Abstract
Purpose:
We sought to determine whether saturation of the index lesion during magnetic resonance imaging-transrectal ultrasound fusion guided biopsy would decrease the rate of pathological upgrading from biopsy to radical prostatectomy.
Materials and Methods:
We analyzed a prospectively maintained, single institution database for patients who underwent fusion and systematic biopsy followed by radical prostatectomy in 2010 to 2016. Index lesion was defined as the lesion with largest diameter on T2-weighted magnetic resonance imaging. In patients with a saturated index lesion transrectal fusion biopsy targets were obtained at 6 mm intervals along the long axis of the index lesion. In patients with a nonsaturated index lesion only 1 target was obtained from the lesion. Gleason 6, 7 and 8–10 were defined as low, intermediate and high risk, respectively.
Results:
Included in the study were 208 consecutive patients, including 86 with a saturated and 122 with a nonsaturated lesion. Median patient age was 62.0 years (IQR 10.0) and median prostate specific antigen was 7.1 ng/ml (IQR 8.0). The median number of biopsy cores per index lesion was higher in the saturated lesion group (4 vs 2, p <0.001). The risk category upgrade rate from systematic only, fusion only, and combined fusion and systematic biopsy results to prostatectomy was 40.9%, 23.6% and 13.8%, respectively. The risk category upgrade from combined fusion and systematic biopsy results was lower in the saturated than in the nonsaturated lesion group (7% vs 18%, p = 0.021). There was no difference in the upgrade rate based on systematic biopsy between the 2 groups. However, fusion biopsy results were significantly less upgraded in the saturated lesion group (Gleason upgrade 20.9% vs 36.9%, p = 0.014 and risk category upgrade 14% vs 30.3%, p = 0.006).
Conclusions:
Our results demonstrate that saturation of the index lesion significantly decreases the risk of upgrading on radical prostatectomy by minimizing the impact of tumor heterogeneity.
Keywords: prostatic neoplasms; image-guided biopsy; neoplasm grading; pathology, surgical; risk
ALTHOUGH the current standard for PCa diagnosisis 12-core TRUS guided (systematic) biopsy, this approach may under grade 38% to 46% of tumors compared to whole mount prostatectomy specimens.1,2 As most treatment decisions rely on accurate assessment of PCa grade in the prostate, a more accurate method of diagnosing PCa is necessary to avoid under treatment of disease.
One such method is the saturation biopsy, a method of systematic biopsy in which a substantially higher number of biopsy cores are obtained from the entire prostate gland.3 While some studies indicate an increased CDR using this method, there is concern that increased unguided sampling of the prostate also leads to increased detection of mostly clinically insignificant disease.4,5
The application of mpMRI has enabled urologists to visualize areas in the prostate suspicious for harboring CS PCa with sensitivity and specificity as high as 93% and 98%, respectively, to detect and exclude CS PCa larger than 0.5 cm.6 In addition to increased lesion detection capability, mpMRI has proved to increase the yield of cancer diagnosis via mpMRI-TRUS fusion guided prostate biopsies when combined with systematic biopsy.7–13
Using transrectal saturation biopsy with mpMRI image guidance a high number of biopsy cores can be taken specifically from an area of abnormality on mpMRI. At our institution this method of image guided saturation biopsy of the largest lesion (index lesion) found on T2-weighted imaging has been used since 2010. By increasing the number of biopsy cores taken from the index lesion we hypothesized that the CDR of fusion biopsy would improve, reducing the upgrade rate at the final pathological evaluation.
MATERIALS AND METHODS
Patient Selection and Data Collection
We prospectively collected and retrospectively reviewed demographic and clinicopathological data on 411 patients who underwent RP between 2010 and 2016 at NIH in Bethesda, Maryland (fig. 1). Patients who underwent prostate mpMRI and a combination of fusion and systematic biopsy prior to RP were included in study. Exclusion criteria were a history of prior therapy for PCa (eg radiation therapy, hormone therapy, etc) or a maximum index tumor diameter less than 1 cm, which was deemed insufficient for consideration for saturation in 61 cases. We defined image guided saturation as obtaining at least 2 biopsy targets from the index tumor with each target 6 mm apart. Therefore, patients with tumors less than 1 cm in the longest dimension were excluded from analysis. Figure 1 shows patient selection for this study.
Figure 1.
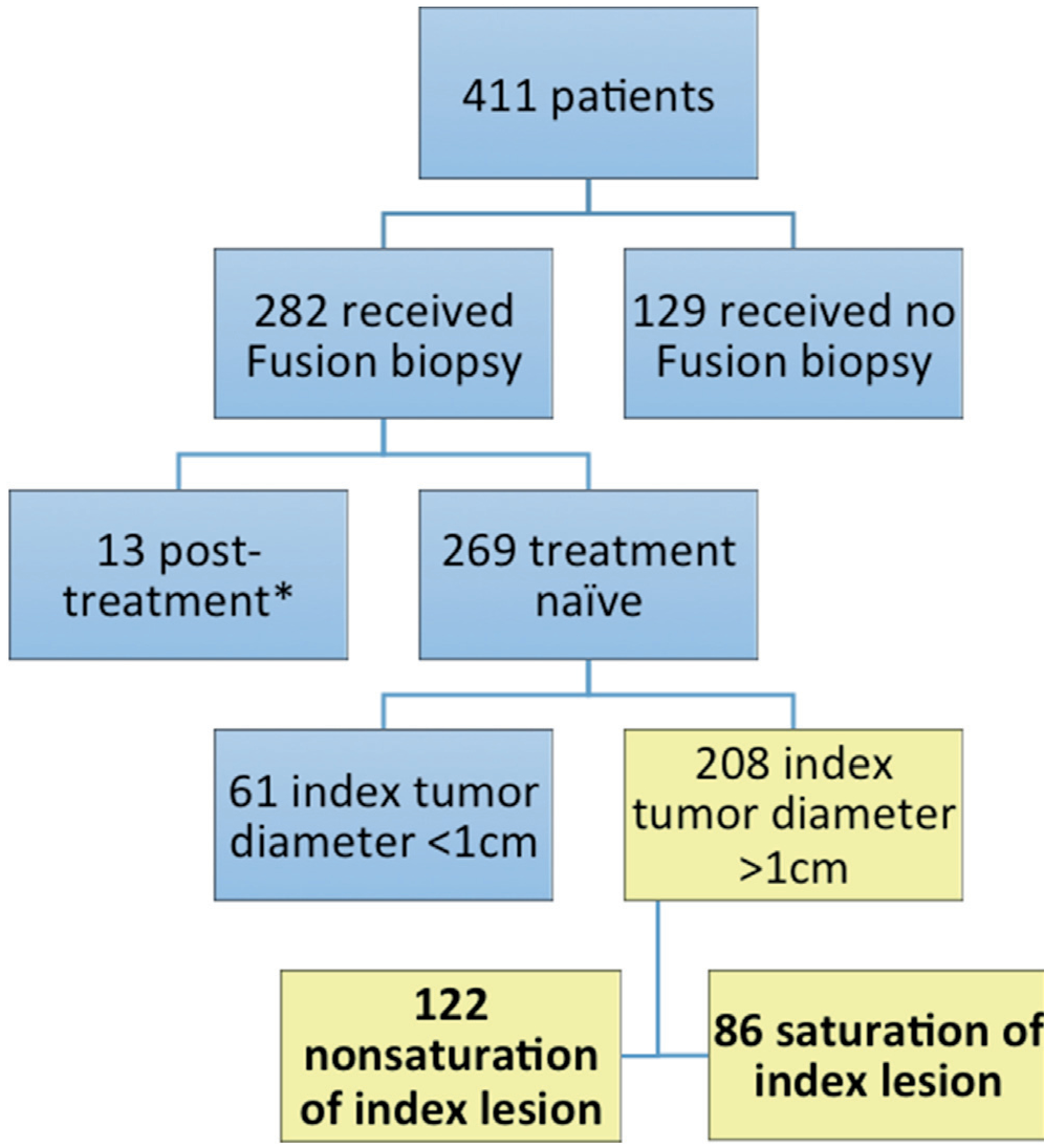
Inclusion and exclusion criteria for entire patient cohort. Asterisk indicates that treatment included radiation and hormone therapy.
Magnetic Resonance Imaging Acquisition and Interpretation
Patients underwent prostate MRI on a 3.0 Tesla Achieva scanner (Philips Healthcare, Andover, Massachusetts), which was performed with a BPX-30 endorectal coil (Medrad, Warrendale, Pennsylvania) and a 16-channel SENSE surface coil (Philips Healthcare) using previously described protocols.14,15 Sequences obtained included triplanar T2-weighted, axial dynamic contrast enhanced and axial diffusion weighted imaging with apparent diffusion coefficient mapping and high B-value diffusion-weighted MRI. All MRI interpretation was performed by 2 highly experienced genitourinary radiologists (BT and PLC) using an in-house MRI interpretation system.7
Although PI-RADS™, version 2 has been in use at our institution since May 2015 along with an in-house reporting system, for this current prospective study we did not use PI-RADS data since patients who underwent MRI prior to May 2015 did not undergo mpMRI interpretation based on PI-RADS, version 2. In each prostate lesion size was approximated using dimensions measured on T2-weighted imaging. The index lesion was defined as the lesion with the largest diameter on T2-weighted imaging that had the highest NIH suspicion score.16 Patients were then retrospectively separated into NSIL and SIL cohorts according to the number of biopsy targets designated to the index lesion.
Biopsy Procedure
All patients underwent transrectal fusion biopsy as previously described.7 A total of 2 biopsy cores were obtained per target designation, including 1 in the axial plane and 1 in the sagittal plane. In the 86 SIL cases targets were placed at 6 mm intervals along the axis of maximal diameter of the index lesion as shown on T2-weighted MRI (fig. 2, A). In the 122 NSIL cases only a single target was used in the center of the index lesion (fig. 2, B). Patients in each cohort also underwent systematic 12-core biopsy at the same session as fusion biopsy.
Figure 2.
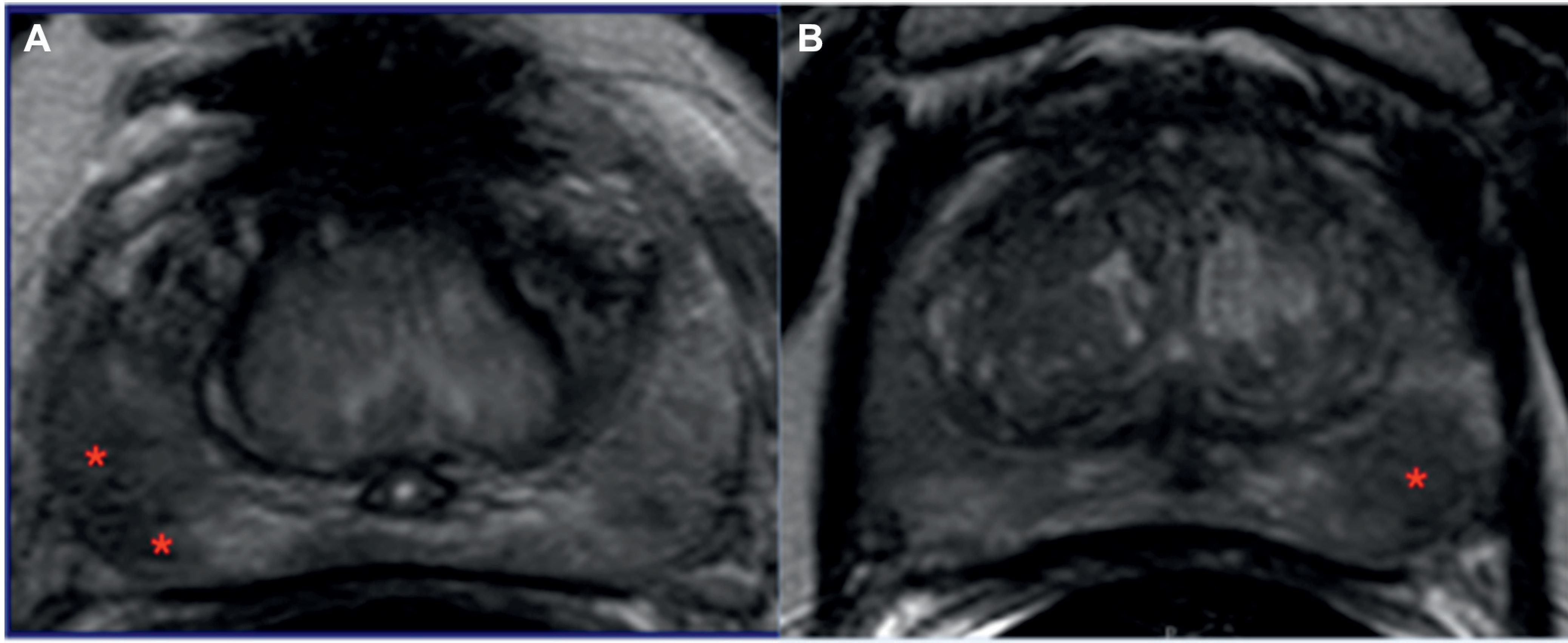
Patients referred for elevated PSA and sextant biopsy done elsewhere which showed Gleason 3 + 3 cancer. A, in 62-year-old male mpMRI at our institution revealed lesion in midline apical-mid anterior transition zone. Two-target mpMRI-TRUS fusion guided biopsy subsequently showed Gleason 3 + 4 cancer in 2 cores plus Gleason 3 + 3 in 1 core. Prostatectomy analysis demonstrated Gleason 3 + 4 disease. B, in 58-year-old male mpMRI at our institution revealed lesion in left apical-mid posterior peripheral zone. Single target mpMRI-TRUS fusion guided biopsy subsequently showed benign prostatic tissue only. Prostatectomy analysis demonstrated Gleason 3 + 4 disease in lesion area.
Evaluations
Pathological.
Pathological data on each preprostatectomy biopsy specimen were analyzed for the highest Gleason score. RP specimens were whole mount mapped to determine the highest Gleason score in the gland. Low, intermediate and high risk categories were assigned to biopsy and whole mount specimens according to the highest Gleason score detected in the specimen, including Gleason 3 + 3, 3 + 4 to 4 + 3 and greater than 4 + 3, respectively. Pathological data on biopsy and RP specimens were then compared for differences in the highest Gleason score or risk category in the 2 specimens. If a whole mount specimen had a higher Gleason score or risk category than the corresponding preRP biopsy, the case was upgraded from biopsy to RP.
Statistical.
Statistical analysis was performed using SPSS®, version 21. Continuous parameters between cohorts were compared by the Mann-Whitney test. The proportion of Gleason score and risk category upgrading was compared by biopsy modality, and between the SIL and NSIL cohorts. The chi-square test was applied to compare biopsy results and upgrading rates between the SIL and NSIL cohorts. The McNemar test was used to compare upgrading rates between fusion biopsy and systematic biopsy in patients.
RESULTS
A total of 208 patients were included in study. Median age was 62 years (IQR 10) and median PSA was 7.1 ng/ml (IQR 8.0). SIL fusion biopsy was performed in 86 patients and the median number of biopsy cores was 4 (IQR 2). Table 1 shows patient demographic, clinical, biopsy and RP pathological data. Clinical stage, prostate volume, race and PSA were similar in the 2 cohorts (table 1).
Table 1.
Demographics, clinical characteristics and biopsy results
| Index Tumor Saturation |
||||
|---|---|---|---|---|
| No | Yes | Overall | p Value | |
| No. pts (%) | 122 (58.6) | 86 (41.4) | 208 | – |
| Median age (IQR) | 61 (10) | 63 (9.8) | 62 (10) | 0.535 |
| Median ng/ml PSA (IQR) | 7.17 (6) | 7.92 (11) | 7.1 (8) | 0.074 |
| No. race (%): | ||||
| White | 74 (60.1) | 54 (62.8) | 128 (61.5) | 0.310 |
| Black | 24 (19.7) | 11 (12.8) | 35 (16.8) | |
| Other | 7 (5.7) | 8 (9.3) | 15 (7.2) | |
| No. clinical stage greater than cT1c (%) | 3 (2.9) | 2 (2.7) | 5 (2.9) | 0.925 |
| No. highest NIH MRI score moderate-high (%) | 100 (96.2) | 70 (97.2) | 170 (96.6) | 0.697 |
| Median ml MRI prostate vol (IQR) | 38 (18) | 37 (19) | 38 (19) | 0.906 |
| Median No. index lesion cores (IQR) | 2 (0) | 4 (2) | 2 (2) | <0.001 |
| No. systematic biopsy Gleason score (%): | 0.217 | |||
| 6 | 35 (28.5) | 22 (25.6) | 57 (27.4) | |
| 7 | 58 (47.2) | 35 (40.7) | 93 (44.7) | |
| 8–10 | 22 (17.9) | 17 (19.8) | 39 (18.8) | |
| No. fusion biopsy Gleason score (%): | 0.003 | |||
| 6 | 17 (13.8) | 11 (12.8) | 28 (13.5) | |
| 7 | 61 (49.6) | 47 (54.7) | 108 (51.9) | |
| 8–10 | 29 (23.6) | 28 (32.6) | 57 (27.4) | |
| No. combined biopsy Gleason score (%): | 0.059 | |||
| 6 | 16 (13.0) | 5 (5.8) | 21 (10.1) | |
| 7 | 76 (61.8) | 49 (57.0) | 125 (60.1) | |
| 8–10 | 31 (25.2) | 32 (37.2) | 64 (30.8) | |
In the entire cohort the proportion of patients who experienced upgrading of Gleason score on prostatectomy according to systematic only, fusion only and combined fusion/systematic biopsy results was 47.1%, 30.3% and 18.8%, respectively. Risk category upgrading followed similar trends with 40.9%, 23.6% and 13.8% of cases upgraded on prostatectomy pathology findings compared to systematic, fusion and combined biopsy results, respectively. When comparing the biopsy results of the 2 techniques, fusion biopsy results were significantly less likely to be upgraded in terms of Gleason score and risk category (each p <0.001, table 2).
Table 2.
Paired analysis of biopsy modalities showing upgrade rates from biopsy to final prostatectomy
| No. mpMRI-TRUS Biopsy (%) |
||
|---|---|---|
| Systematic 12-Core Biopsy | No Prostatectomy Upgrade* | Prostatectomy Upgrade |
| Final prostatectomy Gleason score: | ||
| No upgrade | 86 (41.3) | 24 (11.5) |
| Upgrade | 59 (28.4) | 39 (18.8) |
| Final prostatectomy risk category: | ||
| No upgrade | 102 (49.0) | 21 (10.1) |
| Upgrade | 57 (27.4) | 28 (13.5) |
Pathological data from systematic and mpMRI-TRUS fusion biopsies were analyzed for maximum Gleason score and risk category, and compared with corresponding data from prostatectomy on each patient. If prostatectomy Gleason score or risk category was higher than systematic, fusion or combined biopsy Gleason score or risk category, case was deemed upgraded in corresponding biopsy category. Upgrade rates of each biopsy modality were recorded for patients with saturation biopsy and those with nonsaturation fusion biopsy of index tumor.
McNemar test p <0.001.
Any Gleason score and risk category upgrade from combined fusion/systematic biopsy results were lower in the SIL than in the NSIL group (table 3). While there was no difference in the 2 groups in the upgrade rate based on systematic biopsy, fusion biopsy results were significantly less upgraded in the SIL group (Gleason upgrade 20.9% vs 36.9%, p = 0.014, and risk category upgrade 14% vs 30.3%, p = 0.006).
Table 3.
Gleason score and risk category upgrade rates from biopsy to prostatectomy for different biopsy modalities
| Systematic 12-Core Biopsy |
mpMRI-TRUS Biopsy |
Systematic 12-Core + mpMRI-TRUS Biopsy |
||||
|---|---|---|---|---|---|---|
| Prostatectomy Upgrade | Nonsaturation | Saturation | Nonsaturation | Saturation | Nonsaturation | Saturation |
| Gleason score: | ||||||
| No | 70 (57.4) | 40 (46.5) | 77 (63.1) | 68 (79.1) | 95 (77.9) | 74 (86.0) |
| Yes | 52 (43.1) | 46 (53.5) | 45 (36.9) | 18 (20.9) | 27 (22.1) | 12 (14.0) |
| p Value | 0.122 | – | 0.014 | – | 0.137 | – |
| Risk category: | ||||||
| No | 75 (61.5) | 48 (55.8) | 85 (69.7) | 74 (86.0) | 100 (82.0) | 80 (93.0) |
| Yes | 47 (38.5) | 38 (44.2) | 37 (30.3) | 12 (14.0) | 22 (18.0) | 6 (7.0) |
| p Value | 0.413 | – | 0.006 | – | 0.021 | – |
Pathological data from systematic and mpMRI-TRUS fusion biopsies were analyzed for maximum Gleason score and risk category, and compared with corresponding data from prostatectomy on each patient. If prostatectomy Gleason score or risk category was higher than systematic, fusion or combined biopsy Gleason score or risk category, case was deemed upgraded in corresponding biopsy category. Upgrade rates of each biopsy modality were recorded for patients with saturation biopsy and those with nonsaturation fusion biopsy of index tumor.
DISCUSSION
The ultimate goal of prostate biopsy is to sample the prostate in a manner that enables urologists to make the most informed clinical decisions possible based on accurate biopsy results at the preoperative decision stage. Our results show that increasing the number of biopsy samples from the index tumor improved agreement between biopsy and prostatectomy specimens in Gleason score and in the overall PCa risk category designation. Our strategy borrows from 2 established biopsy techniques, including saturation biopsy and fusion biopsy, to maximize the cancer detection rate of CS disease while avoiding additional biopsies that yield little clinical benefit.3,7
Saturation biopsy, which traditionally involves taking 20 or more cores from the prostate, is based on the premise that increased sampling will result in a lower rate of PCa misdiagnosis compared to traditional transrectal ultrasound guided systematic biopsy.3 However, several studies indicated that simply increasing the number of biopsy cores does not necessarily improve the cancer detection rate.17,18 The optimal number and location of biopsy core placement remain subjects of controversy, and concerns regarding over detection and increased risk of biopsy complications remain barriers to the widespread adoption of whole gland saturation biopsy.19 Image guidance provides a lesion based solution. By focusing additional biopsy cores on the index lesion, it may be possible to achieve more accurate pathological information which is closer to that obtained from prostatectomy.
The index lesion is the tumor with the largest volume that has the highest readout score (eg PI-RADS, Likert, etc) on MRI and the highest Gleason grade. It is generally held that the index tumor drives disease progression and by treating this tumor alone one can achieve results similar to those of treating the whole gland.20–22 At many institutions lesions are now targeted by an additional biopsy using mpMRI-TRUS fusion guidance. Results have shown that urologists who apply this method can detect 30% more high grade cancers and 17% fewer low grade cancers than by systematic biopsy alone.7
Although this is a significant improvement in the cancer detection rate, it may not yet be optimal. Tumor histology is known to show heterogeneity. In a prostatectomy analysis of 61 patients Ruijter et al found that 100% of tumors greater than 2 cm in diameter showed histological heterogeneity.23 Furthermore, histological heterogeneity has proved to be detectable on biopsy. In a recent study Porpiglia et al performed fusion biopsy in 327 patients using 4 to 6 cores per index lesion.24 They found that 26.4% of index lesions that were greater than 8 mm in diameter showed Gleason score heterogeneity compared to biopsy cores taken from the lesion. Mesko et al reported an even higher rate with more than half of 73 mpMRI targets that were biopsied using 2 or more cores showing Gleason score heterogeneity among the cores.25 These results and others suggest that a single fusion biopsy obtained from the index tumor may not accurately represent the aggressiveness of the whole tumor and carries a risk of missing CS disease.26,27 This study comparison of 2 biopsy strategies to detect the highest grade cancer in the prostate in turn allows for more complete assessment of how these strategies would impact the patient overall prognosis.
Using image guided fusion biopsy and saturation sampling we significantly reduced the rate of surgical upgrading compared with that of a single target mpMRI-TRUS fusion biopsy. In comparison, if we had relied on systematic biopsy alone for clinical decision making, we would have upgraded 47% of our cases, which is consistent with the established literature on the upgrade rate of systematic biopsy.2 Adding NSIL fusion biopsy to systematic biopsy (ie a combined biopsy approach) decreased our overall upgrade rate to 22%, which is similar to previous results demonstrating that the combination of fusion biopsy and systematic biopsy has a 19% upgrade rate.28 With the addition of SIL fusion biopsy of the index lesion to systematic biopsy we achieved an even lower 14% rate of Gleason score upgrading. Furthermore, in terms of a more clinically relevant risk category the upgrade rate was only 6% in the SIL group.
There are several limitations inherent to any prospective study such as this. The decision to perform saturation biopsy was made by the reading radiologist. It is likely that some tumor characteristics might have prompted that decision, resulting in potential selection bias. However, the 2 groups were found to be similar in clinical characteristics. Also, we acknowledge that a considerable degree of interreader variability exists among radiologists when identifying tumors on mpMRI. This may have impacted which tumors were designated as the index tumor. However, our approach to image interpretation and target designation is completely standard and our NSIL results are consistent with those of other studies. Collectively this provides strong evidence of improved CDR when additional biopsies are taken from the index lesion.
CONCLUSIONS
We report that in patients who undergo targeted fusion biopsy for suspicion of PCa increasing the number of biopsy cores from the index lesion decreases the rate of Gleason and risk category upgrading at prostatectomy. Using this approach urologists can be more confident in the accuracy of preoperative decision making with a decreased risk of under treating disease.
Abbreviations and Acronyms
- CDR
cancer detection rate
- CS
clinically significant
- mpMRI
multiparametric MRI
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- NSIL
nonSIL
- PCa
prostate cancer
- PI-RADS™
Prostate Imaging Reporting and Data System
- PSA
prostate specific antigen
- RP
radical prostatectomy
- SIL
saturated index lesion
- TRUS
transrectal ultrasound
Footnotes
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
Contributor Information
Brian P. Calio, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Abhinav Sidana, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland; Division of Urology, University of Clincinnati College of Medicine, Cincinnati, Ohio.
Dordaneh Sugano, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Sonia Gaur, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Mahir Maruf, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Amit L. Jain, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Maria J. Merino, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Peter L. Choyke, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Bradford J. Wood, Center for Interventional Oncology, National Cancer Institute and Clinical Center, National Institutes of Health, Bethesda, Maryland.
Peter A. Pinto, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Baris Turkbey, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Kvale R, Moller B, Wahlqvist R et al. : Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int 2009; 103: 1647. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes ET, Sundaram CP, Long R et al. : Biopsy Gleason score: how does it correlate with the final pathological diagnosis in prostate cancer? Br J Urol 1997; 79: 615. [DOI] [PubMed] [Google Scholar]
- 3.Borboroglu PG, Comer SW, Riffenburgh RH et al. : Extensive repeat transrectal ultrasound guided prostate biopsy in patients with previous benign sextant biopsies. J Urol 2000; 163: 158. [PubMed] [Google Scholar]
- 4.Jiang X, Zhu S, Feng G et al. : Is an initial saturation prostate biopsy scheme better than an extended scheme for detection of prostate cancer? A systematic review and meta-analysis. Eur Urol 2013; 63: 1031. [DOI] [PubMed] [Google Scholar]
- 5.Kahl P, Wolf S, Adam A et al. : Saturation biopsy improves preoperative Gleason scoring of prostate cancer. Pathol Res Pract 2009; 205: 259. [DOI] [PubMed] [Google Scholar]
- 6.Ukimura O, Coleman JA, de la Taille A et al. : Contemporary role of systematic prostate biopsies: indications, techniques, and implications for patient care. Eur Urol 2013; 63: 214. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui MM, Rais-Bahrami S, Turkbey B et al. : Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ukimura O, Hirahara N, Fujihara A et al. : Technique for a hybrid system of real-time transrectal ultrasound with preoperative magnetic resonance imaging in the guidance of targeted prostate biopsy. Int J Urol 2010; 17: 890. [DOI] [PubMed] [Google Scholar]
- 9.Marks L, Young S and Natarajan S: MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol 2013; 23: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puech P, Rouviere O, Renard-Penna R et al. : Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsydprospective multicenter study. Radiology 2013; 268: 461. [DOI] [PubMed] [Google Scholar]
- 11.Mendhiratta N, Rosenkrantz AB, Meng X et al. : Magnetic resonance imaging-ultrasound fusion targeted prostate biopsy in a consecutive cohort of men with no previous biopsy: reduction of over detection through improved risk stratification. J Urol 2015; 194: 1601. [DOI] [PubMed] [Google Scholar]
- 12.Calio B, Sidana A, Sugano D et al. : Changes in prostate cancer detection rate of MRI-TRUS fusion vs systematic biopsy over time: evidence of a learning curve. Prostate Cancer Prostatic Dis 2017; 20: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugano D, Sidana A, Calio B et al. : MRI-targeted biopsy: is systematic biopsy obsolete? Can J Urol 2017; 24: 8876. [PubMed] [Google Scholar]
- 14.Greer MD, Choyke PL and Turkbey B: PI-RADSv2: how we do it. J Magn Reson Imaging 2017; 46: 11. [DOI] [PubMed] [Google Scholar]
- 15.Muthigi A, George AK, Sidana A et al. : Missing the mark: prostate cancer upgrading by systematic biopsy over magnetic resonance imaging/ transrectal ultrasound fusion biopsy. J Urol 2017; 197: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey B, Fotin SV, Huang RJ et al. : Fully automated prostate segmentation on MRI: comparison with manual segmentation methods and specimen volumes. AJR Am J Roentgenol 2013; 201: W720. [DOI] [PubMed] [Google Scholar]
- 17.Linder BJ, Frank I, Umbreit EC et al. : Standard and saturation transrectal prostate biopsy techniques are equally accurate among prostate cancer active surveillance candidates. Int J Urol 2013; 20: 860. [DOI] [PubMed] [Google Scholar]
- 18.Jones JS, Patel A, Schoenfield L et al. : Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol 2006; 175: 485. [DOI] [PubMed] [Google Scholar]
- 19.Djavan B, Remzi M and Marberger M: When to biopsy and when to stop biopsying. Urol Clin North Am 2003; 30: 253. [DOI] [PubMed] [Google Scholar]
- 20.Rouviere O, Souchon R, Salomir R et al. : Transrectal high-intensity focused ultrasound ablation of prostate cancer: effective treatment requiring accurate imaging. Eur J Radiol 2007; 63: 317. [DOI] [PubMed] [Google Scholar]
- 21.Gangi A, Tsoumakidou G, Abdelli O et al. : Percutaneous MR-guided cryoablation of prostate cancer: initial experience. Eur Radiol 2012; 22: 1829. [DOI] [PubMed] [Google Scholar]
- 22.Jones JS: Focal or subtotal therapy for early stage prostate cancer. Curr Treat Options Oncol 2007; 8: 165. [DOI] [PubMed] [Google Scholar]
- 23.Ruijter ET, van de Kaa CA, Schalken JA et al. : Histological grade heterogeneity in multifocal prostate cancer. Biological and clinical implications. J Pathol 1996; 180: 295. [DOI] [PubMed] [Google Scholar]
- 24.Porpiglia F, De Luca S, Passera R et al. : Multiparametric magnetic resonance/ultrasound fusion prostate biopsy: number and spatial distribution of cores for better index tumor detection and characterization. J Urol 2017; 198: 58. [DOI] [PubMed] [Google Scholar]
- 25.Mesko S, Marks L, Ragab O et al. : Targeted prostate biopsy Gleason score heterogeneity and implications for risk stratification. Am J Clin Oncol 2016; doi: 10.1097/COC.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukagai T, Namiki T, Namiki H et al. : Discrepancies between Gleason scores of needle biopsy and radical prostatectomy specimens. Pathol Int 2001; 51: 364. [DOI] [PubMed] [Google Scholar]
- 27.Corcoran NM, Hovens CM, Hong MK et al. : Underestimation of Gleason score at prostate biopsy reflects sampling error in lower volume tumours. BJU Int 2012; 109: 660. [DOI] [PubMed] [Google Scholar]
- 28.Le JD, Stephenson S, Brugger M et al. : Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. J Urol 2014; 192: 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]


