Abstract
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic condition in which phosphaturic mesenchymal tumors (PMTs) secrete high levels of fibroblast growth factor 23 (FGF23) into the circulation. This results in renal phosphate wasting, hypophosphatemia, muscle weakness, bone pain, and pathological fractures. Recent studies suggest that fibronectin-fibroblast growth factor receptor 1 (FN1-FGFR1) translocations may be a driver of tumorigenesis. We present a patient with TIO who also exhibited clinical findings suggestive of Cowden syndrome (CS), a rare autosomal dominant disorder characterized by numerous benign hamartomas, as well as an increased risk for multiple malignancies, such as thyroid cancer. While CS is a clinical diagnosis, most, but not all, harbor a mutation in the tumor suppressor gene PTEN. Genetic testing revealed a somatic FN1-FGFR1 translocation in the FGF23-producing tumor causing TIO; however, a germline PTEN mutation was not identified. To our knowledge, this is the first reported case of concurrent TIO and CS.
Keywords: Cowden syndrome, FGF23, FN1-FGFR1, Phosphaturic mesenchymal tumor, PTEN, Tumor-induced osteomalacia
Introduction
Tumor-induced osteomalacia (TIO) is a rare acquired paraneoplastic disorder manifested as hypophosphatemia, renal phosphate wasting, and inappropriately normal or frankly low 1,25-dihydroxyvitamin D levels, resulting in bone pain, recurrent fractures, muscle weakness [1]. The biochemical findings are mediated by ectopic excessive fibroblast growth factor 23 (FGF23) production and secretion into the blood by the causative phosphaturic mesenchymal tumor (PMT). FGF23 is a phosphate and vitamin D-regulating hormone that acts on fibroblast growth factor receptor-1 (FGFR1) in the proximal renal tubule cells, suppressing the activity of the sodium-phosphate cotransporters (NaPi-2a and NaPi-2c) and 25-hydroxyvitamin D3 1-alpha-hydroxylase. The inability to reabsorb phosphate and produce the active form of vitamin D leads to increased renal phosphate excretion and decreased intestinal phosphate absorption, with resultant hypophosphatemia and osteomalacia [2]. Recent work suggests novel FN1-FGFR1 translocations within these PMTs may drive tumorigenesis as well as FGF23 production [3, 4]. Surgical resection is generally curative in patients with an isolated PMT, with a return of serum phosphate and FGF23 to normal levels within a matter of days [1].
Cowden syndrome is a rare autosomal dominant condition with an incidence of approximately 1:200,000 [5]. Characteristic skin lesions of CS include facial trichilemmomas, acral keratoses, and papillomatous lesions. Major and minor criteria include macrocephaly, thyroid lesions/cancer, fibromas, lipomas, genitourinary tumors, and cerebellar dysplastic gangliocytoma. Approximately 80% of Cowden syndrome patients harbor a germline mutation in the tumor suppressor PTEN; however, a mutation is not required for diagnosis. Given the propensity for Cowden patients to develop numerous cancers, much of the management focuses upon early screening to mitigate cancer risk through early detection [6].
To the best of our knowledge, we present the first patient with concurrent TIO and Cowden syndrome.
Case
Clinical features
A 50-year-old Caucasian male was referred to the NIH with an approximately 3-year history of progressively worsening bone pain, recurrent fractures, muscle spasms, fatigue, and difficulty ambulating without assistance. Prior to admission, the patient had a technetium-99 bone scan that revealed lesions in the femoral neck bilaterally, several vertebral bodies, and multiple ribs which were concerning for osteoblastic malignancy. Biopsy of the right femur ruled out malignancy and instead revealed osteopenia and histopathology consistent with a fracture callus. The patient was found to have low serum phosphorus levels (range, 1.8–2 mg/dL; normal, 2.5–4.8), and elevated C-terminal FGF23 levels (287 RU/mL; normal, < 180, Mayo Medical Laboratories, Rochester, MN). TIO was suspected, and the patient was referred to NIH endocrinology. Upon arrival to NIH, tubular resorption of phosphorus was calculated to be 74% (normal > 85%). 1,25-dihydroxyvitamin D was 30 pg/mL (normal, 18–64). Medical therapy was initiated with phosphate and calcitriol with reduction in bone pain and fatigue. Additional co-morbidities included hypertension, dyslipidemia with high cholesterol and triglycerides, morbid obesity, and sleep apnea.
Physical examination revealed multiple firm, moveable, and non-tender subcutaneous masses on the bilateral upper and lower extremities and abdomen suspicious for lipomas. Other findings included a pebbled, cobblestone texture of the upper gingiva; multiple fleshy and warty exophytic papules on the neck, chest, and axillae consistent with acrochordons; depigmentation of the glans penis, scrotum, and right cheek consistent with vitiligo; and macrocephaly (61.3 cm, 50th percentile for height: 58 cm [7]). The patient reported that his mother, sister, and brother had a history of lipomas, and a first degree cousin had a history of thyroid cancer and lipomas. With the suspicion of Cowden syndrome raised, the patient underwent a thyroid ultrasound, which revealed several bilateral echogenic and complex thyroid foci > 1 cm in diameter. A fine needle aspiration of the nodule showed a follicular lesion of unknown significance. MRI of the head was negative for Cowden-associated dysplastic cerebellar gangliocytoma. Skin biopsies from the lower lip (1.5-mm gray papule on the mucosal surface) and mandible were interpreted as angiofibroma and polypoid benign keratosis, respectively (Fig. 1a, b and Fig. 2f,h). CLIA-Lab-certified targeted genetic testing revealed the patient was PTEN mutation-negative.
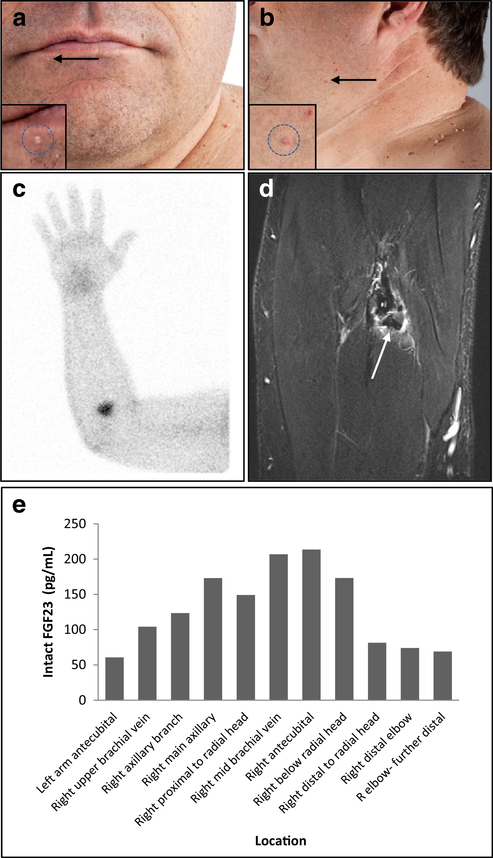
Fig. 1.
a Patient anterior view with numerous pedunculated papules. Arrow denotes biopsy location of a 1.5-mm gray papule on the lower lip interpreted as an angiofibroma. b Numerous pedunculated papules of various sizes distributed around the neck, chest, and bilateral axillae, consistent with acrochordon. Arrow denotes location of mandibular skin shave biopsy interpreted as a polypoid benign keratosis. c SPECT/CT scan after Indium-111-pentetreotide injection with abnormal focal uptake in the right elbow region indicated suspected location of the PMT. d T1 MR imaging of right elbow shows a 3 × 1.5-cm mass of low signal with peripheral enhancement just below the radial head, anterior to the ulna about 2 cm below the elbow. The PMT was located just deep of pronator teres and medial to brachioradialis muscle within the deep fascia adjacent to the biceps tendon. e Intact FGF23 levels ELISA results from plasma collected during venous sampling. Increased FGF23 secretion suggests localization of the tumor to the right antecubital region
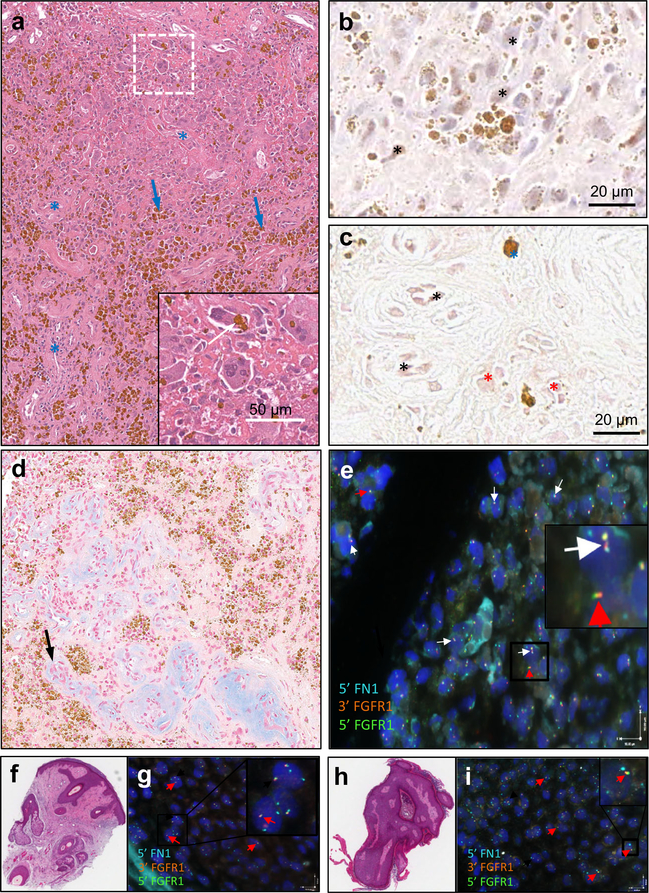
Fig. 2.
a A mixed connective tissue variant type with extensive hemangiopericytoma-like vascular differentiation (blue asterisks) with interstitial fibrosis is seen in the picture. Abundant intracellular and extracellular hemosiderin granular brown deposits are evident (blue arrows), as well as osteoclastic-like giant cells (inset). b The figure shows widespread positive reaction for FGF23 in tumor cells (asterisks). c CD31 is positive in endothelial cells (black asterisks) as well as in tumor cells (red asterisks). Blue asterisks point to brown coarse hemosiderin deposits. d Numerous irregular vascular structures with thickened walls, frequently containing amorphous hyaline or mucous Alcian blue positive deposits (right, black arrow). e Fluorescent in situ hybridization (FISH) of OOM41 FFPE tissue with tri-color probe for 5′ FN1 (CytoAqua), 3′ FGFR1 (CytoGreen), and 5′ FGFR1 (CytoOrange). Examples of abnormal FN1-FGFR1 partners are represented by white arrows. Normal, intact FGFR1 signal is represented by red arrows. 23/65 nuclei (35%) represents a positive sample for FN1-FGFR1 translocation per thresholds proposed by Lee et al. (2015). f H&E of biopsy of right lower lip papule interpreted as an angiofibroma. g FISH of lip papule showing normal, intact FGFR1 and FN1 signals as represented by red arrows. h H&E biopsy of left mandibular skin shave interpreted as a polypoid benign keratotic lesion. i FISH of mandibular skin shave showing normal, intact FGFR1 and FN1 signals represented by red arrows
TIO—tumor localization and resection
Functional and anatomical imaging studies were performed to localize the TIO-causing tumor. A 111-Indium-pentetreotide SPECT/CT scan (octreoscan) identified a discrete focus of uptake in the right antecubital region (Fig. 1c). 18F-fluorodeoxyglucose (18F-FDG)-PET/CT showed multifocal activity in the left knee, femur, calcaneus, right knee, and diffuse abnormal activity at the right elbow. MRI of the right arm identified a 3.0 × 1.5-cm mass with peripheral enhancement on T1 within the soft tissues approximately 2 cm below the anterior right elbow and radial head. The mass was visualized approximately 2.7 cm from the surface of the skin, just deep of pronator teres, medial to brachioradialis, and adjacent to the neurovascular bundle (Fig. 1d). Selective venous sampling was performed to confirm that the lesion was the source of elevated FGF23. Intact FGF23 was measured in our lab by ELISA (Immutopics, Athens, OH) (Fig. 1e).
The tumor was excised with wide margins and serum intact FGF23 levels returned to normal ranges (9.2–31.4 pg/mL) within 24 h. Post-operatively, the serum phosphorus returned to normal within 5 days (3.4–4.9 mg/dL). As expected, after PMT removal [8] 1,25-dihydroxyvitamin D levels transiently rebounded to a maximum of 200 pg/mL, before returning to the normal range.
Pathologic analysis revealed spotty calcifications, mesenchymal cell proliferation with perivascular arrangement, and the presence of focal giant cells, consistent with a PMT. PAS and Alcian blue staining showed the presence of degenerative vessels within the tumor. FGF23 expression was present within tumor cells as demonstrated by immunohistochemistry. Further immunostaining for vascular marker CD31 was positive, illustrating staining in endothelial as well as in tumor cells, suggesting vascular differentiation of the PMT (Figs. 2a–d).
FN1-FGFR1 testing
Fluorescent in situ hybridization (FISH) was performed on formalin-fixed paraffin-embedded samples (FFPE) using a validated, commercially available, research-grade FN1-FGFR1-specific assay kit (CytoTest, Rockville, MD). The aforementioned skin angiofibroma and polypoid benign keratosis lesions revealed normal chromosomal distribution of FN1 and FGFR1 (Fig. 2g, i). In contrast, FISH performed on FFPE samples of the TIO-causing PMT showed positivity for the FN1-FGFR1 translocation as determined by thresholds proposed by Lee et al. (2015) [3] (Fig. 2e).
Discussion
This report represents the first case of the coexistence of Cowden syndrome and TIO. Clinical cure following tumor resection confirmed that the excised PMT was the cause of the disease. Molecular testing demonstrated that the tumor harbored the recently described FN1-FGFR1 translocation, suggesting that FGFR1 signaling played a role in tumorigenesis. The Cowden-associated hamartomas, however, were negative for the FN1-FGFR1 translocation, suggesting that etiology of these tumors was different from the PMT.
While PTEN mutations are the most common cause of CS (approximately 80% of patients), this patient did not have an identified PTEN mutation, suggesting that the molecular etiology may be in a genomic region outside of the coding region of the PTEN gene. Recent studies on PTEN mutation-negative patients have shown that a small fraction (10%) have alterations in the PTEN promoter region, resulting in a decrease in PTEN protein expression and considerable increase in downstream phospho-AKT protein expression. Additional small cohorts of PTEN-mutation patients have been shown to have PTEN deletions that extend into adjacent genes [5]. The FGFR1 gene appears to be a locus prone to deleterious genomic alterations; a number of FGFR1-related genomic changes including translocations, intra-tandem duplications, and activating mutations exist across a variety of neoplastic conditions. [9]
It is speculated that FGFR1 signaling may play a role in PMT pathogenesis. Downstream signaling from FGFR1 includes many pathways, including the AKT pathway. PTEN is a brake on the AKT pathway. Therefore, loss of PTEN tumor suppression may enhance, if not predispose to tumorigenesis, or possibly more aggressive or even malignant PMTs. In fact, we recently reported on a patient with a malignant, metastatic PMT that harbored the FN1-FGFR1 translocation [10]. Of note, mutation analysis of 395 known cancer-causing genes of one of the metastatic lesions found, in addition to the FN1-FGFR1 translocation, a frameshift mutation in PTEN. The change, p.T319fs*5, results in the insertion of a thymidine at c.957_958 and a nonsense mutation of PTEN exon 8 at amino acid 324, and is predicted to cause early termination. It is possible that this previously reported patient with a germline mutation that may inactivate PTEN could be at risk for more aggressive, malignant disease and warrants close observation.
While the currently described patient is PTEN mutation-negative, high levels of phosphorylated AKT and phosphorylated S6 were detected on immunohistochemical staining (data not shown), supporting the concept that the AKT pathway is activated in tumor cells, perhaps due to translocation-mediated signaling through the chimeric FN1-FGFR1 receptor. Future examination of AKT pathway activation in Cowden-associated hamartomatous lesions may lead to greater understanding of the associations between these two rare diseases.
While it is difficult to make a mechanistic association at this point between the coexistence of CS and TIO, perhaps this first reported case of these two syndromes occurring together may prompt clinicians to identify other cases, further investigation of which may aid in understanding not only their association, but also their individual pathogenesis and pathophysiology.
Acknowledgements
The authors would like to thank Reinhard Ebner, Joseph Cheng, Claire Tzou and Dale Whitley of CytoTest Inc. (Rockville, MD) for their technical support and assistance.
Funding information This research was supported in part by the Intramural Research Program of the NIH, NIDCR. This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH by the Doris Duke Charitable Foundation (grant no. 2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and other private donors (for a complete list, visit the foundation website at http://www.fnih.org).
Footnotes
Compliance with ethical standards
Conflict of interest None.
References
- 1.Chong WH, Molinolo AA, Chen CC, Collins MT (2011) Tumor-induced osteomalacia. Endocr Relat Cancer 18(3):R53–R77. 10.1530/ERC-11-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Chong WH, Gafni RI, Collins MT (2012) Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab 23(12):610–618. 10.1016/j.tem.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JC, Jeng YM, Su SY, Wu CT, Tsai KS, Lee CH, Lin CY, Carter JM, Huang JW, Chen SH, Shih SR, Mariño-Enríquez A, Chen CC, Folpe AL, Chang YL, Liang CW (2015) Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol 235(4):539–545. 10.1002/path.4465 [DOI] [PubMed] [Google Scholar]
- 4.Lee JC, Su SY, Changou CA, Yang RS, Tsai KS, Collins MT, Orwoll ES, Lin CY, Chen SH, Shih SR, Lee CH, Oda Y, Billings SD, Li CF, Nielsen GP, Konishi E, Petersson F, Carpenter TO, Sittampalam K, Huang HY, Folpe AL (2016) Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod Pathol 29(11):1335–1346. 10.1038/modpathol.2016.137 [DOI] [PubMed] [Google Scholar]
- 5.Pilarski R (2009) Cowden syndrome: a critical review of the clinical literature. J Genet Couns 18(1):13–27. 10.1007/s10897-008-9187-7 [DOI] [PubMed] [Google Scholar]
- 6.Pilarski R, Eng C (2004) Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet 41(5):323–326. 10.1136/jmg.2004.018036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bale SJ, Amos CI, Parry DM, Bale AE (1991) Relationship between head circumference and height in normal adults and in the nevoid basal cell carcinoma syndrome and neurofibromatosis type I. Am J Med Genet 40(2):206–210. 10.1002/ajmg.1320400217 [DOI] [PubMed] [Google Scholar]
- 8.Chong WH, Andreopoulou P, Chen CC, Reynolds J, Guthrie L, Kelly M, Gafni RI, Bhattacharyya N, Boyce AM, El -Maouche D, Crespo DO, Sherry R, Chang R, Wodajo FM, Kletter GB, Dwyer A, Collins MT (2013) Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J Bone Miner Res 28(6):1386–1398. 10.1002/jbmr.1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo LH, Nelson KN, Meyer AN, Donoghue DJ (2015) Functions of fibroblast growth factor receptors in cancer defined by novel translocations and mutations. Cytokine Growth Factor Rev 26(4): 425–449. 10.1016/j.cytogfr.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 10.Collins M, et al. (2015) Striking response of tumor-induced osteomalacia to the FGFR inhibitor NVP-BGJ398 American Society of Bone and Mineral Research Annual Meeting Seattle. WA.(pp. LB-SA0035 and >SA0035) [Google Scholar]