To the Editor:
IgE antibodies play a crucial role in allergic reactions, including systemic anaphylaxis, by binding to the high-affinity IgE Fc receptor FcεRI on mast cells and basophils and thereby inducing the release of inflammatory mediators.1,2,E1-E3 In contrast, allergen-specific IgG antibodies, induced also in response to allergen-specific immunotherapies, can suppress IgE-mediated anaphylaxis via allergen masking and particularly crosslinking FcεRI with the IgG inhibitory receptor FcγRIIB.1-3,E1,E4,E6 However, when allergen levels are high, for example, medical drugs, IgG antibodies also have the potential to mediate anaphylaxis by crosslinking classical activating FcγRs, which is also controlled by FcγRIIB, on different innate immune cell types.2-4,E5,E7-E14
Hence, analyzing or even enhancing the expression level of the inhibitory FcγRIIB might be a promising approach to predict or prevent IgG-mediated allergic reactions and also IgG-FcγRIIB–controlled-IgE-mediated allergic reactions.
The intravenous immunoglobulin (IVIg), pooled human (hu) serum IgG from healthy donors, has been successfully used in high concentrations (1-2 g/kg) to treat patients with acute flares of inflammatory autoimmune diseases. Importantly, findings in animal autoimmune models have indicated that the therapeutic effect of IVIg/huIgG might be predominantly mediated via its Fc N-sialylated IgG subfraction (Fig 1, A).5,6,E15-E17 Elevating the fraction of sialylated bulk serum IgG antibodies to a certain critical level might be therefore sufficient to attenuate inflammatory autoimmune conditions.6,E15,E17 Functionally, it has been indicated in mice that sialylated huIgG antibodies with irrelevant specificities (or sialylated huIgG1 Fc portions) can interact with the sugar-binding C-type lectin receptor SIGN-R1 (specific intercellular adhesion molecule-3 (ICAM-3) grabbing nonintegrin-related 1) on marginal zone macrophages resulting in the expression of IL-33, which activates basophils to produce IL-4, which in turn upregulates FcγRIIB on effector macrophages in mice.5,7,E15,E18
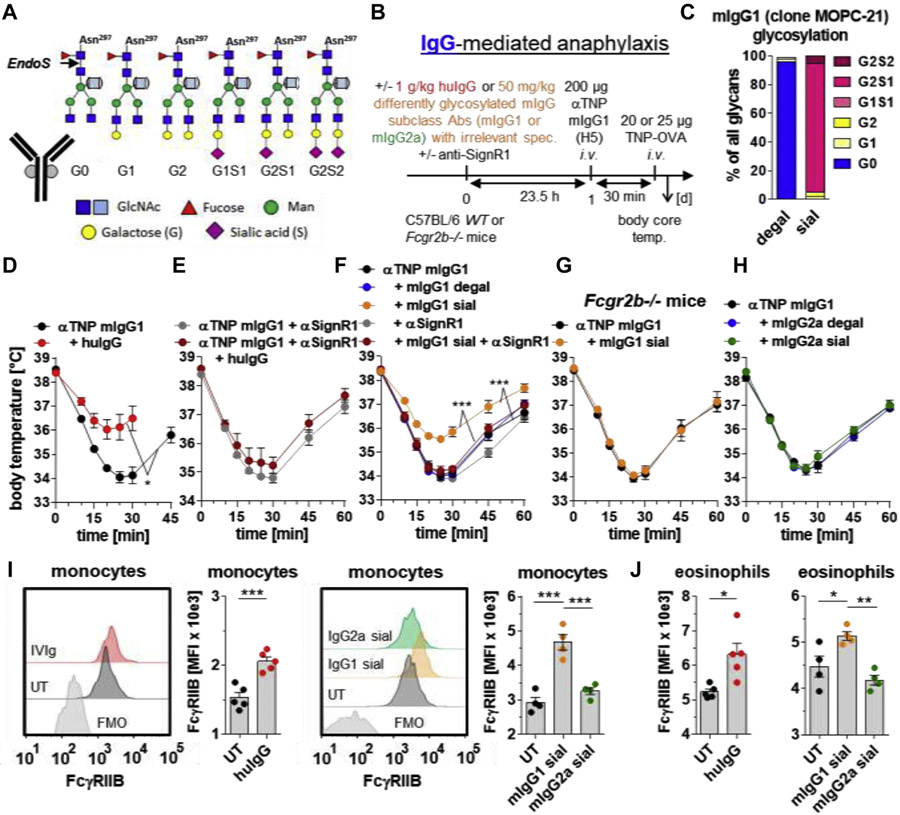
FIG 1.
Enrichment of bulk serum IgG with sialylated murine IgG1, but not IgG2a, with irrelevant specificity attenuates IgG1-mediated anaphylaxis in a SIGN-R1– and FcγRIIB-dependent manner. A, The conserved biantennary N-glycan (4 N-acetylglucosamines [dark blue] and 3 mannoses [green]) at Asn 297 in the IgG Fc part can be modified by fucose (red), bisecting GlcNAc (light blue), galactose (G; yellow), and sialic acid (S; magenta). The cleavage site of EndoS used for IgG glycan analysis is depicted. B, Experimental design of the IgG-mediated 30-minute anaphylaxis model used in the experiments shown in Fig 1, D-H. IgG1-mediated anaphylaxis was induced i.v. with 200 μg of anti (α)-TNP murine (m) IgG1 (clone H5) and subsequent (30 minutes later) i.v. injection of 20 or 25 μg of TNP-OVA. C, Fc glycosylation profiles of in vitro desialylated plus degalactosylated (degal) and galactosylated plus sialylated (sial) mIgG1 antibodies (clone MOPC-21) with irrelevant specificity. D and E, When indicated, intraperitoneal injection of huIgG (IVIg; 1 g/kg) and/or i.v. injection of anti (α)-SIGN-R1 into WT mice. IgG1-mediated anaphylaxis was induced 23.5 hours later. F-H, When indicated, i.v. injection of in vitro galactosylated plus sialylated (sial) or desialylated plus degalactosylated (degal) mIgG1 (clone MOPC-21; 50 mg/kg) or mIgG2a (clone C1.18.4; 50 mg/kg) with irrelevant specificities and/or αSIGN-R1 into (Fig 1, F and H) WT or (Fig 1, G) Fcgr2b−/− mice. IgG1-mediated anaphylaxis was induced 23.5 hours later. The severity of anaphylaxis in all experiments was measured by determining the changes in the body core/rectal temperature on the indicated time points after antigen challenge. n = 4-5 for all groups. I and J, When indicated, intraperitoneal injection of huIgG (IVIg; 1 g/kg) or i.v. injection of in vitro galactosylated plus sialylated (sial) mIgG1 (clone MOPC1; 50 mg/kg) or mIgG2a (clone C1.18.4; 50 mg/kg) with irrelevant specificities into WT mice to analyze FcγRIIB expression (MFI)on blood (Fig 1, I SSC low/CD11b+/F40/80+ classical monocytes and (Fig 1, J) SSC high/GR-1+ (not high) eosinophils by flow cytometry 24 hours later, including overlay histograms of FcγRIIB expression on classical monocytes from representative mice of each group (including FMO controls). Dots represent single mice. Abs, Antibodies; i.v., intravenous/intravenously; MFI, mean fluorescent intensity; OVA, ovalbumin; spec., specificities; temp, temperature; UT, untreated; WT, wild-type.
Here, we tested the capacity of high amounts of huIgG (1 g/kg) or lower amounts of highly sialylated murine (m) or huIgG subclass antibodies (10-50 mg/kg) with irrelevant specificities to enhance FcγRIIB expression on blood immune cells and to attenuate IgG-mediated anaphylaxis and IgG-FcγRIIB–controlled-IgE-mediated anaphylaxis in mice.
IgG-mediated anaphylaxis was induced by intravenous injection of 200 μg of anti–2,4,6-trinitrophenyl (TNP) mIgG1 mAbs (clone H5),2,8,9,E19-E21 followed by intravenous challenge with 20 or 25 μg of TNP-coupled ovalbumin 30 minutes later to allow formation of immune complexes (Fig 1, B).
We first verified key cellular and molecular players of anti-TNP mIgG1-mediated anaphylaxis. We confirmed that Gr-1–expressing cells (containing monocytes, neutrophils, and eosinophils) are critical for induction of anaphylaxis (see Fig E1, A and B, in this article’s Online Repository at www.jacionline.org).4 To assess the role of activating and inhibitory Fcγ receptors, we tested mice deficient in the signaling receptor subunit, the FcR γ–chain, of activating FcγRs (Fcerg1−/−), or in FcγRIIB (Fcgr2b−/−), respectively. Notably, murine IgG1 interacts only with the activating FcγRIII/FcR γ–chain complex but not with FcγRI- or FcγRIV-containing complexesE12 Indeed, FcR γ–chain-deficient mice were protected from IgG1-mediated anaphylaxis (Fig E1, C), whereas animals lacking the inhibitory receptor FcγRIIB showed exacerbated symptoms compared with controls (Fig E1, D).2,4
To test the effect of bulk IgG Fc sialylation on IgG-mediated anaphylaxis, we injected intraperitoneally high amounts of huIgG (IVIg; 1 g/kg) or intavenously lower amounts of highly galactosylated plus sialylated (sialylated; sial) versus desialylated plus degalactosylated (degal) mIgG1 mAbs (clone MOPC-21 [50 mg/kg]E22 or clone MRC OX-7 [10 mg/kg]9,E23) with irrelevant specificities 23.5 hours before induction of the 30-minute anti-TNP mIgG1-mediated anaphylaxis model described above (Fig 1, B and C; see Fig E2, A and B, in this article’s Online Repository at www.jacionline.org).
The unspecific huIgG as well as sialylated mIgG1 antibodies attenuated the anti-TNP mIgG1-mediated anaphylaxis, whereas the degal mIgG1 antibodies had no effect (Fig 1, D and F, and Fig E2, B and C). Notably, the IgG sialylation-mediated inhibition required SIGN-R1 (Fig 1, E and F) and FcγRIIB (Fig 1, G), whereas SIGN-R1 had no influence on the anti-TNP mIgG1-mediated anaphylaxis itself (Fig E2, D). We could not revoke the SIGN-R1–dependent effect with anti–IL-4 or anti–IL-33R blocking antibodies (data not shown), suggesting further as yet unclear SIGN-R1–dependent inhibitory pathways.
Interestingly, sialylated mIgG2a mAbs (clone C1.18.4; 50 mg/kg) with irrelevant specificitiesE24 failed to suppress the anti-TNP mIgG-mediated anaphylaxis (Fig 1, H, and Fig E2, E), further indicating that, in contrast to mIgG1 or mIgG2b,2,8,9,E20,E21,E25,E26 effector functions of mIgG2a antibodies might be less dependent on Fc glycosylation.2,E25,E27 Instead, in vitro galactosylated plus sialylated purified serum huIgG4 attenuated the anti-TNP mIgG1-induced anaphylaxis (Fig E2, F and G).
We further observed that sole intraperitoneal injection of huIgG (IVIg; 1 g/kg) or intravenous injection of sialylated mIgG1 (clone MOPC-21; 50 mg/kg), but not IgG2a (clone C1.18.4, 50 mg/kg), mAbs upregulated FcγRIIB expression on blood classical monocytes and eosinophils, which was detected by flow cytometry 24 hours later (Fig 1, I and J). FcγRIIB expression on murine neutrophils was too low to identify significant differences (data not shown).
Importantly, timing, rather than antigen specificity, is critical for inhibitory effects of sialylated mIgG1 antibodies. Indeed, when applied 1 day in advance, both mIgG1 antibodies with irrelevant specificity (Fig 1, F) as well as antigen-specific (ie, anti-TNP) mIgG1 antibodies (see Fig E3, A-C, in this article’s Online Repository at www.jacionline.org),2, when sialylated, suppressed anti-TNP mIgG1-mediated anaphylaxis. In contrast, Fc sialylation had no significant effect on the ability of antigen-specific mIgG1 antibodies to induce anaphylactic reactions in the 30-minute model (Fig E3, D and E). We therefore conclude that this delay might be critical for modulating FcγRIIB expression in an IgG Fc sialylation–dependent manner.
Similarly, sialylated mIgG1 (clone MOPC-21; 50 mg/kg) with irrelevant specificity enhanced the FcγRIIB-dependent2 inhibitory effect of antigen-specific mIgG1 antibodies in an IgE-mediated anaphylaxis model (Fig 2, A-C). These observations suggested that sialylated IgG with irrelevant specificity might also upregulate FcγRIIB expression on FcεRI-expressing blood cells. Indeed, we observed an upregulation of FcγRIIB on FcεRI-expressing blood basophils 24 hours after application of huIgG or sialylated muIgG1, but not sialylated mIgG2a antibodies, with irrelevant specificities, in a SignR1-dependent manner (Fig 2, D).
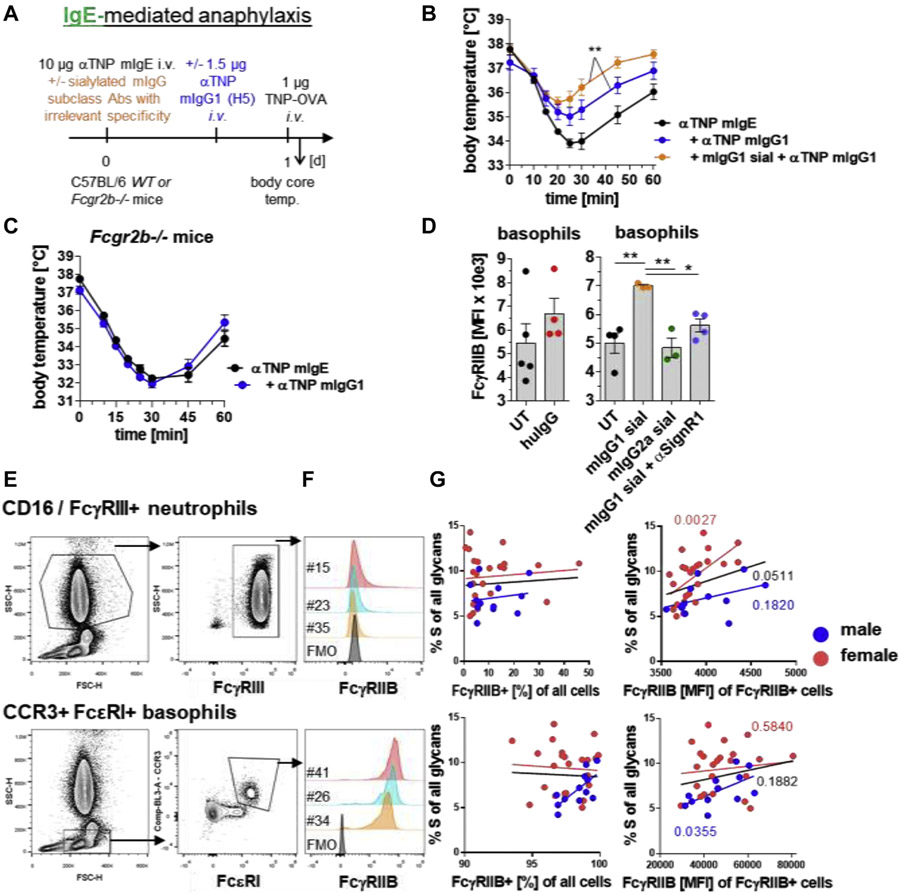
FIG 2.
Blood IgG sialylation levels are predictive for FcγRIIB expression levels and also attenuate IgG-FcγRIIB–controlled IgE-mediated anaphylaxis. A, Experimental design of the IgE-mediated anaphylaxis model used in the experiments shown in parts B and C. IgE-mediated anaphylaxis was induced i.v. with 10 μg of anti (α)-TNP murine (m) IgE (clone IgELa2) and i.v. injection of 1 μg of TNP-OVA 24 hours later. B and C, When indicated, i.v. injection of in vitro galactosylated plus sialylated (sial) mIgG1 (clone MOPC-21; 50 mg/kg) with irrelevant specificity and/or i.v. injection of 1.5 μg of αTNP mIgG1 (clone H5) into (Fig 2, B) WT or (Fig 2, C) Fcgr2b−/− mice. IgE-mediated anaphylaxis was induced as described in Fig 2, A. The severity of anaphylaxis in all experiments was measured by determining the changes in the body core/rectal temperature on the indicated time points after antigen challenge. n = 4-5 for all groups. D, When indicated, intraperitoneal injection of huIgG (IVIg; 1 g/kg) or i.v. injection of mIgG1 (clone MOPC-21; 50 mg/kg) or mIgG2 (clone C1.18.4; 50 mg/kg) with irrelevant specificities and/or αSIGN-R1 into WT mice to analyze FcαRIIB expression (MFI) on blood CD49b+/FcεRI+ basophils 24 hours later. n = 3-5 for all groups. Dots represent single mice. E, Human blood cell staining and gating strategies as indicated. F, Overlay histograms of FcγRIIB expression of the indicated cell populations and samples. G, Correlation of FcγRIIB expression on human neutrophils and basophils with serum IgG Fc sialylation levels. For correlating the expression levels of FcγRIIB with the IgG Fc sialylation levels, we focused on the percentages of FcγRIIB-expressing cells and the MFI of FcγRIIB on FcγRIIB-positive cells. FMO, Flourescence minus one; FSC-H, forward scatter-height; i.v., intravenous/intravenously; MFI, mean fluorescent intensity; SSC-H, side scatter-height; temp., temperature; WT, wild-type; UT, untreated.
Besides IVIg treatment, physiological levels of IgG Fc sialylation already vary between individuals in the blood,6 which might in turn modulate the expression of Fcγ receptors on immune cells and, in turn, influence an individual’s susceptibility to IgG-mediated or IgG-FcγRIIB–controlled-IgE-mediated allergic reactions or other IgG antibody–mediated diseases.
We therefore assessed in a pilot study serum IgG Fc glycosylation and the expression levels of the inhibitory receptor FcγRIIB and the activating FcγRIIA, FcγRIII(A+B), and FcεRI on peripheral human blood neutrophils, monocytes, basophils, and/or B cells of 36 volunteers (24 females and 12 males; for details, see this article’s Methods section in the Online Repository at www.jacionline.org). Because IgG sialylation levels are distinct between the 2 sexes, we analyzed male and female data separately.
Intriguingly, we identified significant positive associations between the levels of serum IgG Fc sialylation and the expression levels of FcγRIIB on neutrophils in female donors and on basophils in male donors (Fig 2, E-G; see Tables E1 and E2 and Fig E4 in this article’s Online Repository at www.jacionline.org). Although a larger cohort of donors is required to clearly establish the connection between IgG sialylation and FcγRIIB expression in human leukocytes, these results are well in line with our findings in mice.
The data suggest that natural or modified levels of blood IgG Fc N-sialylation regulate the expression level of the inhibitory receptor FcγRIIB on immune cells and might protect individuals via this way not only from inflammatory autoimmune conditions but also from IgG-mediated as well as IgG-FcγRIIB–controlled-IgE-mediated allergic reactions. Thus, IgG-inducing allergen-specific immunotherapy in patients suffering from IgE-mediated allergic diseases might be more promissing and successful in patients showing higher bulk serum IgG sialylation levels, which has to be investigated.
Although this effect of bulk serum IgG Fc sialylation was dependent on SIGN-R1 in mice, we could not revoke this effect with anti–IL-4 or anti–IL-33R blocking antibodies, suggesting further as yet unclear SIGN-R1–dependent inhibitory pathways to upregulate FcγRIIB. Interestingly, this inhibitory effect of bulk serum IgG sialylation was mediated by mIgG1, but not mIgG2a antibodies, suggesting IgG subclass–specific roles in mice.
These findings might explain earlier observations of protective effects of IVIg in the context of allergyE27-E29 and might help to develop diagnostic, prognostic, and/or therapeutic tools for controlling IgG-mediated as well as IgG-FcγRIIB–controlled-IgE-mediated allergic reactions and IgG-dependent inflammatory disorders in general.
Supplementary Material
Acknowledgments
We thank Mattias Collin for providing EndoS, Birgitta Heyman for the anti-TNP IgG1 (clone H5) hybridoma cells, Rudolf Manz for the anti–GR-1 mAbs (clone RB6-8C5), and Robina Thurmann and Kathleen Kuhrwahn for technical assistance. Human blood from healthy donors was collected with the approval of and in accordance with regulatory guidelines and ethical standards set by the University of Lübeck.
M.E. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—grant numbers 257739680 (EH 221/8-1), 398859914 (EH 221/10-1), 222374435 (international Research Training Group - iRTG 1911), 49701054 (Germany’s Excellence Strategies - EXC 306) and 390884018 (EXC 2167, Precision Medicine in Chronic Inflammation [PMI])—and the VolkswagenStiftung (grant no. 97301). A.L. received junior grants from the University of Lüubeck and the EXC 306. Y.C.B. and H.B.L. were members and J.P., L.D., S.L., and G.-M.L. were associated members of the RTG 1727. A.E. and J.R. were members of the iRTG 1911.
Disclosure of potential conflict of interest: M. Ehlers received grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—grant numbers 257739680 (EH 221/8-1), 398859914 (EH 221/10-1), 222374435 (international iRTG 1911), 49701054 (Germany’s Excellence Strategies - EXC 306) and 390884018 (EXC 2167, Precision Medicine in Chronic Inflammation [PMI])—and the Volkswagenstiftung (grant no. 97301) for this work and grants from the DFG—grant numbers 400912066 (EH 221/11-1), 269234613 (Clinical Research Unit 303, EH 221/9-1), 179309734 (RTG 1727)—and the Else Kroener-Fresenius-Foundation (2014_A91) for other works; and is employed by the University of Lüubeck. A. Leliavski received junior grants from the University of Lüubeck, and the EXC 306 for other works. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Berings M, Karaaslan C, Altunbulakli C, Gevaert P, Akdis M, Bachert C, et al. Advances and highlights in allergen immunotherapy: on the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol 2017;140:1250–67 [DOI] [PubMed] [Google Scholar]
- 2.Epp A, Hobusch J, Bartsch YC, Petry J, Lilienthal GM, Koeleman CAM, et al. Sialylation of IgG antibodies inhibits IgG-mediated allergic reactions. J Allergy Clin Immunol 2018;141:399–402.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol 2016;137:1674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutier H, Gillis CM, Iannascoli B, Godon O, England P, Sibilano R, et al. IgG subclasses determine pathways of anaphylaxis in mice. J Allergy Clin Immunol 2017;139:269–80.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006;313:670–3. [DOI] [PubMed] [Google Scholar]
- 6.Maddur MS, Kaveri SV, Bayry J. Circulating normal IgG as stimulator of regulatory T cells: lessons from intravenous immunoglobulin. Trends Immunol 2017;38:789–92. [DOI] [PubMed] [Google Scholar]
- 7.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 2011;475:110–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, et al. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J Allergy Clin Immunol 2012;129:1647–55. [DOI] [PubMed] [Google Scholar]
- 9.Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest 2013;123:3788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.