Abstract
BACKGROUND:
Platelet transfusion is among the most useful therapeutic tools in modern clinical settings which mean that ensuring an adequate supply is of paramount importance.
AIM:
The aim of our study was to record the use and wastage of platelet concentrates (PCs) in Greece, so as to come up with evidence-based interventions.
METHODS:
The study was conducted during May and June 2015. We evaluated the use of random-donor platelets (RDPs) and single-donor apheresis platelets (SDPs). We analyzed such parameters as hospital department and diagnosis, indication for transfusion, PCs' age at the time of transfusion, and wastage rate.
RESULTS:
We used data from 21 hospitals across the country. A total of 12,061 RDPs and 1189 SDPs were transfused, with an average of 4.84 (±2.72) and 1.12 (±2.73) units per episode, respectively. Most patients had been admitted to the internal medicine and hematology departments. The transfusions were mostly given prophylactically, usually in cases of acute leukemia, and mostly on the day before expiration. Wastage rate was 16.75% for RPDs and 2.70% for SDPs, primarily because of the expiration of the use-by date.
CONCLUSIONS:
This is the first national survey regarding platelet transfusion in Greece. Since most patients were admitted in internal medicine and hematology departments, we recommend that the staff of the abovementioned departments should undergo training on contemporary transfusion guidelines. Platelet discard rate could further be lowered through the centralization of inventory management along with the extension of the lifetime of PCs by means of emerging technologies.
Keywords: National, platelet, platelet products, platelets transfusion, survey
Introduction
Nowadays, platelet transfusion is a useful therapeutic and preventive method in modern clinical settings. The most common indications for platelet transfusion are thrombocytopenia resulting from chemotherapy and bone marrow failure syndromes, with increased risk of bleeding and as a way of preventing bleeding prior to surgical procedures.[1,2] However, the cost of transfusing platelets (PLTs) is among the highest in transfusion medicine and their specific characteristics, such as special storage requirements and relatively short shelf life, mean that they are not always freely available.[2,3]
Inappropriate use of PLTs is another important reason for the scarcity of platelet concentrates (PCs) which results in transfusion centers often being unable to cover the patients' needs. Setting proper guidelines for platelet transfusion should have solved the problem of inappropriate use. A plethora of guidelines has been introduced in many countries, including Greece.[4,5,6,7,8] However, despite existing national and institutional guidelines, in the majority of clinical settings, doctors' compliance to these guidelines varies significantly from 43% to 89% for both therapeutic and prophylactic platelet transfusion.[3,9,10]
Furthermore, data from previous years showed that platelet usage is constantly rising. Reasons for this increase, at both regional and national level, include the fact that an aging population implies an increase in the prevalence of malignancies, along with the fact that there is an increased use of antiplatelet agents in cardiac disease. Thus, it seems clear that the proportion of the population requiring platelet products for transfusion is likely to rise over the coming decades, while the proportion eligible for donations of blood and platelet products is likely to fall.[6,11]
Hence, we conducted this study to assess and evaluate the production, distribution, use, and wastage rate of platelet products in Greece to identify the parameters that contribute to their proper use and sufficiency. Special national characteristics, such as the decentralization of transfusion services and geographical particularities, have been taken into consideration. Given that Greece very often cannot cover its high national transfusion needs, results, and conclusions of this survey will be used to inform educational initiatives on appropriate platelet use, thus improving platelet availability and decreasing platelet wastage rate.
Methods
This study was conducted by the Working Committee of Transfusion Medicine and Apheresis of the Hellenic Society of Hematology. An electronic data collection form (Excel 2016, Microsoft/Corp, WA, USA) was used, and all transfusion services in Greece were invited to participate in the study. Data collection was conducted from May to June 2015 using the aforementioned data forms that were filled by the participating centers. The collected data consisted of the number of platelet units produced, transfused, and discarded. Platelet units were separately recorded for those originating from random donor platelets (RDPs) and those retrieved during apheresis from single-donor platelets (SDPs). A RDP unit is a platelet component derived from a single whole blood donation prepared from platelet-rich plasma (PRP method). Both RDPs and SDPs met the requirements of the European Directorate for the Quality of Medicines and HealthCare of the Council of Europe (EDQM).[12] Maximum storage time for PCs in Greece is 5 days. The data recorded included the patients' departments, diagnosis, indication for transfusion (bleeding vs. prophylaxis), the transfusion episodes, and the age of the platelet unit at the time of transfusion. The transfusion of several PLTs units at the same time was recorded as the transfusion dose given in a single episode. Numbers of platelet units produced in and imported to each transfusion center and discharge rates were separately recorded as well. Data regarding national platelet units' supplies were provided by the Hellenic National Blood Transfusion Center, which is responsible for the national inventory blood management along with the implementation of the blood products' molecular testing.
Electronic spreadsheet data forms (Excel 2016, Microsoft/Corp, WA, USA) were also used to perform part of the analysis. Additional Statistical analysis was performed using the SAS software version 9.3 for Windows (SAS Institute Inc., USA).
Results
From 97 transfusion services located within equal number of hospitals that have been invited to join this study, 21 (21.6%) have responded positively. Twelve out of 21 hospitals are located in Athens, and the remaining nine are general hospitals located in cities outside Athens (Thessaloniki, Katerini, Kavala, Alexandroupolis, Loannina, Agrinio, Patras, and Heraklion) covering most of the country as they were geographically equally distributed. After being invited to participate in this study, all 21 hospitals voluntarily contributed with data covering the entire 2-month period. The total number of platelet units evaluated was 13,250; 12,061 RDPs and 1189 SDPs. Numbers of RDPs and SDPs for every contributing hospital are listed in Table 1.
Table 1.
Hospital contributing to this study and their respective platelet units
| RDPs | SDPs | |||||||
|---|---|---|---|---|---|---|---|---|
| Produced RDPs | Imported RDPs | Total RDPs | RDPs (%) | Produced SDPs | Imported SDPs | Total SDPs | SDPs (%) | |
| HA1 | 1348 | 1348 | 11.18 | 147 | 147 | 12.36 | ||
| HA2 | 1272 | 1272 | 10.55 | 242 | 242 | 20.35 | ||
| HA3 | 81 | 731 | 812 | 6.73 | 105 | 15 | 120 | 10.09 |
| HA4 | 655 | 82 | 737 | 6.11 | 84 | 1 | 85 | 7.15 |
| HA5 | 651 | 60 | 711 | 5.90 | 47 | 47 | 3.95 | |
| HA6 | 338 | 70 | 408 | 3.38 | 95 | 3 | 98 | 8.24 |
| HA7 | 341 | 341 | 2.83 | 47 | 47 | 3.95 | ||
| HA8 | 307 | 10 | 317 | 2.63 | 0.00 | |||
| HA9 | 115 | 160 | 275 | 2.28 | 0.00 | |||
| HA10 | 179 | 41 | 220 | 1.82 | 73 | 73 | 6.14 | |
| HA11 | 111 | 13 | 124 | 1.03 | 0.00 | |||
| HA12 | 17 | 64 | 81 | 0.67 | 0.00 | |||
| HOA1 | 1494 | 33 | 1527 | 12.66 | 44 | 3 | 47 | 3.95 |
| HOA2 | 66 | 66 | 0.55 | 0.00 | ||||
| HOA3 | 14 | 14 | 0.12 | 0.00 | ||||
| HOA4 | 259 | 259 | 2.15 | 45 | 45 | 3.78 | ||
| HOA5 | 992 | 49 | 1041 | 8.63 | 28 | 28 | 2.35 | |
| HOA6 | 85 | 85 | 0.70 | 0.00 | ||||
| HOA7 | 10 | 10 | 0.08 | 0.00 | ||||
| HOA8 | 1028 | 483 | 1511 | 12.53 | 124 | 124 | 10.43 | |
| HOA9 | 902 | 902 | 7.48 | 86 | 86 | 7.23 | ||
| Totals | 10,255 | 1806 | 12,061 | 100.00 | 1167 | 22 | 1189 | 100.00 |
HA=Hospital in Athens, HOA=Hospital outside Athens, AH=Athens Hospital, AH1=Laikon Hospital, AH2=Evangelismos Hospital, AH3=Metaxa Anticancer Hospital, AH4=General Hospital “ATTIKON,” AH5=General Hospital Sismanogleio, AH6=St. Savvas Oncology Hospital, AH7= “Amalia Fleming” Hospital, AH8=General Hospital “Saint Panteleimon,” AH9= “Saints Anargyroi” Hospital, AH10=General Hospital Alexandra, AH11=General Hospital Nea Ionia “Agia Olga,” AH12=Aretaeio University Hospital, HOA1=University Hospital of Thessaloniki AHEPA, HOA2=General Hospital of Katerini, HOA3=General Hospital of Kavala, HOA4=University Hospital of Alexandroupolis, HOA5=University Hospital of Loannina, HOA6=“Hatzikosta” General Hospital of Loannina, HOA7=General Hospital of Agrinio, HOA8=University Hospital of Patras, HOA9=University Hospital of Heraklion, RDPs=Random donors platelets, SDPs=Single-donor platelets
The overall number of PLTs units reported by the hospitals in Athens was 7505 (6646 RDPs and 859 SDPs), while the units reported by the hospitals outside Athens were 5745 (5415 RDPs and 330 SDPs). Imported PLTs units from other blood services constituted the 15.0% and 1.9% of RDPs and SDPs, respectively [Table 1].
Seven hundred and four patients received RDPs. The majority of those patients was admitted to the internal medicine and hematology departments (30.26% and 26.28%, respectively). These patients received 12,061 RDPs in 2494 episodes, leading to an average of 4.84 (±2.73) RDPs/episode. Patients in cardiac surgery and emergency departments received the most RDPs/episode (6.69 ± 3.02 and 6.5 ± 2.83, respectively), and patients in neonatology units received the least RDPs/episode (2.06 ± 1.11). In most cases, 83.68% (2087/2494) one transfusion episode was recorded per day, in 16% (407/2494) two episodes, while there was no recorded case of more than two transfusion episodes per day. The majority of episodes occurred in the internal medicine and hematology departments in 36.09% and 33.48%, respectively. Other departments and their respective results can be seen in detail in Table 2. It is worth noticing that the number of transfused RDPs per episode varied widely between hospitals from 3.51 in HA1 to 7.31 in HOA1, P < 0.0001 was depicted in Table 3.
Table 2.
Distribution of patients receiving random donors platelets and single-donor platelets grouped by hospital department
| Department | RDPs | SDPs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Patients (%) | Number of consumed RDPs | Consumed RDPs (%) | Number of episodes | Episodes (%) | RDPs per episode (average) | Patients | Patients (%) | Number of consumed SDPs | Consumed SDPs (%) | Number of episodes | Episodes (%) | SDPs per episode (average) | |
| Bone marrow/solid organ transplantation unit | 14 | 1.99 | 382 | 3.17 | 67 | 2.69 | 4.39 | 3 | 0.91 | 61 | 5.13 | 41 | 3.85 | 1.49 |
| Cardiac surgery | 110 | 15.63 | 950 | 7.88 | 142 | 5.69 | 6.69 | 31 | 9.45 | 45 | 3.78 | 35 | 3.28 | 1.29 |
| Coronary unit | 2 | 0.28 | 12 | 0.10 | 4 | 0.16 | 3.00 | |||||||
| Emergency room | 3 | 0.43 | 26 | 0.22 | 4 | 0.16 | 6.50 | |||||||
| Gastroenterology | 6 | 0.85 | 40 | 0.33 | 10 | 0.40 | 4.00 | |||||||
| Hematology | 185 | 26.28 | 3857 | 31.98 | 835 | 33.48 | 4.62 | 157 | 47.87 | 646 | 54.33 | 591 | 55.44 | 1.09 |
| Intensive care units (adult) | 69 | 9.80 | 787 | 6.53 | 156 | 6.26 | 5.04 | |||||||
| Intensive care units (pediatric) | 1 | 0.14 | 10 | 0.08 | 3 | 0.12 | 3.33 | 1 | 0.30 | 10 | 0.84 | 10 | 0.94 | 1.00 |
| Internal medicine | 214 | 30.40 | 4205 | 34.86 | 900 | 36.09 | 4.67 | 93 | 28.35 | 293 | 24.64 | 268 | 25.14 | 1.09 |
| Neonatology | 26 | 3.69 | 130 | 1.08 | 63 | 2.53 | 2.06 | 3 | 0.91 | 3 | 0.25 | 3 | 0.28 | 1.00 |
| Neurosurgery | 4 | 0.57 | 19 | 0.16 | 4 | 0.16 | 4.75 | 1 | 0.30 | 1 | 0.08 | 1 | 0.09 | 1.00 |
| Obstetrics - gynecology | 1 | 0.14 | 5 | 0.04 | 1 | 0.04 | 5.00 | - | - | - | - | - | - | - |
| Oncology | 47 | 6.67 | 481 | 3.99 | 101 | 4.05 | 4.88 | 11 | 3.35 | 28 | 2.35 | 24 | 2.25 | 1.17 |
| Orthopedics | 7 | 0.99 | 43 | 0.36 | 9 | 0.36 | 4.78 | - | - | - | - | - | - | - |
| Pediatrics | 15 | 2.13 | 150 | 1.24 | 35 | 1.40 | 4.64 | 16 | 2.44 | 49 | 2.06 | 47 | 2.20 | 1.04 |
| Pneumatology | 4 | 0.57 | 30 | 0.25 | 8 | 0.32 | 3.75 | 1 | 0.30 | 1 | 0.08 | 1 | 0.09 | 1.00 |
| Private hospitals | 18 | 2.56 | 123 | 1.02 | 28 | 1.12 | 4.39 | 3 | 0.91 | 4 | 0.34 | 4 | 0.38 | 1.00 |
| Special care units | 2 | 0.28 | 33 | 0.27 | 8 | 0.32 | 4.13 | 18 | 5.49 | 27 | 2.27 | 23 | 2.16 | 1.17 |
| Special infections | 2 | 0.28 | 25 | 0.21 | 6 | 0.24 | 4.17 | 1 | 0.30 | 1 | 0.08 | 1 | 0.09 | 1.00 |
| Surgery | 50 | 7.10 | 697 | 5.78 | 117 | 4.69 | 5.96 | 11 | 1.68 | 20 | 0.84 | 18 | 0.84 | 1.28 |
| Urology | 3 | 0.43 | 56 | 0.46 | 14 | 0.56 | 4.00 | |||||||
| Total | 704* | 100** | 12,061 | 100.00 | 2494 | 100** | 4.84 | 328* | 100** | 1189 | 100.00 | 1066 | 100** | 1.12 |
*This is the exact number of patients in this column, a patient admitted to two different clinics is counted one time for every clinic, **Percentages in this column are calculated considering that a patient is different if admitted in two or more clinics. RDPs=Random donors platelets, SDPs=Single-donor platelets
Table 3.
Transfused random donors platelets per episode and transfusion indication for both random donors platelets and single-donor platelets grouped by hospital
| Hospital | RDPs | SDPs from apheresis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RDPs per episode | Bleeding | Prophylactic | NR | Total | Bleeding | Prophylactic | NR | Total | |
| HA1 | 3.51 | 208 | 1140 | 1348 | 145 | 2 | 147 | ||
| HA2 | 4.28 | 713 | 552 | 7 | 1272 | 242 | 242 | ||
| HA3 | 4.98 | 98 | 714 | 812 | 16 | 104 | 120 | ||
| HA4 | 4.07 | 99 | 638 | 737 | 9 | 76 | 85 | ||
| HA5 | 5.01 | 170 | 541 | 711 | 6 | 41 | 47 | ||
| HA6 | 5.44 | 74 | 334 | 408 | 8 | 90 | 98 | ||
| HA7 | 5.17 | 148 | 129 | 64 | 341 | 9 | 37 | 1 | 47 |
| HA8 | 4.34 | 9 | 308 | 317 | |||||
| HA9 | 5.09 | 89 | 186 | 275 | |||||
| HA10 | 5.00 | 76 | 144 | 220 | 9 | 64 | 73 | ||
| HA11 | 5.17 | 5 | 119 | 124 | |||||
| HA12 | 3.86 | 38 | 43 | 81 | |||||
| HOA1 | 7.31 | 629 | 898 | 1527 | 7 | 40 | 47 | ||
| HOA2 | 5.08 | 41 | 25 | 66 | |||||
| HOA3 | 4.67 | 14 | 14 | ||||||
| HOA4 | 5.40 | 48 | 211 | 259 | 1 | 44 | 45 | ||
| HOA5 | 5.00 | 343 | 698 | 1041 | 6 | 22 | 28 | ||
| HOA6 | 4.47 | 39 | 46 | 85 | |||||
| HOA7 | 5.00 | 10 | 10 | ||||||
| HOA8 | 5.30 | 966 | 544 | 1 | 1511 | 83 | 41 | 124 | |
| HOA9 | 4.93 | 31 | 871 | 902 | 86 | 86 | |||
| Totals | 3824 | 8165 | 72 | 12,061 | 299 | 889 | 1 | 1189 | |
RDPs=Random donors platelets, SDPs=Single-donor platelets, HA=Hospital in Athens, HOA=Hospital outside Athens, NR=Nonreported
Three hundred and twenty-eight patients received SDPs; most of those were treated in hematology and internal medicine departments; 47.87% and 28.35%, respectively [Table 2]. The total number of SDPs transfused was 1189 in 1066 episodes, leading to an average of 1.12 (±2.73) SDPs/episode. Patients in transplantation and cardiac surgery departments received most SDPs/episode (1.49 ± 0.29 and 1.29 ± 0.47) [Table 2].
The most common diagnosis for RDPs transfusion was acute leukemia, myelodysplastic syndrome, solid tumors, lymphoma, and cardiovascular procedures in 32.12% ± 1.82%, 10.87% ± 1.21%, 9.86% ± 1.16%, 8.06% ± 1.06%, and 6.01% ± 0.93%, of the episodes, respectively. SDPs were transfused mostly in patients with acute leukemia (51.41%), lymphoma (11.91%), transplantation patients (6.00%), Multiple myeloma (6.94%), solid tumors (5.53%), myelodysplastic syndromes (4.78%), and cardiovascular procedures (3.47%). Patients with aneurysms were found to receive the highest number of RDPs/episode reaching 11.08 ± 6.00 and premature infants received the least with 2.11 ± 1.27 RDPs/episode [Table 4].
Table 4.
Random donors platelets and single-donor platelets per episode grouped by diagnosis
| Diagnosis | RDPs | SDPs | ||||||
|---|---|---|---|---|---|---|---|---|
| RDPs | Episodes | RDPs per episode (average) | Episodes (%) | SDPs | Episodes | SDPs per episode (average) | Episodes (%) | |
| Acute leukemia | 3777 | 801 | 4.72 | 32.12 | 594 | 548 | 1.08 | 51.41 |
| Anemia - thrombocytopenia | 261 | 64 | 4.08 | 2.57 | 7 | 7 | 1.00 | 0.66 |
| Aneurysm | 133 | 12 | 11.08 | 0.48 | 1 | 1 | 1.00 | 0.09 |
| Aplastic anemia | 149 | 33 | 4.52 | 1.32 | 4 | 4 | 1.00 | 0.38 |
| Autoimmune disease | 71 | 15 | 4.73 | 0.60 | 1 | 1 | 1.00 | 0.09 |
| Cardiovascular procedure | 787 | 131 | 6.01 | 5.25 | 48 | 37 | 1.30 | 3.47 |
| Gastrointestinal bleeding | 76 | 18 | 4.22 | 0.72 | 1 | 1 | 1.00 | 0.09 |
| Idiopathic Thrombocytopenia | 95 | 20 | 4.75 | 0.80 | ||||
| Infant hemolytic disease | 32 | 12 | 2.67 | 0.48 | ||||
| Liver cirrhosis | 34 | 7 | 4.86 | 0.28 | 1 | 1 | 1.00 | 0.09 |
| Lymphoma | 913 | 201 | 4.54 | 8.06 | 143 | 127 | 1.13 | 11.91 |
| Multiple myeloma | 496 | 113 | 4.39 | 4.53 | 80 | 74 | 1.08 | 6.94 |
| Myelodysplastic syndrome | 1348 | 271 | 4.97 | 10.87 | 57 | 51 | 1.12 | 4.78 |
| Myelohyperproliferative disease | 49 | 8 | 6.13 | 0.32 | 1 | 1 | 1.00 | 0.09 |
| Noncardiovascular surgical procedure | 267 | 66 | 4.05 | 2.65 | 10 | 9 | 1.11 | 0.84 |
| Obstetric bleeding | 20 | 3 | 6.67 | 0.12 | ||||
| Preterm infant | 80 | 38 | 2.11 | 1.52 | 2 | 2 | 1.00 | 0.19 |
| Sepsis | 553 | 110 | 5.51 | 4.41 | 23 | 18 | 1.62 | 1.68 |
| Solid tumor | 1245 | 246 | 5.06 | 9.86 | 63 | 59 | 1.07 | 5.53 |
| Thrombocytopenia (other) | 671 | 167 | 4.02 | 6.70 | 20 | 20 | 1.00 | 1.88 |
| Transplantation BM/solid organ | 513 | 84 | 6.11 | 3.37 | 90 | 64 | 1.41 | 6.00 |
| Trauma | 94 | 18 | 5.22 | 0.72 | 1 | 1 | 1.00 | 0.09 |
| Other causes | 327 | 82 | 3.99 | 3.29 | 38 | 37 | 1.03 | 3.47 |
| Not reported | 70 | 16 | 4.38 | 0.64 | 4 | 4 | 1.00 | 0.38 |
| Totals | 12,061 | 2494* | 4.78 | 100 | 1189 | 1066* | 1.11 | 100 |
*Exact number of patients, if a patient is treated for more than one reasons in different clinics, then he/she is counted separately for each reason. BM=Bone marrow
Both RDPs and SDPs were transfused mostly prophylactically to prevent bleeding (8165/12,061 RDPs – 68.11% and 889/1189 SDPs – 74.83%). The rest of the PLTs units in each group was administered as therapy to already established bleeding, that concerned bleeding cases during cardiovascular surgery (61.89%) or other surgery interventions (15.17%), aneurysm rapture (9.62%), gastrointestinal bleeding (6.48%), trauma (5%), and obstetric hemorrhage (1.85%). A very small portion of RDPs and SDPs (0.6% and 0.3%, respectively) was transfused for reason that has not been reported [Table 3].
The age of PLT units at the time of transfusion varied from 1 to 5 days. RDPs were more frequently transfused at the 5thand 2ndday in 27.05% and 20.01%, respectively. SDPs were more frequently transfused at the age of 5 and 4 days in 26.16% and 22.71% of units, respectively, as shown in Figure 1. Both RDPs and SDPs at the time of transfusion have a similar distribution among age groups. Except for day 4, there was no statistically significant difference in the percentages of RDP and SDPs when compared for every individual transfusion day (test for proportions: P = 0.7089, 0.5187, 0.5595, 0.0182, and 0.5316 from day 1 to day 5). Hospitals demonstrate very different results regarding age at the time of transfusion, and this is common to both RDPs as well as to SDPs (data not shown). It was not found a relation of the hospital size and the transfusion day. On the contrary, it was found that hospitals located in Athens transfused the majority of RDPs in day 5 (transfusion percentages per day 1–5: 9.15%, 20.12%, 20.55%, 20.28%, and 29.90%), while hospitals outside Athens had a more balanced approach (transfusion percentages per day 1–5: 21.99%, 19.89%, 15.36%, 19.19%, and 23.56%; P < 0.05). Similar picture was observed for SDPs, whereas in Athens hospitals, the percentages transfused per day were: 8.73%, 18.16%, 17.58%, 24.80%, and 30.73%, and in hospitals outside Athens were 29.39%, 21.82%, 17.27%, 17.27%, and 14.24% (day 1–5, respectively, P < 0.05).
Figure 1.
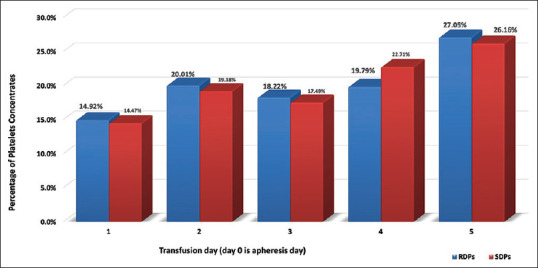
Percentages of RDPs and RDPs transfused according to transfusion day. RDPs = Random-donors platelets, SDPs = Single-donor platelets
In total, 2427 RDPs were discarded out of the total number of RDPs evaluated in our study representing a wastage rate of 16.75%. The wastage rate for SDPs was 2.70% (1189 transfused plus 33 discarded). Data regarding the reason for discarding PLTs were available for 1703 out of 2427 RDPs and for 30 out of 33 SDPs. The most prevalent reason was the expiration of the lifetime for both RDPs and SDPs (1073/1703=63.01% and 21/30=70.00%, respectively). The rest of the reasons of discarding RDPs were distributed as follows: component contaminated with red cells 278/1703 (16.32%), component out of specification 119/1703 (6.99%), positivity for infectious disease 74/1703 (4.35%), broken bag 43/1703 (2.52%), high suspicion for infection 31/1703 (1.82%), component used for quality control 22/1703 (1.29%), indirect Coombs positivity 2/1703 (0.12%), and other causes 61/1703 (3.58%). The reasons for SDP discard apart from expiration of lifetime included component out of specification (4/30, 13.33%) and other causes (5/30 16.67%).
Discussion
Twenty-one hospitals participated in our study, out of the 70 that report transfusing PLTs, according to data provided by the Hellenic National Blood Transfusion Center. The sample size consisted of 13,250 platelet units; 12,061 RDPs and 1189 SDPs transfused over a period of 2 months, May and June 2015. At a national level, PLT units transfused over a 2-month period in 2015 averaged 28,093 RDPs and 3142 SDPs. The PLT units evaluated to account for 42.93% of RDPs, and for 37.85% of SDPs transfused in Greece over the corresponding time period. Thus, the total sample size was considered representative, and hence, the analysis is assumed to have led to safe conclusions (with a 95% confidence interval, margin of error 0.65% for all samples, and 0.67% for RDPs and 2.24% for SDPs).
Blood transfusion services in Greece are still decentralized and are located in almost every hospital. At present, the National Blood Transfusion Centre is moving to restructure the system and centralize the blood products' production and supply. However, until this happens, every hospital's blood transfusion service is responsible for the whole blood transfusion chain (recruitment of donors, collection, and serological testing of donated blood, preparation, storage and issue of various blood products).[13] Thus, both RDPs and SDPs are generally produced in the same hospital, where they are transfused at rates that reach 85.01% and 98.14%, respectively. Therefore, production and transfusion of PLTs units are high in major hospitals and mainly in Athens, where population density is higher, and in some regional university Hospitals. This is to be expected as these same hospitals attract the majority of patients, for example, HA2 (18.87%), HA1 (8.65%) located in Athens, and HOA8 (8.79%) located in Patras as well as HOA1 (8.51%) located in Thessaloniki (data not shown). This concentration of patients and platelet transfusion needs in specific hospitals could be the result of the fact that these hospitals have very large hematology departments, where patients from other geographical areas are also treated.[14] The majority of patients receiving PLTs transfusions were admitted to either internal medicine or hematology departments (28% and 24%, respectively), as in many Greek hospitals, especially outside larger towns, hematology wards are subunits of internal medicine departments.[14]
According to our data, the vast majority of PCs is produced from whole blood donations (PRP method), while a small portion comes from apheresis. Regarding the type of RDPs products, there is a major difference between Greece and other European countries and Canada, where pooled buffy coat (BC) PCs in additive solution are widely used. By contrast, PCs resuspended in plasma (including both apheresis and PRP derived) are the norm in the United States and China. However, in these countries, most of the PCs transfused involve apheresis PLTs (97.23% SDPs, 2.77% RDPs), while the opposite is true in Greece (91.2% RDPs, 9.8% SDPs), as only 13 out of the 21 hospitals in the study have the necessary equipment and the expertise to produce PCs by apheresis.[15,16,17]
We found that the majority of PLTs (both RDPs and SDPs) were used in patients with hematological disease (64.19% and 76.11%), malignancies (10.32% and 5.30%), and those undergoing cardiovascular procedures (6.53% and 4.04%). The most common diagnosis within hematology was acute leukemia, (outside the setting of stem cell transplantation) followed by myelodysplastic syndromes and lymphomas. Our results can be compared to those of similar reports in the UK, which provide relevant information on the clinical diagnosis of platelet recipients and which give largely similar results.[16,18] According to Charlton et al. and Pendry and Davies, the largest number of PLTs (54% and 57%) was also given to patients with hematological disease including acute leukemia followed by transplantation allogeneic and autologous.[16,18] In our study, patients undergoing transplantation were preferably transfused with SDPs. This finding could probably be explained as this practice is consistent with the current guidelines, to minimize the risk of human leukocyte antigens and human platelet antigens alloimmunization, to prevent RhD alloimmunization, and to manage platelet transfusion refractoriness.[19]
According to our study, the average number of RDPs and SDPs transfused per episode was 4.84 (±2.73) and 1.12 (±2.73), respectively. The mean adult standard platelet dose is 2.2 x 1011platelets/m2.[4,20] Although parameters regarding the mean platelet yield in RDPs and SDPs were not included in our study, all participating transfusion services produce PCs according to the “Guide to the preparation, use and quality assurance of Blood Components” by EDQM and accordingly perform regular quality controls.[12] Thus, the mean platelet dose transfused in Greece (corresponding to five RDPs and one SDP) seems to be equivalent to the standard adult dose defined above. As expected, preterm neonates in neonatology departments received the lowest number of RDPs/episode, while patients with aneurysms the highest.
Platelet transfusion has both therapeutic and prophylactic purposes depending on whether bleeding is already established, or there is a significant risk of it occurring. Approximately two-thirds of RDPs (66%) and three quarters (75%) of SDPs were transfused prophylactically, to prevent bleeding. This phenomenon was evident in almost all participating hospitals in our study as depicted in Table 4 and was more prominent in hospitals with large hemato-oncology units treating patients with hypoproliferative thrombocytopenia. These data are in accordance with the results reported in the North of England, where 72% of platelet doses were given for prophylactic purposes, with the majority of the above given without any planned procedure.[16] However, the platelet count threshold for prophylactic platelet transfusion was not assessed in our study. It would be interesting to see the laboratory threshold for prophylactic platelet transfusion in Greece in a future audit, as different guidelines define the need for platelet transfusion for patients with therapy-induced hypoproliferative thrombocytopenia, in the absence of active bleeding at different platelet counts, ranging from 10 or less to 50 × 109/L.[19,21,22,23,24]
It is well known that platelet products are frequently wasted due to the expiration of their use-by date, which is attributed to a combination of an uncertain daily demand and a short shelf life. The majority of both RDPs and SDPs in Greece were transfused on day 5, just a day before expiration. Accordingly, the discard rate of 16.75% for RDPs and 2.70% for SDPs was mainly attributed to their expiration. In ten European countries, the mean discard rate for PLTs (regardless of their method of production) was estimated at 13.7%.[24] Regarding RDPs, recently published data indicate that the RDPs wastage rate in Iran varied between 18.5% and 10.5% before and after specific intervention.[25] In another study conducted in the Central India, 37% of PLT units were discarded,[26] while in Malaysia PLT wastage, excluding units discarded due to the expiry date and transfusion-transmitted diseases, was estimated at 6%.[27] Regarding SDPs in the US, the wastage rate calculated only for shelf life expiration was 10.3%, while in the UK, it was 3.8%.[16,28] It is worth mentioning that our results include wastage due to seropositivity for transfusion-transmitted diseases, and to perform regular quality controls, that are not included in the majority of the studies evaluating blood wastage. Considering the heterogeneity of the above-mentioned studies and the diversity of factors evaluated, our results could be considered to be at a very similar level. Nevertheless, the wastage rate could further be reduced by means of a central inventory management system in our country, which the Hellenic National Blood Transfusion Center is planning to implement in the near future.
Efforts to prolong platelet lifetime to decrease wastage are continuing. Recently, published data revealed that BC-derived PLT in AS stored for 7 days at 22°C are safe regarding the risk of a positive blood culture.[29] Additional pathogen inactivation systems in platelet products reduce bacterial contamination and provide a safeguard against the risk of emerging and reemerging pathogens.[30] Furthermore, cold storage and cryopreservation of PLTs may facilitate the extension of the products' shelf life to weeks and even years, and they may also provide the benefit of the PLTs being more hemostatically effective than conventionally stored PLTs.[31] In support of these, it has already been shown that PLTs stored at 4°C for 10–14 days have a better hemostatic and biochemical profile than those stored for 5 days in 22°C.[32]
Conclusions
The present study is the first national survey regarding platelet transfusion in Greece. The majority of patients receiving PLTs transfusions are admitted to either internal medicine or hematology departments, and they are treated for hematological diseases or other malignancies. Thus, it would seem sensible that members of the hematology and internal medicine clinical teams should be the target for educational and training initiatives on contemporary transfusion guidelines, implemented locally or at a national level. Platelet discard rate could further be lowered by the centralization of the inventory management in combination with the expansion of lifetime of PCs through the use of emerging technologies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Verma A, Agarwal P. Platelet utilization in the developing world: Strategies to optimize platelet transfusion practices. Transfus Apher Sci. 2009;41:145–9. doi: 10.1016/j.transci.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Estcourt LJ, Birchall J, Lowe D, Grant-Casey J, Rowley M, Murphy MF. Platelet transfusions in haematology patients: Are we using them appropriately? Vox Sang. 2012;103:284–93. doi: 10.1111/j.1423-0410.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- 3.Pérez Vaquero MÁ, Gorria C, Lezaun M, López FJ, Monge J, Eguizabal C, et al. Optimization of the management of platelet concentrate stocks in the Basque country using mathematical simulation. Vox Sang. 2016;110:369–75. doi: 10.1111/vox.12377. [DOI] [PubMed] [Google Scholar]
- 4.Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365–94. doi: 10.1111/bjh.14423. [DOI] [PubMed] [Google Scholar]
- 5.Australian and New Zealand Society of Blood Transfusion. Sydney. 2nd ed. Australia: Australian and New Zealand Society of Blood Transfusion Ltd; 2011. [Last accessed on 2018 Jun 30]. Guidelines for the Administration of Blood Products; pp. 1–56. Available from: https://www.anzsbt.org.au/data/documents/guidlines/ANZSBT_Guidelines_Administration_Blood_Products_2ndEd_Dec_2011_Hyperlinks.pdf . [Google Scholar]
- 6.Walters G. NHSBT Portfolio of Blood Components and Guidance for their Clinical Use. SPECIFICATION SPN223/8 2016 ed UK. 2016. [Last accessed on 2018 Jun 30]. pp. 1–95. Available from: http://hospital.blood.co.uk/media/28748/spn223.pdf .
- 7.Australian and New Zealand Society of Blood Transfusion. Sydney. 1st ed. Australia: Australian & New Zealand Society of Blood Transfusion Ltd; 2016. [Last accessed on 2018 Jun 25]. Guidelines for Transfusion and Immunohaematology Laboratory Practice; pp. 1–63. Available from: https://www.anzsbt.org.au/data/documents/guidlines/GuidelinesforTransfusionandImmunohaematology LaboratoryPractice_1ed_Nov20_.pdf . [Google Scholar]
- 8.Grouzi E, Spiliotopoulou I. Athens. 1st ed. Greece: Working Committee of Transfusion Medicine & Apheresis, Hellenic Society of Hematology; 2010. [Last accessed on 2018 Jun 20]. Guidelines for the Administration of Blood Products; pp. 1–64. Available from: http://www.eae.gr/images/files/GUIDE.LINES.pdf . [Google Scholar]
- 9.Sonnekus PH, Louw VJ, Ackermann AM, Barrett CL, Joubert G, Webb MJ, et al. An audit of the use of platelet transfusions at universitas academic hospital, bloemfontein, South Africa. Transfus Apher Sci. 2014;51:44–52. doi: 10.1016/j.transci.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Lin YC, Chang CS, Yeh CJ, Wu YC. The appropriateness and physician compliance of platelet usage by a computerized transfusion decision support system in a medical center. Transfusion. 2010;50:2565–70. doi: 10.1111/j.1537-2995.2010.02757.x. [DOI] [PubMed] [Google Scholar]
- 11.Greinacher A, Fendrich K, Hoffmann W. Demographic changes: The impact for safe blood supply. Transfus Med Hemother. 2010;37:141–8. doi: 10.1159/000313949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Directorate for the Quality of Medicines & Health Care (EDQM), Council of Europe. Guide to the Preparation, use and Quality Assurance of Blood Components Recommendation No. R (95) 15. 18th ed. Strasbourg, France: Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM); 2015. [Last accessed on 2018 Jun 30]. Available from: http://wwwedqmeu . [Google Scholar]
- 13.Valsami S, Grouzi E, Pouliakis A, Fountoulaki-Paparisos L, Kyriakou E, Gavalaki M, et al. Red blood cell transfusions in Greece: Results of a survey of red blood cell use in 2013. Turk J Haematol. 2017;34:52–8. doi: 10.4274/tjh.2016.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Economou C, Kaitelidou D, Karanikolos M, Maresso A. Greece: Health system review. Health Syst Transit. 2017;19:1–66. [PubMed] [Google Scholar]
- 15.Lozano M, Heddle N, Williamson LM, Wang G, AuBuchon JP, Dumont LJ. Practices associated with ABO-incompatible platelet transfusions: A BEST collaborative international survey. Transfusion. 2010;50:1743–8. doi: 10.1111/j.1537-2995.2010.02642.x. [DOI] [PubMed] [Google Scholar]
- 16.Charlton A, Wallis J, Robertson J, Watson D, Iqbal A, Tinegate H, et al. Where did platelets go in 2012.A survey of platelet transfusion practice in the North of England? Transfus Med. 2014;24:213–8. doi: 10.1111/tme.12126. [DOI] [PubMed] [Google Scholar]
- 17.Liang XH, Zhou SH, Fan YX, Meng QL, Zhang ZY, Gao Y, et al. A survey of the blood supply in China during 2012-2014. Transfus Med. 2017;11:12492. doi: 10.1111/tme.12492. [DOI] [PubMed] [Google Scholar]
- 18.Pendry K, Davies T. An audit of use and wastage in the North West of England and North Wales: Where have all the platelets gone? Blood Transplant Matters. 2010;34:17–9. [Google Scholar]
- 19.Schiffer CA, Bohlke K, Delaney M, Hume H, Magdalinski AJ, McCullough JJ, et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2018;36:283–99. doi: 10.1200/JCO.2017.76.1734. [DOI] [PubMed] [Google Scholar]
- 20.Pietersz RN, Reesink HW, Panzer S, Gilbertson MP, Borosak ME, Wood EM, et al. Prophylactic platelet transfusions. Vox Sang. 2012;103:159–76. doi: 10.1111/j.1423-0410.2012.01595.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: A clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 22.Tosetto A, Balduini CL, Cattaneo M, De Candia E, Mariani G, Molinari AC, et al. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia: Guidelines of the Italian society for haemostasis and thrombosis (SISET) Thromb Res. 2009;124:e13–8. doi: 10.1016/j.thromres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 23.van de Weerdt EK, Peters AL, Goudswaard EJ, Binnekade JM, van Lienden KP, Biemond BJ, et al. The practice of platelet transfusion prior to central venous catheterization in presence of coagulopathy: A national survey among clinicians. Vox Sang. 2017;112:343–51. doi: 10.1111/vox.12498. [DOI] [PubMed] [Google Scholar]
- 24.Fasano RM, Josephson CD. Platelet transfusion goals in oncology patients. Hematology Am Soc Hematol Educ Program. 2015;2015:462–70. doi: 10.1182/asheducation-2015.1.462. [DOI] [PubMed] [Google Scholar]
- 25.Javadzadeh Shahshahani H, Taghvai N. Blood wastage management in a regional blood transfusion centre. Transfus Med. 2017;27(Suppl 5):348–53. doi: 10.1111/tme.12433. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Sharma SM, Ingole NS, Gangane N. Analysis of reasons for discarding blood and blood components in a blood bank of tertiary care hospital in central India: A prospective study. Int J Med Public Health. 2014;4:72–4. [Google Scholar]
- 27.Morish M, Ayob Y, Naim N, Salman H, Muhamad NA, Yusoff NM. Quality indicators for discarding blood in the national blood center, Kuala Lumpur. Asian J Transfus Sci. 2012;6:19–23. doi: 10.4103/0973-6247.95045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan L, Tian X, Gombar S, Zemek AJ, Krishnan G, Scott R, et al. Big data modeling to predict platelet usage and minimize wastage in a tertiary care system. Proc Natl Acad Sci U S A. 2017;114:11368–73. doi: 10.1073/pnas.1714097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreuger AL, Rostgaard K, Middelburg RA, Kerkhoffs JH, Edgren G, Erikstrup C, et al. Storage time of platelet concentrates and risk of a positive blood culture: A nationwide cohort study. Transfusion. 2018;58:16–24. doi: 10.1111/trf.14401. [DOI] [PubMed] [Google Scholar]
- 30.Katus MC, Szczepiorkowski ZM, Dumont LJ, Dunbar NM. Safety of platelet transfusion: Past, present and future. Vox Sang. 2014;107:103–13. doi: 10.1111/vox.12146. [DOI] [PubMed] [Google Scholar]
- 31.Waters L, Cameron M, Padula MP, Marks DC, Johnson L. Refrigeration, cryopreservation and pathogen inactivation: An updated perspective on platelet storage conditions. Vox Sang. 2018;113:317–28. doi: 10.1111/vox.12640. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Yin W, Zhang Y, Sun Y, Ma T, Gu S, et al. Evaluation of the advantages of platelet concentrates stored at 4°C versus 22°C. Transfusion. 2018;58:736–47. doi: 10.1111/trf.14462. [DOI] [PubMed] [Google Scholar]


