Abstract
Purpose:
We examined the additional value of preoperative prostate multiparametric magnetic resonance imaging and transrectal ultrasound/multiparametric magnetic resonance imaging fusion guided targeted biopsy when performed in combination with clinical nomograms to predict adverse pathology at radical prostatectomy.
Materials and Methods:
We identified all patients who underwent 3 Tesla multiparametric magnetic resonance imaging prior to fusion biopsy and radical prostatectomy. The Partin and the MSKCC (Memorial Sloan Kettering Cancer Center) preradical prostatectomy nomograms were applied to estimate the probability of organ confined disease, extraprostatic extension, seminal vesicle invasion and lymph node involvement using transrectal ultrasound guided systematic biopsy and transrectal ultrasound/multiparametric magnetic resonance imaging fusion guided targeted biopsy Gleason scores. With radical prostatectomy pathology as the gold standard we developed multivariable logistic regression models based on these nomograms before and after adding multiparametric magnetic resonance imaging to assess any additional predictive ability.
Results:
A total of 532 patients were included in study. When multiparametric magnetic resonance imaging findings were added to the systematic biopsy based MSKCC nomogram, the AUC increased by 0.10 for organ confined disease (p <0.001), 0.10 for extraprostatic extension (p = 0.003), 0.09 for seminal vesicle invasion (p = 0.011) and 0.06 for lymph node involvement (p = 0.120). Using Gleason scores derived from targeted biopsy compared to systematic biopsy provided an additional predictive value of organ confined disease (Δ AUC 0.07, p = 0.003) and extraprostatic extension (Δ AUC 0.07, p = 0.048) at radical prostatectomy with the MSKCC nomogram. Similar results were obtained using the Partin nomogram.
Conclusions:
Magnetic resonance imaging alone or in addition to standard clinical nomograms provides significant additional predictive ability of adverse pathology at the time of radical prostatectomy. This information can be greatly beneficial to urologists for preoperative planning and for counseling patients regarding the risks of future therapy.
Keywords: prostatic neoplasms, prostatectomy, image-guided biopsy, risk assessment, nomograms
Accurate PCa preoperative staging is essential for patient counseling as well as treatment planning. For example, in cases of suspected EPE or SVI external beam radiation therapy may be preferred over RP or brachytherapy due to the risks of incomplete resection or under dosing, respectively.1 Preoperative knowledge of EPE may also inform surgical management, potentially allowing for modified surgical techniques such as a wider resection margin or even nerve resection to maximize oncologic efficacy and minimize the risk of positive surgical margins.2
Prediction models that combine clinical stage, serum PSA levels and Gleason grade in the biopsy specimens are commonly used in clinical practice to predict the pathological stage of PCa and, thus, aid in preoperative decision making. The Partin nomogram3 and the MSKCC preradical prostatectomy nomogram4 are examples of validated predictive tools that are widely used for patient counseling.
Multiparametric MRI offers multiple advantages that can be incorporated into existing nomograms, including imaging data on multiple adverse features and the ability to perform Tbx to detect more clinically significant cancer. There is a clear benefit of mpMRI and Tbx for diagnosing clinically significant disease over that of Sbx alone. Siddiqui et al found that Tbx can detect up to 30% more high grade disease and less indolent cancer compared with Sbx.5 Ahmed et al found that mpMRI had greater sensitivity than standard TRUS (up to 92% vs up to 60%) in the ability to rule out clinically significant disease.6 However, it is uncertain whether the upgrading that occurs due to mpMRI guided biopsies helps predict adverse pathology at surgery.
Due to the high costs of MRI and the high prevalence of PCa mpMRI poses a significant financial burden on society.7–10 As a result there is still debate regarding the role of MRI for the preoperative evaluation of PCa. Guidelines from the AUA (American Urological Association),11 the EAU (European Association of Urology)12 and the NCCN® (National Comprehensive Cancer Network®)13 suggest a role for MRI, especially in the setting of high risk disease, but they do not provide definite indications for application.
Many clinically used nomograms have been validated using standard TRUS guided biopsies. However, studies of the additional value of prostate mpMRI and Tbx to validated clinical nomograms are scarce.14–17 In this study we examined the additional value of prostate mpMRI data and Tbx pathology data to the MSKCC4 and Partin3 nomograms to predict adverse pathological features on final pathology. In addition, due to the increased performance of Tbx in clinical practice we sought to determine whether using pathology data from Tbx compared to Sbx would provide additional benefit to predict these adverse pathological features on RP in these nomograms.
MATERIALS AND METHODS
Patient Selection
All patients were enrolled in an institutional review board approved protocol and provided informed consent. A prospectively maintained database was queried for all patients who underwent mpMRI, Sbx and/or Tbx and RP from May 2007 to September 2017. We collected baseline demographics, including age, PSA, digital rectal examination, biopsy and mpMRI findings, including the NIH suspicion score, EPE, SVI and the largest lesion diameter.
PI-RADS™ v2 has been in use at our institution since May 2015 along with the previously validated NIH suspicion score.18 However, in the current study we did not use PI-RADS v2 data since patients who underwent mpMRI prior to May 2015 did not undergo prospective mpMRI interpretation based on PI-RADS v2. Patients with insufficient mpMRI or biopsy data were excluded from study. Any patients with prior radiation or hormonal therapy were also excluded.
Staging Nomograms
The likelihood of OCD, EPE, SVI and LNI according to the 2010 Partin nomogram3 and the MSKCC preradical prostatectomy nomogram4 were recorded based on pretreatment characteristics, including PSA level, Gleason Grade Group and clinical stage. Sbx as well as Tbx results were used with each of these nomograms.
Imaging and Biopsy Protocol
Diagnostic mpMRI of the prostate was performed with a 3 Tesla Achieva scanner (Philips, Cleveland, Ohio) using a Medrad® BPX-30 endorectal coil and a 16-channel SENSE cardiac surface coil (Philips) as previously described.19 Prostate mpMRIs were evaluated prospectively by 2 radiologists with extensive prostate MRI experience. In most cases mpMRI incorporated triplanar T2-weighted, diffusion weighted, dynamic contrast-enhanced and magnetic resonance spectroscopy sequences. These sequences were combined to produce a 3-point NIH PCa suspicion score of 1—low, 2—moderate or 3—high as previously validated.18
Patients then underwent an outpatient prostate biopsy session, which included Tbx of all MRI suspicious lesions with a minimum of 2 cores sampled in the axial and sagittal planes using an end fire TRUS probe.20 Additionally, all patients underwent Sbx at the same time. All biopsy and RP pathology findings were reviewed by a single genitourinary pathologist. Robot-assisted RP was performed by a single urologist.
Statistical Analysis
Multivariable logistic regression was done to estimate the association of clinical variables and mpMRI findings with the prediction of adverse pathological features. Using the Sbx and Tbx results we calculated the Partin3 and MSKCC4 nomogram estimates of OCD, EPE, SVI and LNI, and incorporated these estimates into multivariable modeling to create a ROC curve. Multiparametric MRI results were then added to each model and the multivariable modeling was reassessed.
The AUC values before and after adding mpMRI results to each model were compared by the Delong method. Nomogram predictions using Sbx and Tbx results were also compared. Statistical significance was considered at p <0.05. All analyses were calculated with Stata/IC™ 13.
RESULTS
Of the 552 patients who underwent RP 20 were excluded from study due to insufficient data or prior PCa treatment. All remaining 532 patients underwent preoperative mpMRI prior to RP and were included in study. Of these patients 327 underwent Tbx plus Sbx and the remaining 205 underwent only Sbx. On the final pathology evaluation EPE, SVI and LNI were found in 115 (21.6%), 37 (7.0%) and 31 patients (6.2%), respectively.
Table 1 summarizes the distribution of preoperative clinical variables. Median age in this group was 61 years (IQR 56–66) and median PSA was 6.2 ng/ml (IQR 4.3–9.9). Of the patients 101 (19.0%) had suspected EPE, 24 (4.5%) had suspected SVI and none had suspected LNI on mpMRI. In the overall cohort the mean ± SD largest prostate lesion diameter was 1.6 ± 0.7 cm on mpMRI.
Table 1.
Preoperative clinical variables
| Median age (IQR) | 61 | (56–66) |
| No. clinical stage (%):* | ||
| cT1 | 483 | (90.8) |
| cT2 | 49 | (9.2) |
| Median ng/ml PSA (IQR) | 6.2 (4.3–9.9) | |
| No. systematic biopsy Grade Group (%): | ||
| 1 | 190 | (36.0) |
| 2 | 201 | (37.8) |
| 3 | 46 | (8.6) |
| 4 | 73 | (13.8) |
| 5 | 22 | (3.8) |
| No. targeted biopsy Grade Group (%): | ||
| 1 | 82 | (25.1) |
| 2 | 112 | (34.3) |
| 3 | 41 | (12.5) |
| 4 | 76 | (23.2) |
| 5 | 16 | (4.9) |
| mpMRI findings:† | ||
| No. low NIH suspicion score (%) | 52 | (9.8) |
| No. intermediate NIH suspicion score (%) | 254 | (47.7) |
| No. high NIH suspicion score (%) | 226 | (42.5) |
| No. suspected extraprostatic extension (%) | 101 | (19.0) |
| No. suspected seminal vesicle invasion (%) | 24 | (4.5) |
| Mean ± SD largest lesion diameter (cm) | 1.6 ± 0.7 | |
No cT3 disease.
No suspected lymph node invasion.
The mpMRI findings were then incorporated into the MSKCC4 and Partin3 nomograms with Sbx results using multivariable logistic regression (supplementary table, http://jurology.com/). These models incorporated mpMRI findings of the NIH suspicion score, EPE, SVI and the largest lesion diameter. A smaller lesion diameter on mpMRI was the strongest predictor of OCD in the Sbx Partin plus mpMRI model (OR 0.64, p = 0.046) and in the Sbx MSKCC plus mpMRI model (OR 0.63, p = 0.039).
When looking at nonorgan confined disease, mpMRI findings yielded more predictive ability than either of the 2 nomograms alone. The largest lesion diameter on mpMRI was the strongest predictor of EPE at RP in the Sbx Partin3 plus mpMRI model and the Sbx MSKCC4 plus mpMRI model (OR 2.33, p <0.001 and OR 2.07, p = 0.001, respectively). The largest lesion diameter on mpMRI was also the strongest predictor of LNI at RP in the Sbx Partin plus mpMRI model and the Sbx MSKCC plus mpMRI model (OR 2.10, p = 0.017 and OR 2.09, p = 0.013, respectively). SVI on mpMRI was the strongest predictor of SVI on RP in the Sbx Partin plus mpMRI model and in the Sbx MSKCC plus mpMRI model (OR 28.10, p <0.001 and OR 35.86, p <0.001, respectively).
Table 2 shows a comparison of MSKCC nomogram4 AUCs before and after adding mpMRI results. The combined mpMRI and Sbx MSKCC model outperformed the Sbx MSKCC nomogram alone. When comparing the Sbx MSKCC and the Sbx MSKCC plus mpMRI predictive models, the AUC was 0.70 vs 0.80 (p <0.001) for OCD, 0.70 vs 0.80 (p = 0.003) for EPE, 0.81 vs 0.90 (p = 0.011) for SVI and 0.82 vs 0.88 (p = 0.120) for LNI. Similarly the combined mpMRI and Tbx MSKCC model outperformed the Tbx MSKCC nomogram alone. When comparing the Tbx MSKCC and Tbx MSKCC plus mpMRI predictive models, the AUC was 0.77 vs 0.82 (p = 0.046) for OCD, 0.77 vs 0.82 (p = 0.045) for EPE, 0.76 vs 0.85 (p = 0.065) for SVI and 0.88 vs 0.94 (p = 0.023) for LNI.
Table 2.
| Nomogram | mpMRI Model AUC (95% CI) | Systematic TRUS Biopsy |
Targeted TRUS Biopsy |
||||
|---|---|---|---|---|---|---|---|
| Nomogram AUC (95% CI) | Nomogram + mpMRI AUC (95% CI) | p Value | Nomogram AUC (95% CI) | Nomogram + mpMRI AUC (95% CI) | p Value | ||
| MSKCC: | |||||||
| Organ confined disease | 0.78 (0.72e0.83) | 0.70 (0.64e0.76) | 0.80 (0.75e0.85) | <0.001* | 0.77 (0.73e0.84) | 0.82 (0.76e0.87) | 0.046* |
| Extraprostatic extension | 0.78 (0.71e0.82) | 0.70 (0.63e0.77) | 0.80 (0.74e0.85) | 0.003* | 0.77 (0.73e0.83) | 0.82 (0.75e0.87) | 0.045* |
| Seminal vesicle invasion | 0.86 (0.75e0.92) | 0.81 (0.71e0.88) | 0.90 (0.83e0.96) | 0.011* | 0.76 (0.67e0.86) | 0.85 (0.72e0.93) | 0.065 |
| Lymph node invasion | 0.87 (0.77e0.92) | 0.82 (0.70e0.89) | 0.88 (0.83e0.94) | 0.120 | 0.88 (0.84e0.95) | 0.94 (0.91e0.97) | 0.023* |
| Partin: | |||||||
| Organ confined disease | 0.78 (0.72e0.83) | 0.73 (0.67e0.78) | 0.81 (0.75e0.86) | 0.001* | 0.78 (0.74e0.84) | 0.83 (0.76e0.88) | 0.030* |
| Extraprostatic extension | 0.78 (0.71e0.82) | 0.66 (0.59e0.71) | 0.80 (0.73e0.83) | <0.001* | 0.67 (0.61e0.75) | 0.79 (0.73e0.85) | 0.003* |
| Seminal vesicle invasion | 0.86 (0.75e0.92) | 0.80 (0.71e0.89) | 0.88 (0.82e0.96) | 0.022* | 0.83 (0.69e0.88) | 0.87 (0.77e0.96) | 0.452 |
| Lymph node invasion | 0.87 (0.77e0.92) | 0.85 (0.68e0.88) | 0.91 (0.85e0.96) | 0.0710 | 0.89 (0.84e0.97) | 0.93 (0.86e0.97) | 0.085 |
Significant at p <0.05.
A similar analysis was done to compare the AUCs of the Partin nomogram3 before and after adding mpMRI results (table 2). The combined mpMRI and Sbx Partin model outperformed the Sbx Partin nomogram alone. When comparing the Sbx Partin and Sbx Partin plus mpMRI predictive models, the AUC was 0.73 vs 0.81 (p = 0.001) for OCD, 0.66 vs 0.80 (p <0.001) for EPE, 0.80 vs 0.88 (p = 0.022) for SVI and 0.85 vs 0.91 (p = 0.071) for LNI. Similarly the combined mpMRI and Tbx Partin model outperformed the Tbx Partin nomogram alone. When comparing the Tbx Partin and Tbx Partin plus mpMRI predictive models, the AUC was 0.78 vs 0.83 (p = 0.030) for OCD, 0.67 vs 0.79 (p = 0.003) for EPE, 0.83 vs 0.87 (p = 0.452) for SVI and 0.89 vs 0.93 (p = 0.085) for LNI.
The figure shows a comparison of the Sbx and Tbx MSKCC4 and Partin3 nomogram ROC curves before and after adding mpMRI for the overall prediction of nonorgan confined disease, including EPE, SVI or LNI.
Figure.
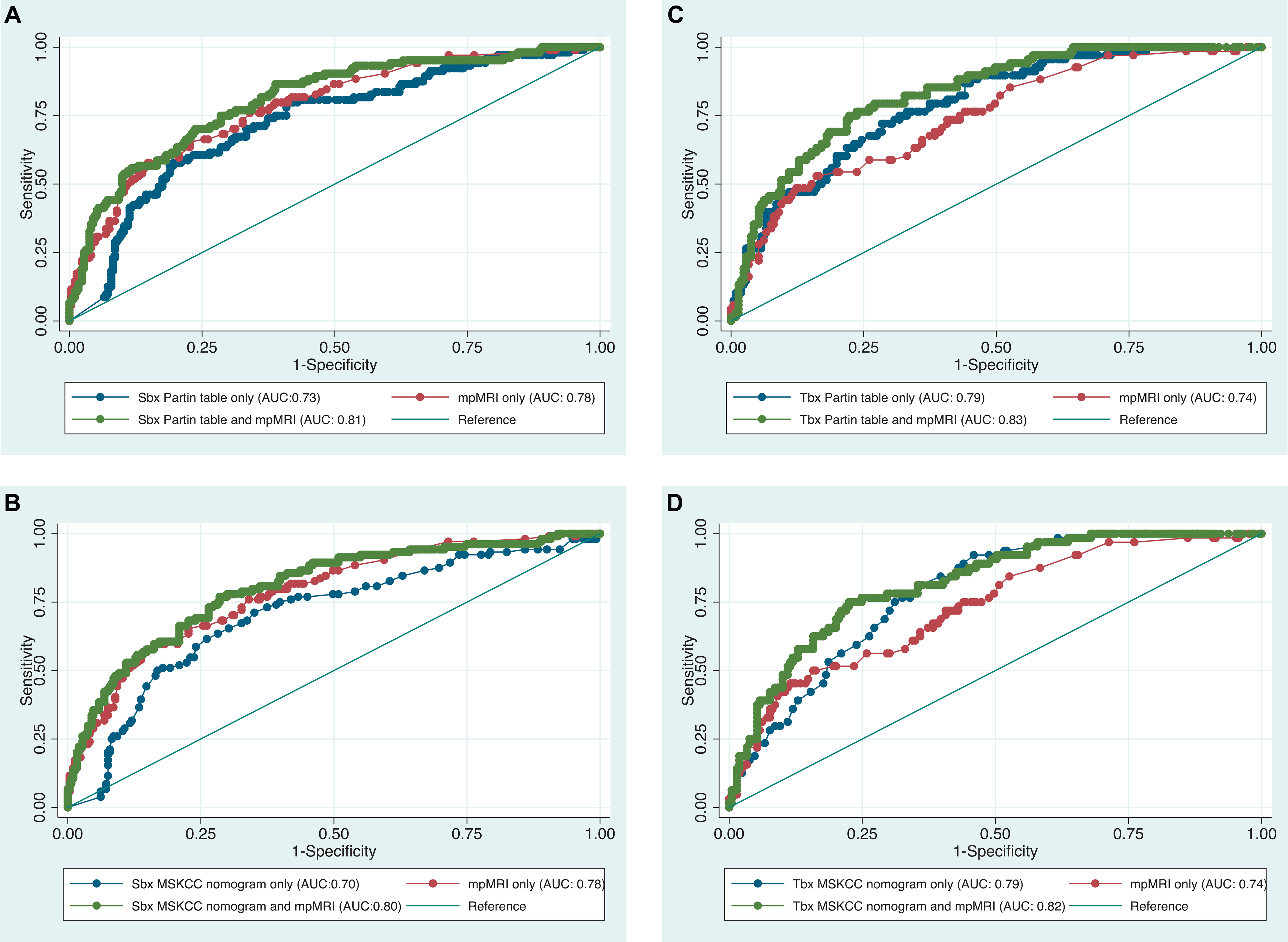
ROC curves of Partin3 and MSKCC4 nomograms by biopsy type with and without adding mpMRI results to predict nonorgan confined disease. A, SBx and Partin nomogram. B, Sbx and MSKCC nomogram. C, Tbx and Partin nomogram. D, Tbx and MSKCC nomogram.
Finally, the Tbx and Sbx nomograms were compared by ROC analysis. When comparing the Tbx MSKCC and Sbx MSKCC nomograms,4 the AUC was 0.77 vs 0.70 (p = 0.003) for OCD, 0.77 vs 0.70 (p = 0.048) for EPE, 0.76 vs 0.81 (p = 0.820) for SVI and 0.88 vs 0.82 (p = 0.174) for LNI. When comparing the Tbx Partin and Sbx Partin nomograms,3 the AUC was 0.78 vs 0.73 (p=0.009) for OCD, 0.67 vs 0.66 (p = 0.230) for EPE, 0.83 vs 0.80 (p = 0.214) for SVI and 0.89 vs 0.85 (p = 0.374) for LNI.
DISCUSSION
In the setting of Sbx our results demonstrated that mpMRI provides additional value to theMSKCC4 and Partin3 nomograms to predict adverse pathology at RP. Adding mpMRI findings to these staging nomograms significantly improved the prediction of OCD, EPE and SVI on final pathology. A nonsignificant increase in LNI prediction was seen, which may have been due to the relatively low event rate of LNI as well as the low sensitivity of mpMRI for LNI detection.21–23
While it is important that new tests and imaging modalities be assessed in relation to existing tests or nomograms, studies exploring the additional value of mpMRI to these nomograms are scarce and not all in agreement.15,16 In contrast to our series, in a retrospective study Morlacco et al compared the predictive accuracy of MRI and clinical models, including the Partin nomogram3 and the CAPRA (Cancer of the Prostate Risk Assessment) score, for PCa staging and found significantly improved accuracy after adding MRI to the models.15 One of the main differences between the 2 studies is that in the study by Morlacco et al the overall cohort had more advanced disease with a 42.3%, 30.7% and 16.0% rate of EPE, SVI and LNI compared to 21.6%, 7.0% and 6.2%, respectively, in our study. We found rates of EPE, SVI and LNI similar to those in previously published studies.2,17,24,25 In the study by Morlacco et al the greater ratio of patients with adverse pathology findings may have been due to mpMRI ordered based on higher clinical suspicion of adverse pathology rather than to a standardized protocol in every patient.15
The additional value obtained by adding mpMRI results to the Tbx based nomograms was statistically significant for predicting OCD, EPE and LNI using the MSKCC nomogram4 and for OCD and EPE prediction using the Partin nomogram.3 However, the additional value obtained by adding mpMRI results to the Tbx based nomograms was generally not as pronounced as when using the combined mpMRI Sbx nomogram models. The higher Gleason score in targeted biopsies may have been due to incorporating the higher risk into the nomograms, resulting in less significant improvements in the value of adding further mpMRI data to preoperative predictions.
In a recent study of 236 patients Weaver et al found that prostate MRI added no additional value to the MSKCC nomogram4 for PCa staging.16 It is not clear from that study whether Tbx or Sbx results were used for risk prediction with the MSKCC nomogram, which we observed was an important distinction. Other differences in the 2 studies include the involvement of 9 radiologists for reading mpMRI in the study by Weaver et al16 compared to only 2 radiologists in our series. The increased number of radiologists likely negatively impacted overall MRI accuracy as prior research has shown substantial interobserver variability in prostate MRI interpretation.26,27 Additionally, the Weaver et al cohort had a greater rate of advanced disease than expected based on previous studies with a 35%, 14% and 8% rate of EPE, SVI and LNI, respectively.16
Finally, using pathology derived from Tbx compared to Sbx provided significant additional value for predicting OCD on RP when applying the Partin3 and MSKCC4 nomograms, and for predicting EPE when applying MSKCC nomogram only. The increased predictive ability of EPE when using Tbx compared to Sbx in the MSKCC but not the Partin3 nomogram may have been due to the fact that the MSKCC nomogram incorporates additional variables, including the percent of positive cores on biopsy as a surrogate for tumor volume, which would be improved on Tbx, while the Partin nomogram does not incorporate additional variables.5,28 This suggests a role for updated versions of commonly used clinical nomograms in the era of mpMRI and Tbx.
Our study has several limitations, including the fact that it was a single center study. Additionally, PI-RADS v2 was not used to assess mpMRI uniformly since this study spanned the introduction of PI-RADS v2. Instead, a validated 3-point system was used which was shown to behave similarly to PI-RADS v2.29 Our study also had a small sample size compared to the original Partin3 and MSKCC4 nomograms, which included more than 1,000 patients each. However, with 532 patients we report one of the largest studies of the additional value of mpMRI to clinical staging nomograms.
CONCLUSIONS
Multiparametric MRI alone or in addition to existing validated risk stratification tools, including the Partin3 and the MSKCC4 nomograms, provides significant additional predictive ability for adverse pathological features at the time of RP. Updated versions of commonly used clinical nomograms reflecting mpMRI and Tbx results may be necessary to better reflect preoperative risk stratification. By using this information urologists can better counsel patients regarding this outcome after surgery and the potential need for any future therapies.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of NIH (National Institutes of Health), National Cancer Institute, Center for Cancer Research and the Center for Interventional Oncology, and the NIH Medical Research Scholars Program, supported by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, alumni of student research programs and other individual supporters via contributions to the Foundation for the NIH.
The NIH and Philips Healthcare have a cooperative research and development agreement, and share intellectual property in the field.
Abbreviations and Acronyms
- EPE
extraprostatic extension
- LNI
lymph node involvement
- mpMRI
multiparametric MRI
- MRI
magnetic resonance imaging
- MSKCC
Memorial Sloan Kettering Cancer Center
- NIH
National Institutes of Health
- OCD
organ confined disease
- PCa
prostate cancer
- PI-RADS™
Prostate Imaging Reporting and Data System
- PSA
prostate specific antigen
- RP
radical prostatectomy
- Sbx
systematic TRUS guided biopsy
- SVI
seminal vesicle invasion
- Tbx
TRUS/mpMRI fusion guided targeted biopsy
- TRUS
transrectal ultrasound
- v2
version 2
Footnotes
Financial interest and/or other relationship with Philips InVivo and National Institutes of Health Cooperative Research and Development Agreement.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
Contributor Information
Kareem N. Rayn, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Jonathan B. Bloom, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Samuel A. Gold, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Graham R. Hale, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Joseph A. Baiocco, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Sherif Mehralivand, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland; Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland; Department of Urology and Pediatric Urology, University Medical Center Mainz, Johannes Gutenberg University Mainz, Mainz, Germany.
Marcin Czarniecki, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Vikram K. Sabarwal, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Vladimir Valera, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Bradford J. Wood, Center for Interventional Oncology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Maria J. Merino, Laboratory of Pathology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Peter Choyke, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Baris Turkbey, Molecular Imaging Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Peter A. Pinto, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland; Center for Interventional Oncology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Boehmer D, Maingon P, Poortmans P et al. : Guidelines for primary radiotherapy of patients with prostate cancer. Radiother Oncol 2006; 79: 259. [DOI] [PubMed] [Google Scholar]
- 2.Preisser F, Marchioni M, Nazzani S et al. : The impact of lymph node metastases burden at radical prostatectomy. Eur Urol Focus 2018; doi: 10.1016/j.euf.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Isharwal S, Haese A et al. : Prediction of patient-specific risk and percentile cohort risk of pathological stage outcome using continuous prostate-specific antigen measurement, clinical stage and biopsy Gleason score. BJU Int 2011; 107: 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Memorial Sloan Kettering Cancer Center: Prediction Tools/Prostate Cancer Nomograms: Pre-Radical Prostatectomy. Available at https://www.mskcc.org/nomograms/prostate/pre_op. Accessed May 8, 2018.
- 5.Siddiqui MM, Rais-Bahrami S, Turkbey B et al. : Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed HU, El-Shater Bosaily A, Brown LC et al. : Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815. [DOI] [PubMed] [Google Scholar]
- 7.Woo S, Cho JY, Kim SY et al. : Extracapsular extension in prostate cancer: added value of diffusion-weighted MRI in patients with equivocal findings on T2-weighted imaging. AJR Am J Roentgenol 2015; 204: W168. [DOI] [PubMed] [Google Scholar]
- 8.Gupta RT, Faridi KF, Singh AA et al. : Comparing 3-T multiparametric MRI and the Partin tables to predict organ-confined prostate cancer after radical prostatectomy. Urol Oncol 2014; 32: 1292. [DOI] [PubMed] [Google Scholar]
- 9.Penson DF, Litwin MS and Aaronson NK: Health related quality of life in men with prostate cancer. J Urol 2003; 169: 1653. [DOI] [PubMed] [Google Scholar]
- 10.Quintana L, Ward A, Gerrin SJ et al. : Gleason misclassification rate is independent of number of biopsy cores in systematic biopsy. Urology 2016; 91: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanda MG, Cadeddu JA, Kirkby E et al. : Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specifc care options. J Urol 2018; 199: 990. [DOI] [PubMed] [Google Scholar]
- 12.Santis D, Henry A, Joniau S et al. : EAU 2017. Guidelines on Prostate Cancer. Patient Represent. Available at https://uroweb.org/wpcontent/uploads/09-Prostate-Cancer_2017_web.pdf. Accessed January 17, 2018.
- 13.National Comprehensive Cancer Network®: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 17, 2018.
- 14.Wang L, Hricak H, Kattan MW et al. : Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology 2006; 238: 597. [DOI] [PubMed] [Google Scholar]
- 15.Morlacco A, Sharma V, Viers BR et al. : The incremental role of magnetic resonance imaging for prostate cancer staging before radical prostatectomy. Eur Urol 2017; 71: 701. [DOI] [PubMed] [Google Scholar]
- 16.Weaver JK, Kim EH, Vetter JM et al. : Prostate magnetic resonance imaging provides limited incremental value over the Memorial Sloan Kettering Cancer Center pre-radical prostatectomy nomogram. Urology 2018; 113: 119. [DOI] [PubMed] [Google Scholar]
- 17.Grivas N, Hinnen K, de Jong J et al. : Seminal vesicle invasion on multi-parametric magnetic resonance imaging: Correlation with histopathology. Eur J Radiol 2018; 98: 107. [DOI] [PubMed] [Google Scholar]
- 18.Rais-Bahrami S, Siddiqui MM, Turkbey B et al. : Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013; 190: 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkbey B, Pinto PA, Mani H et al. : Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology 2010; 255: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong CW, Rais-Bahrami S, Walton-Diaz A et al. : Comparison of magnetic resonance imaging and ultrasound (MRI-US) fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU Int 2015; 115: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taupitz M: Imaging of lymph nodes—MRI and CT. In: MRI and CT of the Female Pelvis. Edited by Hamm B and Forstner R. Berlin: Springer; 2007; pp 321–329. [Google Scholar]
- 22.Roy C, Le Bras Y, Mangold L et al. : Small pelvic lymph node metastases: evaluation with MR imaging. Clin Radiol 1997; 52: 437. [DOI] [PubMed] [Google Scholar]
- 23.Tiguert R, Gheiler EL, Tefilli MV et al. : Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology 1999; 53: 367. [DOI] [PubMed] [Google Scholar]
- 24.Pak S, Park S, Ryu J et al. : Preoperative factors predictive of posterolateral extracapsular extension after radical prostatectomy. Korean J Urol 2013; 54: 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephenson AJ, Scardino PT, Eastham JA et al. : Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006; 98: 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruprecht O, Weisser P, Bodelle B et al. : MRI of the prostate: interobserver agreement compared with histopathologic outcome after radical prostatectomy. Eur J Radiol 2012; 81: 456. [DOI] [PubMed] [Google Scholar]
- 27.Mullerad M, Hricak H, Wang L et al. : Prostate cancer: detection of extracapsular extension by genitourinary and general body radiologists at MR imaging. Radiology 2004; 232: 140. [DOI] [PubMed] [Google Scholar]
- 28.Schoots IG, Roobol MJ, Nieboer D et al. : Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015; 68: 438. [DOI] [PubMed] [Google Scholar]
- 29.Gaur S, Harmon S, Mehralivand S et al. : Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging 2018; doi: 10.1002/jmri.26025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


