedia of Neuroscience , ed. Squire LR, pp. 741–49. Oxford, UK: Academic [Google Scholar]
- Paller KA, Voss JL. 2004. Memory reactivation and consolidation during sleep. Learn. Mem. 11(6):664–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalambros NA, Santostasi G, Malkani RG, Braun R, Weintraub S, et al.2017. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front. Hum. Neurosci. 11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalambros NA, Weintraub S, Chen T, Grimaldi D, Santostasi G, et al. 2019. Acoustic enhancement of sleep slow oscillations in mild cognitive impairment. Ann. Clin. Transl. Neurol. 6(7):1191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Winson J. 1989. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 9(8):2907–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. 2018. Stress, sleep, and the selective consolidation of emotional memories. Curr. Opin. Behav. Sci. 19:36–43 [Google Scholar]
- Pitman RK, Shalev AY, Orr SP. 2000. Posttraumatic stress disorder: emotion, conditioning, and memory. In The New Cognitive Neurosciences , ed. Gazzaniga MS, pp. 1133–47. Cambridge, MA: MIT Press. 2nd ed. [Google Scholar]
- Plihal W, Born J. 1997. Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 9(4):534–47 [DOI] [PubMed] [Google Scholar]
- Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, et al. 2017. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Commun. 8:15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. 2013. About sleep’s role in memory. Physiol. Rev. 93(2):681–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J. 2007. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315(5817):1426–29 [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B. 2012. REM sleep instability—a new pathway for insomnia? Pharmacopsychiatry 45(5):167–76 [DOI] [PubMed] [Google Scholar]
- Ritter SM, Strick M, Bos MW, Baaren RBV, Dijksterhuis A. 2012. Good morning creativity: task reactivation during sleep enhances beneficial effect of sleep on creative performance. J. Sleep Res. 21(6):643–47 [DOI] [PubMed] [Google Scholar]
- Roediger HL, Karpicke JD. 2006. Test-enhanced learning: taking memory tests improves long-term retention. Psychol. Sci. 17(3):249–55 [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Herman JH, Bowe-Anders C, Tauber ES. 1978. The effects of sustained alterations of waking visual input on dream content. In The Mind in Sleep: Psychology and Psychophysiology , ed. Arkin AM, Antrobus JS, Ellman SJ, pp. 295–349. Hillsdale, NJ: Erlbaum [Google Scholar]
- Rothschild G 2019. The transformation of multi-sensory experiences into memories during sleep. Neurobiol. Learn. Mem. 160:58–66 [DOI] [PubMed] [Google Scholar]
- Rothschild G, Eban E, Frank LM. 2017.A cortical-hippocampal-cortical loop of information processing dur-ing memory consolidation. Nat. Neurosci. 20(2):251–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumis DK, Frank LM. 2015. Hippocampal sharp-wave ripples in waking and sleeping states. Curr. Opin. Neurobiol. 35:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, Paller KA. 2009. Strengthening individual memories by reactivating them during sleep. Science 326(5956):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KEG, Osburn S, Paller KA, Beeman M. 2019. Targeted memory reactivation during sleep improves next-day problem solving. Psychol. Sci. 30(11):1616–24. Corrigendum. 2020. Psychol. Sci. 31(8):1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, McDevitt EA, Chen L, Norman KA, Mednick SC, Rogers TT. 2017. Sleep benefits memory for semantic category structure while preserving exemplar-specific information. Sci. Rep. 7(1):14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönauer M, Alizadeh S, Jamalabadi H, Abraham A, Pawlizki A, Gais S. 2017. Decoding material-specific memory reprocessing during sleep in humans. Nat. Commun. 8:15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönauer M, Brodt S, Pöhlchen D, Breßmer A, Danek AH, Gais S. 2018. Sleep does not promote solving classical insight problems and magic tricks. Front. Hum. Neurosci. 12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten DI, Pereira SIR, Tops M, Louzada FM. 2017. State of the art on targeted memory reactivation: Sleep your way to enhanced cognition. Sleep Med. Rev. 32:123–31 [DOI] [PubMed] [Google Scholar]
- Schreiner T, Doeller CF,Jensen O, Rasch B, Staudigl T. 2018. Theta phase-coordinated memory reactivation reoccurs in a slow-oscillatory rhythm during NREM sleep. Cell Rep. 25(2):296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner T, Rasch B. 2015. Boosting vocabulary learning by verbal cueing during sleep. Cereb. Cortex 25(11):4169–79 [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20(1):11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan LK, Gjorgieva E, Paller KA, Kahnt T, Gottfried JA. 2018. Odor-evoked category reactivation in human ventromedial prefrontal cortex during sleep promotes memory consolidation. eLife 7:e39681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon KCNS, Gómez RL, Nadel L. 2018. Losing memories during sleep after targeted memory reactivation. Neurobiol. Learn. Mem. 151:10–17 [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. 2003. Communication between neocortex and hippocampus during sleep in rodents. PNAS 100(4):2065–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271(5257):1870–73 [DOI] [PubMed] [Google Scholar]
- Smith C 1995. Sleep states and memory processes. Behav. Brain Res. 69(1–2):137–45 [DOI] [PubMed] [Google Scholar]
- Squire LR. 1992. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J. Cogn. Neurosci. 4(3):232–43 [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. 2011. The cognitive neuroscience of human memory since H.M. Annu. Rev. Neurosci. 34:259–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Bergmann TO, Bonnefond M, van derMeij R, Jensen O, et al. 2015. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18(11):1679–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A, Pawlowski M. 2019. Depression and sleep. Int. J. Mol. Sci. 20(3):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. 1993. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13(8):3252–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Scott L, Rittenhouse C, Hobson JA. 1999. Sleep-induced changes in associative memory. J. Cogn. Neurosci. 11(2):182–93 [DOI] [PubMed] [Google Scholar]
- Tambini A, Davachi L. 2019. Awake reactivation of prior experiences consolidates memories and biases cognition. Trends Cogn. Sci. 23(10):876–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen J, Lambon Ralph MA, Lewis PA. 2017. Targeted memory reactivation of newly learned words during sleep triggers REM-mediated integration of new memories and existing knowledge. Neurobiol. Learn. Mem. 137:77–82 [DOI] [PubMed] [Google Scholar]
- Tempesta D, Socci V, De Gennaro L, Ferrara M. 2018. Sleep and emotional processing. Sleep Med. Rev. 40:183–95 [DOI] [PubMed] [Google Scholar]
- Tilley AJ. 1979. Sleep learning during stage 2 and REM sleep. Biol. Psychol. 9(3):155–61 [DOI] [PubMed] [Google Scholar]
- Timofeev I, Chauvette S. 2017. Sleep slow oscillation and plasticity. Curr. Opin. Neurobiol. 44:116–26 [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. 2014. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81(1):12–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. 2006. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol. Learn. Mem. 86(2):241–47 [DOI] [PubMed] [Google Scholar]
- van Dongen EV, Takashima A, Barth M, Zapp J, Schad LR, et al. 2012. Memory stabilization with targeted reactivation during human slow-wave sleep. PNAS 109(26):10575–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden WM, George SA, Urpa L, Kehoe M, Liberzon I, Poe GR. 2015. Sleep alterations following exposure to stress predict fear-associated memory impairments in a rodent model of PTSD. Exp. Brain Res. 233(8):2335–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux J-P, Rodriguez E,Martinerie J. 2001.The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2(4):229–39 [DOI] [PubMed] [Google Scholar]
- Vaz AP, Inati SK, Brunel N, Zaghloul KA. 2019. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363(6430):975–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger R, Rose M, Wagner U, Yordanova J, Kolev V. 2013. Insights into sleep’s role for insight: studies with the number reduction task. Adv. Cogn. Psychol. 9(4):160–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Eastman KE. 2000. The case against memory consolidation in REM sleep. Behav. Brain Sci. 23(6):867–76 [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. 2006. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave sleep. J. Neurosci. 26(21):5665–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. 2004. Sleep inspires insight. Nature 427(6972):352–55 [DOI] [PubMed] [Google Scholar]
- Walker MP. 2017. Why We Sleep: Unlocking the Power of Sleep and Dreams. New York: Simon & Schuster [Google Scholar]
- Walker MP, Brakefield T,Morgan A, Hobson JA, Stickgold R. 2002. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35(1):205–11 [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. 2009. Overnight therapy? The role of sleep in emotional brain processing. Psychol. Bull. 135(5):731–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Clouter A, Chen Q, Shapiro KL, Hanslmayr S. 2018. Single-trial phase entrainment of theta oscillations in sensory regions predicts human associative memory performance. J. Neurosci. 38(28):6299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassing R, Lakbila-Kamal O, Ramautar JR, Stoffers D, Schalkwijk F, Van Someren EJW. 2019. Restless REM sleep impedes overnight amygdala adaptation. Curr. Biol. 29(14):2351–58.e4 [DOI] [PubMed] [Google Scholar]
- Weigenand A, Mölle M, Werner F, Martinetz T, Marshall L. 2016. Timing matters: Open-loop stimulation does not improve overnight consolidation of word pairs in humans. Eur. J. Neurosci. 44(6):2357–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Florczak SM, Weintraub S, Mesulam M-M, Marshall L, et al. 2015. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol. Aging 36(9):2577–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE,Mander BA, Florczak SM, Weintraub S, Mesulam M-M, et al. 2012. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 18(3):490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, McGaugh JL, Zee PC, Warby SC, et al. 2020. Highly superior autobiographical memory is associated with superior sleep physiology. Paper presented at the 61st Annual Meeting of the Psychonomic Society, online, Nov. 19–21 [Google Scholar]
- Williams JMG, Barnhofer T, Crane C, Herman D, Raes F, et al. 2007. Autobiographical memory specificity and emotional disorder. Psychol. Bull. 133(1):122–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265(5172):676–79 [DOI] [PubMed] [Google Scholar]
- Winson J 1985. Brain and Psyche: The Biology of the Unconscious. New York: Doubleday [Google Scholar]
- Winson J. 2004. To sleep, perchance to dream. Learn. Mem. 11(6):659 [Google Scholar]
- Yonelinas AP, Ranganath C, Ekstrom AD, Wiltgen BJ. 2019. A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat. Rev. Neurosci. 20(6):364–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-S, Gujar N, Hu P, Jolesz FA, Walker MP. 2007. The human emotional brain without sleep—a prefrontal amygdala disconnect. Curr. Biol. 17(20):R877–78 [DOI] [PubMed] [Google Scholar]


