Abstract
Purpose
The purpose of our study was to assess 18F–DCFBC PET/CT, a PSMA targeted PETagent, for lesion detection and clinical management of biochemical relapse in prostate cancer patients after primary treatment.
Methods
This is a prospective IRB-approved study of 68 patients with documented biochemical recurrence after primary local therapy consisting of radical prostatectomy (n = 50), post radiation therapy (n = 9) or both (n = 9), with negative conventional imaging. All 68 patients underwent whole-body 18F–DCFBC PET/CT, and 62 also underwent mpMRI within one month. Lesion detection with 18F–DCFBC was correlated with mpMRI findings and pre-scan PSA levels. The impact of 18F–DCFBC PET/CT on clinical management and treatment decisions was established after 6 months’ patient clinical follow-up.
Results
Forty-one patients (60.3%) showed at least one positive 18F–DCFBC lesion, for a total of 79 lesions, 30 in the prostate bed, 39 in lymph nodes, and ten in distant sites. Tumor recurrence was confirmed by either biopsy (13/41 pts), serial CT/MRI (8/41) or clinical follow-up (15/41); there was no confirmation in five patients, who continue to be observed. The 18F–DCFBC and mpMRI findings were concordant in 39 lesions (49.4%), and discordant in 40 lesions (50.6%); the majority (n = 32/40) of the latter occurring because the recurrence was located outside the mpMRI field of view. 18F–DCFBC PET positivity rates correlated with PSA values and 15%, 46%, 83%, and 77% were seen in patients with PSA values <0.5, 0.5 to <1.0, 1.0 to <2.0, and ≥2.0 ng/ mL, respectively. The optimal cut-off PSA value to predict a positive 18F–DCFBC scan was 0.78 ng/mL (AUC = 0.764). A change in clinical management occurred in 51.2% (21/41) of patients with a positive 18F–DCFBC result, generally characterized by starting a new treatment in 19 patients or changing the treatment plan in two patients.
Conclusions
18F–DCFBC detects recurrences in 60.3% of a population of patients with biochemical recurrence, but results are dependent on PSA levels. Above a threshold PSA value of 0.78 ng/mL, 18F–DCFBC was able to identify recurrence with high reliability. Positive 18F–DCFBC PET imaging led clinicians to change treatment strategy in 51.2% of patients.
Keywords: 18F-DCFBC, PSMA, Prostate cancer, Biochemical recurrence, PSMA-based PETimaging
Introduction
Following initial diagnosis, most patients with prostate cancer (PCa) are treated with either radical prostatectomy, external beam radiation, brachytherapy or active surveillance [1, 2]. While many patients can be cured with definitive local therapy, between 20 and 50% of patients have biochemical recurrence (BCR) of disease [3, 4], defined as a rising serum prostate-specific antigen (PSA) typically without findings on conventional imaging (computed tomography or bone scan). The PSA test is very sensitive for recurrent prostate cancer and, in the absence of normal prostate tissue, is a significant indicator of prostate cancer recurrence. Left untreated, BCR can progress to distant metastatic disease, and; therefore, early salvage radiation is recommended while the PSA is still low [5]. Therefore, it is important that sites of recurrence be accurately identified and treated at PSAvalues less than 1.0 ng/ml.
In the setting of BCR, standard of care imaging, i.e., computed tomography (CT), bone scintigraphy, or magnetic resonance imaging (MRI) are insensitive and nonspecific for localizing recurrent cancer [6]. Thus, serum PSA can reach high levels by the time conventional imaging becomes positive [7, 8]. These limitations have stimulated the development of new molecular imaging probes. Among these is a small radioligand targeting prostate-specific membrane antigen (PSMA), a transmembrane protein overexpressed in more aggressive prostate cancers [9]. To date, a variety of PSMA-targeted imaging probes have been developed which demonstrate improved sensitivity and specificity for prostate cancer detection in patients with BCR and metastatic PCa [10–12]. Most experience has been reported for 68Ga-labeled probes, but there is growing experience with 18F–labeled PSMA probes. The latter have the advantage of longer half-life, enabling centralized production in a cyclotron facility as well as more favorable positron energies for imaging. 18F–DCFBC, is a first generation PSMA targeted PET agent, which demonstrates high affinity for prostate cancer [13]. Here we describe our experience with this agent in 68 patients with BCR in order to determine its sensitivity as a function of PSA level and understand how a positive PSMA PET scan affects treatment decisions, especially when used in conjunction with multiparametric MR imaging (mpMRI).
Materials and methods
Patient selection and follow-up
This is a prospective, HIPAA compliant, single institution study, approved by the local institutional review board (IRB) with written informed consent. Inclusion criteria included patients with biochemically recurrent prostatic adenocarcinoma defined as PSA ≥ 0.2 ng/ml after radical prostatectomy, or any documented PSA progression after an initial nadir in postradiation therapy patients. All patients had documented biochemical recurrence, with a mean PSA level of 4.4 ± 7.3 ng/ml (range 0.2 to 37.4 ng/mL); and mean PSA doubling time of 4.8 ± 3.8 months (range 0.5 to 18 months). Conventional imaging modalities, particularly CT and bone scan, were negative in all cases. Exclusion criteria included contraindications to PET/CTscan or MRI, patients with serum creatinine greater than two times the upper limit of normal, total bilirubin greater than two times the upper limit of normal, and/or liver transaminases (ALT, AST) greater than three times the upper limit of normal.
Between July 2014 and November 2016, 68 patients (mean age 64 years, range: 51–74 years) signed the consent form, and underwent 18F–DCFBC PET/CT imaging at 1 h and at 2 h p.i. Prior treatments consisted of radical prostatectomy (n = 50), radiation therapy (n = 9) or a combination of both (n = 9). Of the 68 patients undergoing PET/CT, 62 also underwent mpMRI of the prostate at 3 T within 1 month. Patients were followed for a median of 10.1 ± 6.9 months [range 3–27.2 mo] with serum PSA levels, conventional imaging and biopsy.
Patients were contacted before and after 18F–DCFBC-PET/ CTscan to evaluate for side effects. Electronic medical record reviews and discussions with clinicians were used to establish if a change in patient management occurred as a consequence of the 18F–DCFBC PET/CT scan. We dichotomized patients as to whether they were on treatment or not before the 18F– DCFBC PET/CT scan, and then determined whether patients continued with the same treatment, started a new treatment or continued to be followed without treatment.
Imaging protocol
18F—DCFBC PET/CT imaging protocol
18F–DCFBC PET/CT imaging was performed on a 3-D time of flight (TOF) mode Philips Gemini TF camera (Philips, Cleveland OH), with an 18 cm coronal and a 57 cm axial FOV. Data were reconstructed with a relaxed list mode ordered subset expectation maximization (LMOSEM) TOF-based algorithm [14] using three iterations and 33 subsets. The scanner uses CT based attenuation correction; along with random, normalization, dead time and a model-based scatter correction [14] for anatomical correlation and attenuation correction purposes.
18F–DCFBC was synthesized under good manufacturing practices (GMP) as previously described [13, 15]; additional information is given in Online Resource 1. Each dose underwent quality control testing before injection to ensure the proper dose and specific activity; average radiochemical purity was 96.5% ± 1.9%, with an average specific activity of 2915 ± 1856 mCi/μmole.
Each patient received an IV bolus injection of 18F– DCFBC, mean dose 292.3 MBq [7.9 mCi] (range 255.3– 299.7 MBq [6.9–8.1 mCi]), followed by static whole body PET/CT images at ~60 min and 120 min post-injection (2 min/bed position). Low dose transmission CT scans (120 KV, 60 mAs) were acquired prior to each PET scan for anatomical correlation and co-registration purposes.
Patient’s vital signs were obtained prior to 18F–DCFBC injection, and at 10 and 30 min post-injection and directly after the last PET scan. The patients were queried regarding potential subjective adverse events during scan and immediately after, and the next day via telephone query.
Two board certified nuclear medicine physicians prospectively read all data sets independently, resolving any disagreements by consensus. 18F–DCFBC PET/CT scans were reviewed using a MIM workstation (version 6.5.6, MIM Software Inc., Cleveland, OH). Maximal Intensity Projection (MIP) PET image, as well as axial, coronal, and sagittal PET, CT and PET/CT images were reviewed. Any abnormal focus of 18F–DCFBC uptake higher than the surrounding background and not associated with physiological uptake was considered positive for recurrent prostate cancer, and each was classified as local recurrence, lymph node metastases or distant metastatic sites. The Standard Uptake Value (SUV) was based on the mean value of a volume-of-interest (VOI) defined by 80% threshold of the maximum pixel value that was recorded (SUV80%).
MRI protocol
MRI studies were performed using an endorectal (BPX-30, Medrad, Pittsburgh, PA) and a 16-channel anterior cardiac coil (SENSE, Philips Medical Systems, Best, The Netherlands) on a 3 T magnet (Achieva, Philips Medical Systems, Best, the Netherlands) without prior bowel preparation. The endorectal coil was inserted using a semi-anesthetic gel (Lidocaine, Akorn Inc., Lake Forest, IL) while the patient was in the left lateral decubitus position. The balloon surrounding the coil was distended with perfluorocarbon (Fluorinert FC-770, 3 M, St. Paul, MN) to a volume of approximately 45 mL. The MRI protocol included tri-planar T2 W turbo spin echo (TSE), diffusion weighted (DW) MRI (ADC maps and b2000 DW MRI), axial pre-contrast T1 W, axial 3D T1-weighted fast field echo dynamic contrast-enhanced MRI (DCE MRI) sequences [16].
Statistical methods
Descriptive values were expressed as the mean ± standard deviation (SD). The non-parametric Wilcoxon test was used to compare pre-scan PSA values for patients with positive vs negative 18F–DCFBC PET/CT scans. Receiver-operating-characteristic (ROC) analysis was performed to assess the ability of pre-scan PSA in distinguishing between a 18F– DCFBC PET positive and negative result. True-positive rate (TPR) was defined as the proportion of 18F–DCFBC PET/CT positive results above pre-scan PSA threshold, and false-positive rate (FPR) was defined as the proportion of 18F– DCFBC PET/CT negative results above the pre-scan PSA threshold. Area under the curve (AUC) was estimated and the 95% confidence interval was based on the Delong method [17]. The impact of a positive 18F–DCFBC PET/CT result on the patient’s treatment strategy was assessed. After each 18F– DCFBC PET/CT scan, patients were followed with regards to whether they continued the same treatment, started a new treatment, discontinued treatment, or continued surveillance without treatment. A p-value <0.05 was considered significant. All the statistical and graphical analysis were performed using the R version 3.2.5 software.
Results
All 68 patients completed the 18F–DCFBC PET/CT examination without adverse events. Forty-one patients (60.3%) showed at least one positive lesion, for a total of 79 lesions. Sites of recurrence included the prostate bed/anastomosis (n = 30), lymph nodes (n = 39), and distant sites (n = 10). Prostate bed recurrences occurred after both radical prostatectomy and radiotherapy [Fig. 1] and included six patients with seminal vesical recurrence [Fig. 2].
Fig. 1.
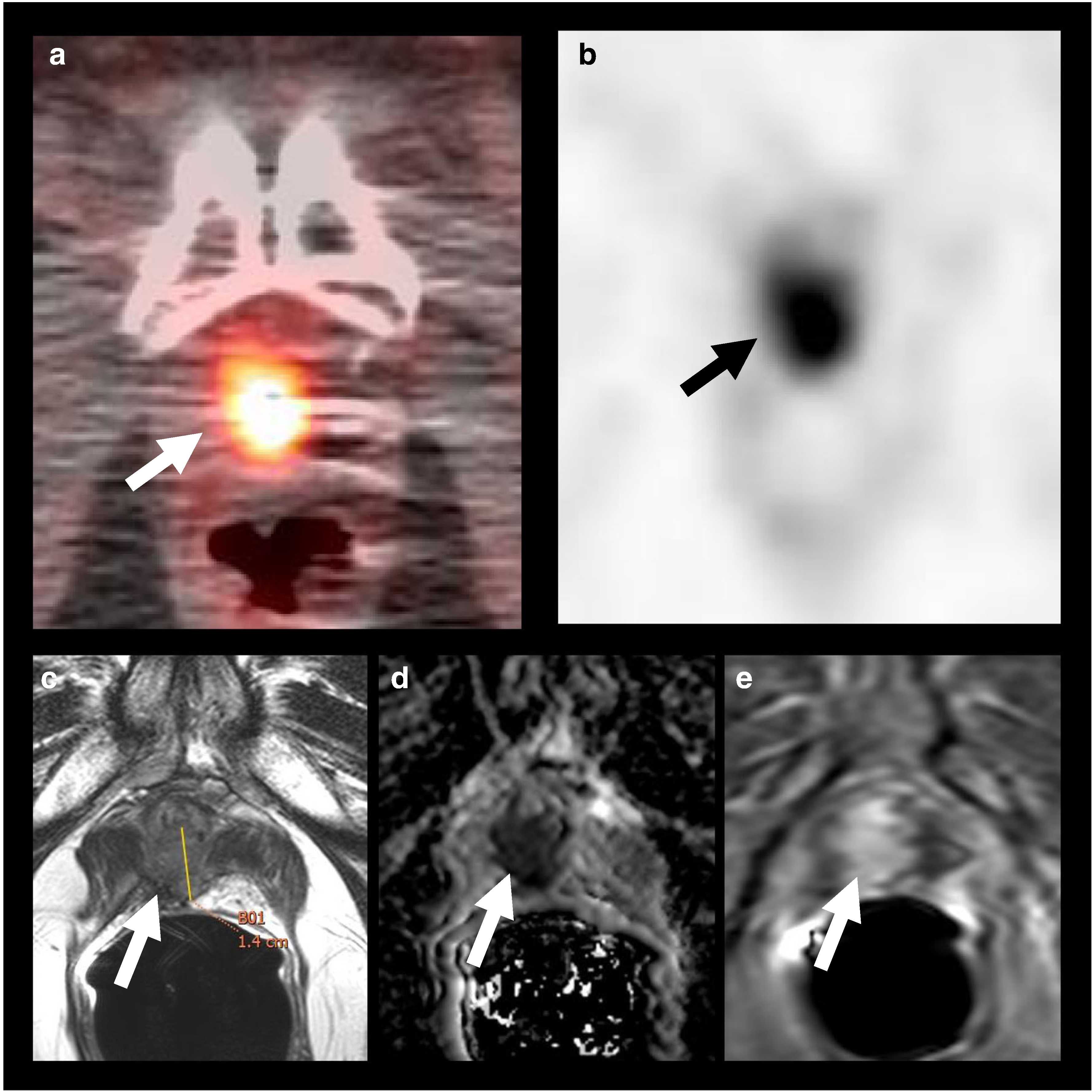
18F–DCFBC PET/CT imaging and mpMRI demonstrating recurrent malignancy at the prostatectomy bed: 71-year-old man, with history of prostate cancer, Gleason 7 (4 + 3), status post-prostatectomy, with pre-scan PSA of 0.86 mg/mL. 18F–DCFBC axial fused PET/CT (a), and PET (b) images demonstrate a focus of abnormal DCFBC uptake at the prostatectomy bed (arrows), which was concordant with MR imaging findings, as seen on T2W MRI (c), ADC map (d) and DCE RMI (e); tumor recurrence was confirmed by biopsy, and patient started hormone therapy
Fig. 2.
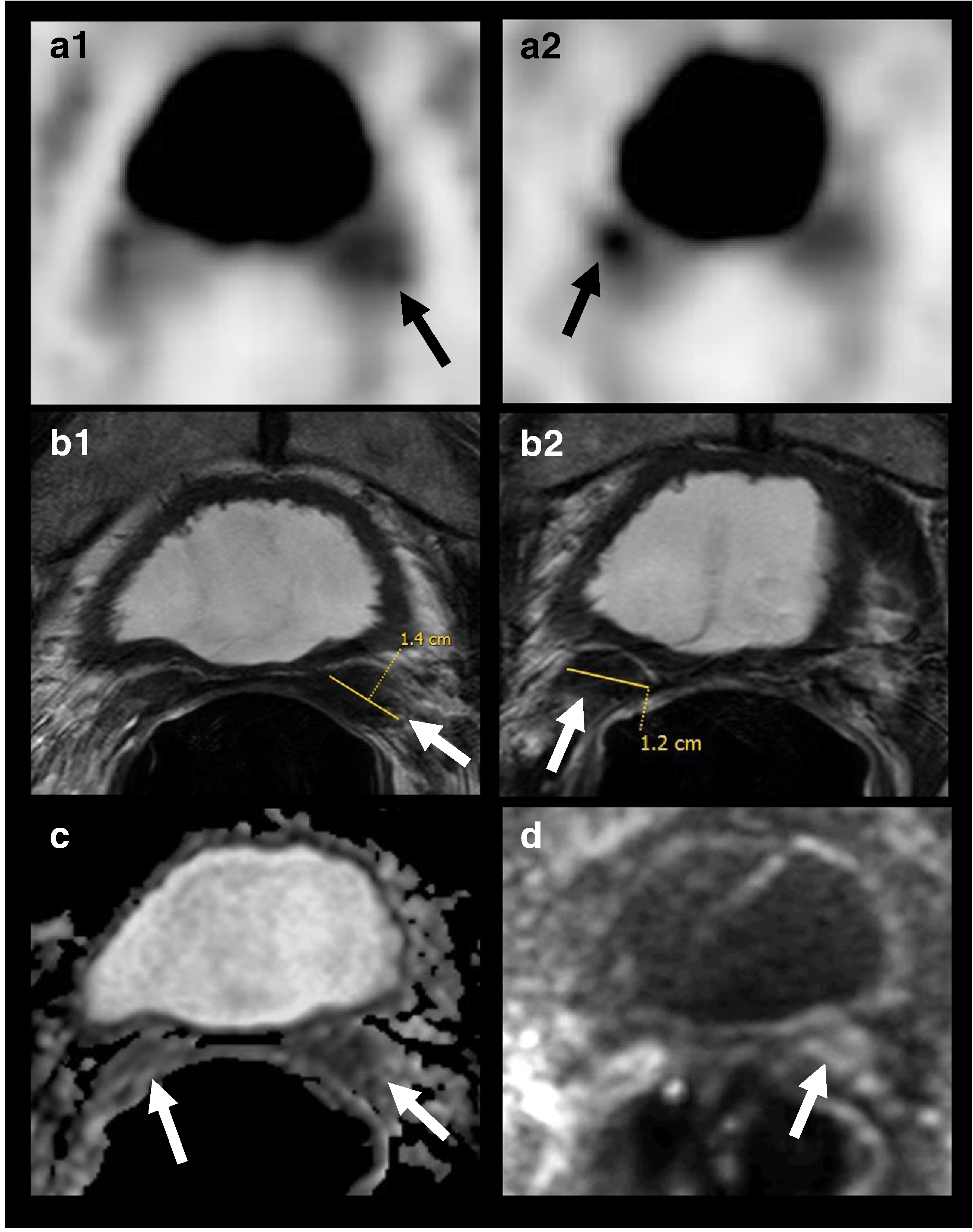
18F–DCFBC PET/CT imaging and mpMRI demonstrating seminal vesicles involvement years after radical prostatectomy: 60-year-old man, with history of prostate cancer, Gleason 7 (4 + 3), status post radical prostatectomy 10 years ago, with pre-scan PSA of 4.7 mg/mL. 18F–DCFBC PET (A1, A2) images demonstrate focal abnormal DCFBC in the bilateral seminal vesicles (arrows), concordant with the MR imaging findings, as seen on T2W MRI (B1, B2), ADC map (C) and b=2000 s/mm2 DW RMI (D); tumor recurrence was confirmed by biopsy, and seminal vesicles surgical resection was performed
Uptake was slightly higher at 2 h p.i. with SUV80% ranging from 2.2 to 12.3, with an average SUV80% of 4.6 ± 2.1 for prostate bed lesions, 5.5 ± 2.9 for lymph nodes and of 3.3 ± 1.3 for distant sites. 18F–DCFBC lesion detection rates were 15% (n = 2/13), 46% (n = 6/13), 83% (n = 10/12), and 77% (n = 23/30) for PSAvalues <0.5, 0.5 to <1.0, 1.0 to <2.0, and ≥2.0 ng/mL, respectively. The mean pre-scan PSA values were significantly higher for patients with positive 18F– DCFBC PET/CT findings, than for patients with negative scan results (6.6 ± 8.89 ng/ml vs 1.22 ± 1.37 ng/mL; Wilcoxon test, p < 0.001). By contrast, there was no significant correlation between PSA doubling time and lesion detection by 18F–DCFBC PET (Wilcoxon test, p = 0.5). We also assessed the benefit of performing 18F–DCFBC PET/CT based on the pre-scan PSA value: an ROC analysis was performed for the homogenous cohort of surgical patients (n = 59) to assess the ability of pre-scan PSA to distinguish between positive and negative scans. The area under the operating characteristic curve (AUC) was 76.4% (95% CI: 63.8%–89.0%). The optimal cut-off pre-scan PSA value to predict a positive 18F–DCFBC scan was 0.78 ng/mL, which maximized the difference between TPR and FPR, i.e., at this PSA value, the TPR was 87.5% (28/32) and the FPR was 33.3% (9/27) [Fig. 3].
Fig. 3.
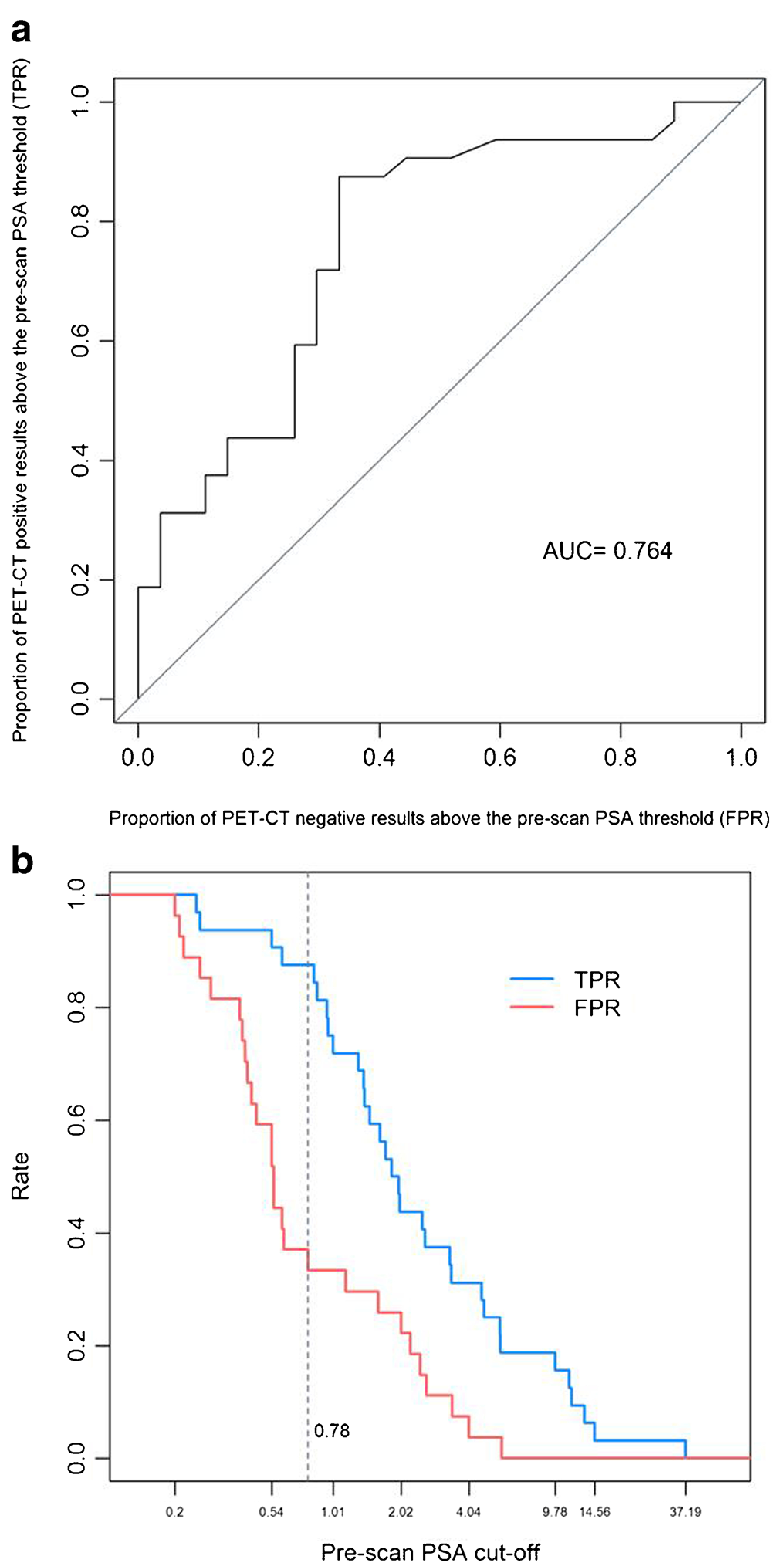
a. ROC analysis performed for surgical patients (n = 59) to assess the ability of pre-scan PSA in distinguishing between 18F–DCFBC PET/ CT positive and negative result, with an AUC (area under the operating characteristic curve) of 76.4%. b. The optimal cut-off pre-scan PSA, which maximizes the difference between the true-positive rate (TPR) and the false-positive-rate (FPR), was 0.78 ng/mL, i.e., at pre-scan PSA of 0.78 ng/mL, TPR was 87.5% (28/32) and FPR was 33.3% (9/27)
PET and mpMRI findings were often complementary although the limited coverage of mpMRI prevented correlation with PET findings in many cases. For instance, if a node was identified outside the field of view of the mpMRI, it was nonetheless included as discordant. The 18F–DCFBC and mpMRI findings were concordant for 39 lesions (49.4%), and discordant for 40 lesions (50.6%); in 32 of the discordant cases, the PET finding was outside the mpMR field of view [Fig. 4].
Fig. 4.
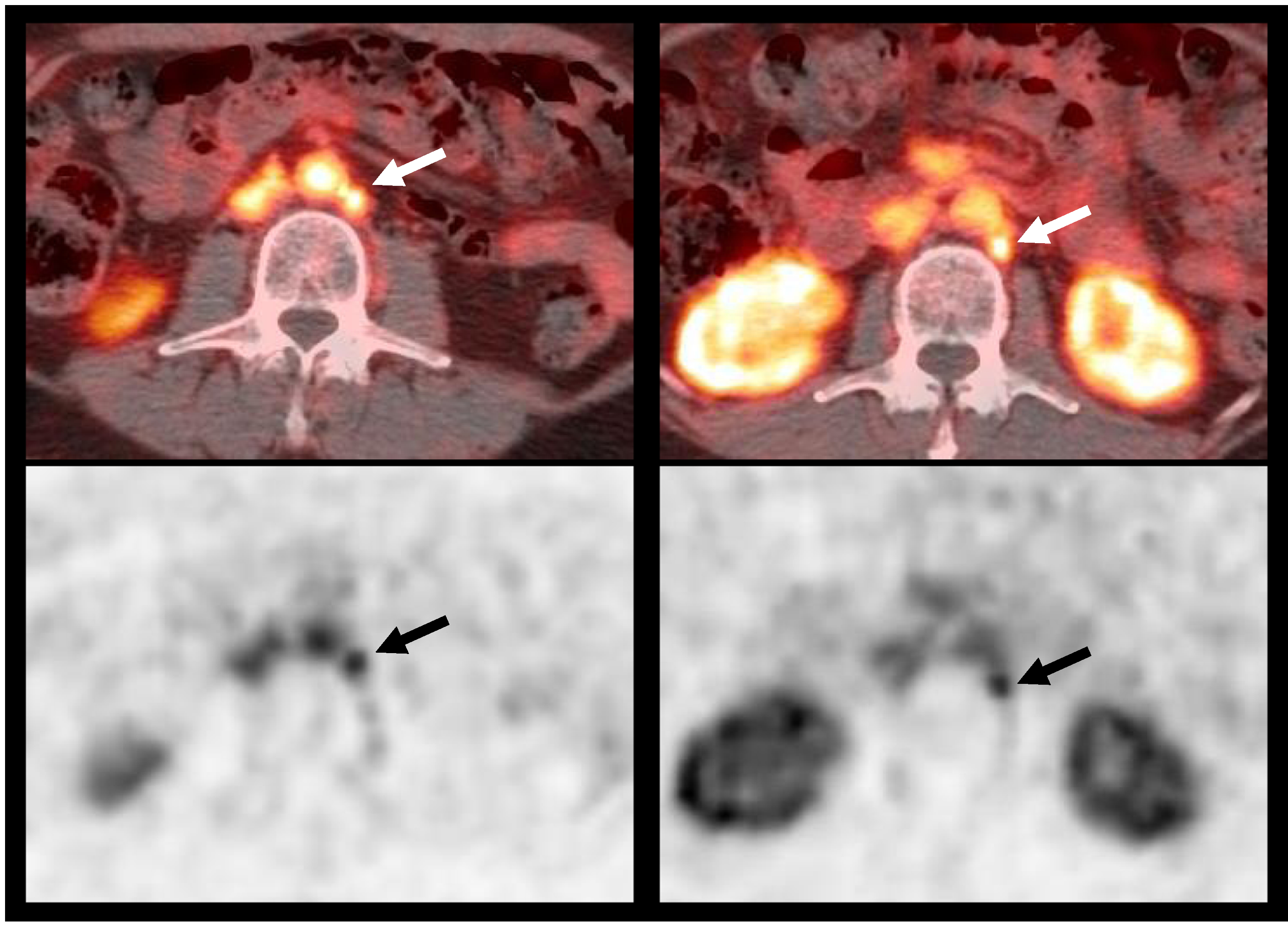
18F-DCFBC PET/CT imaging demonstrating nodal involvement: 61-year-old male, with history of prostate cancer, Gleason 7, status postprostatectomy 2 years ago, with pre-scan PSA of 1.31 mg/mL. 18F–DCFBC fused PET/CT (top) and PET (bottom) images demonstrate two small 9 mm and 7 mm left paraaortic lymph nodes (arrows); biopsy confirmed tumor recurrence. Patient started treatment with enzalutamide.
Biopsy was available for 18 patients, 17 with positive scans (13 of them positive on both PET and mpMRI), and one with a positive mpMRI only. Biopsy was performed in ten prostate bed sites, six lymph nodes and two distant sites (one lung nodule and one rib). Prostate malignancy was confirmed in 72.2% (13/18) of these patients, ten of them were both 18F– DCFBC PET and mpMRI positive, and in three the 18F– DCFBC PET was positive only. Therefore, biopsy confirmed malignancy in 76.5% (13/17) of the 18F–DCFBC PET positive lesions. For the remaining patients in whom biopsy was not performed, serial CT/MR imaging (8/41) or follow-up clinical assessment (15/41) were the means of confirming malignancy; there was no confirmation of recurrent disease in 5/41 patients, who continue under surveillance after the PET scan.
Apositive 18F–DCFBC PET, led to a change in treatment strategy plan in 51.2% (21/41) of patients, characterized by starting a new treatment in 19 patients or changing the treatment plan in two patients. The new treatment strategies included salvage radiotherapy in combination or not with other therapies (n = 12), brachytherapy (n = 3), salvage lymph node dissection (n = 3) or additional chemotherapy (n = 3). Among these patients, eight had biopsy confirmation, and two demonstrated progressive enlargement of masses on serial CT/MR follow-up imaging; the remainder were followed clinically. Conversely, a negative 18F–DCFBC examination led to cancellation of planned treatment in 48.1% (13/27) of patients, who continued to be observed rather than have an intervention. Thus, in the overall cohort of patients, 18F–DCBFC PET imaging, with either a positive or a negative result, lead to change of therapy in 50% (34/68) of the patients.
Discussion
In patients with low but rising PSA after definitive local therapy, it is important to identify the sites of recurrence early, so as to maximize the effects of treatment, particularly salvage radiation. Prior studies have shown that the effectiveness of salvage radiotherapy increases at lower PSA levels, however, the inability to localize the recurrence often stymies the decision to undergo salvage radiotherapy [18]. The aim of this study was to determine the sensitivity of 18F–DCFBC PET/ CT imaging in identifying recurrent prostate cancer and assess its impact on patient management. Over 60% of our patients with BCR had a positive 18F–DCFBC PET scan despite negative conventional imaging. The sensitivity of 18F–DCFBC PET was increased with higher PSA levels: sensitivity of 15%, 46%, 83%, and 77% for PSA levels of less than <0.5, 0.5 to <1.0, 1.0 to <2.0, and ≥2.0 ng/mL, respectively.
Many different formulations of PSMA targeting PET ligands have been proposed. Similar encouraging results to ours have been demonstrated in patients with BCR using 68Ga labeled PSMA ligands [19–21]. For instance, in a retrospective analysis of BCR patients, Afshar-Oromieh and collaborators detected lesions in 32 of 37 (86.5%) patients, 68.8% of them with PSA values of 2.82 ng/mL or less using a 68Ga PSMA PET agent [22]. Excellent sensitivity for 68Ga PSMA-ligand was shown by Eiber et al., who demonstrated a detection rate of 58% for PSA levels below 0.5 ng/ml, compared to 15% in our cohort with 18F– DCFBC PET imaging, and similarly, a detection rate of 73% for PSA levels below 1.0 ng/ml. vs 46% in our series [21]. Similar scan sensitivities were seen in patients recurring after radiation therapy [23]. Thus, the results for 68Ga PSMA PET imaging are somewhat better than 18 F-DCFBC mostly because of the higher background signal of this agent due to considerable blood-pool activity, which could have limited the detection of lymph node metastases in retroperitoneum and pelvis. A second-generation compound, 18F–DCFPyl, is now entering clinical trial evaluation [24] and appears to have greatly improved sensitivity, perhaps even superior to 68Ga-labeled compounds, at least based on one recent study [25].
Despite the relatively lower sensitivity, we were able to carefully record the impact of a positive PSMA PET scan on clinician behavior. What we observed suggested that this type of targeted imaging will have a profound impact on patient management. In this study, a positive 18F–DCFBC PET/CT scan led to changes in treatment in 51% of patients and even a negative scan resulted in the cancellation of planned treatment in 48% of such patients. Naturally, it is premature to conclude that such decisions were necessarily of benefit to the patient; however, the profound impact on treatment strategy is quite clear. Similar to our study, Albisinni et al. [26] retrospectively investigated the clinical impact of 68Ga labeled PSMA PET/CT imaging in 131 patients, showing changes in treatment strategy in 76% of patients [26]. Other groups have demonstrated that 68Ga-PSMA-PET/CT changed therapy in 51–63% of patients [27]. Thus, this agent could have a profound impact on the way BCR patients are clinically managed. It should be noted that none of these studies have complete histologic validation due to the difficulty of sampling lesions that are often located in hard-to-reach locations. Only 76% of the positive lesions in our study proved to be cancer, but it is quite likely that some of the lesions were missed by biopsy due to their small size and awkward location.
Unlike other studies, most of our patients also underwent mpMRI. This proved to be helpful in better localizing the site of increased uptake on 18F–DCFBC PET. This was especially true for recurrent lesions in the prostate bed where CT is often difficult to interpret, whereas mpMRI permits more accurate localization. Our experience would suggest that PSMA PET agents obtained in patients with BCR should be interpreted with the benefit of mpMRI or perhaps even combined into one study on a PET/MRI device.
Our study has a number of limitations. As noted above, most cases did not have histologic validation. This is unfortunately a common problem of studies involving BCR as the location of the lesion is often deep within the pelvis and, therefore, difficult to sample. Not only are there practical issues, but there are also ethical issues regarding the need to put a patient through a biopsy for research purposes only. Additionally, as with most other studies, our sample is heterogeneous, with patients undergoing several different primary cancer treatments (surgery, radiotherapy, or both) with a wide variation in PSA values. As these agents are used more commonly, it should be possible to have entire cohorts of BCR with similar treatment histories with which to better understand the performance of PSMA PET under a variety of conditions. Finally, although significant changes in treatment plan were observed after 18F–DCFBC PET/CT scan, it is unclear whether those decisions were beneficial. In this regard, it would be of interest to investigate the clinical outcomes in a randomized setting where PSMA PET is either offered or not offered to patients. We may rapidly be approaching the point where such a study is no longer considered ethical.
Conclusion
18F–DCFBC is a first-generation fluorine labeled PET compound that provides useful information in the setting of biochemical recurrence of prostate cancer. Using an optimal PSA threshold PSA value of 0.78 ng/mL, 18F–DCFBC PET imaging demonstrated very high sensitivity with only a modest false positive rate. Furthermore, 18F–DCFBC PET markedly influenced clinician behavior resulting in alterations in treatment in 51% of patients with a positive scan and approximately 48% of patients with a negative scan.
Supplementary Material
Acknowledgments
Funding This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00259-017-3818-x) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest Authors have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59(4):572–83. [DOI] [PubMed] [Google Scholar]
- 2.Pfister D, Bolla M, Briganti A, Carroll P, Cozzarini C, Joniau S, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014;65(6):1034–43. [DOI] [PubMed] [Google Scholar]
- 3.Briganti A, Karnes RJ, Gandaglia G, Spahn M, Gontero P, Tosco L, et al. Natural history of surgically treated high-risk prostate cancer. Urol Oncol. 2015;33(4):163 e7–13. [DOI] [PubMed] [Google Scholar]
- 4.Punnen S, Cooperberg MR, D’Amico AV, Karakiewicz PI, Moul JW, Scher HI, et al. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013;64(6):905–15. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–9. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179(3):906–10. discussion 10 [DOI] [PubMed] [Google Scholar]
- 7.Rouviere O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol. 2010;20(5):1254–66. [DOI] [PubMed] [Google Scholar]
- 8.Novo JF, Lopez SP, Aguilo FL, Miranda EF. Diagnostic methodology for the biochemical recurrence of prostate cancer after brachytherapy. Arch Esp Urol. 2006;59(10):1063–7. [PubMed] [Google Scholar]
- 9.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–61. [DOI] [PubMed] [Google Scholar]
- 10.Rowe SP, Gorin MA, Allaf ME, Pienta KJ, Tran PT, Pomper MG, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19(3):223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe SP, Gage KL, Faraj SF, Macura KJ, Cornish TC, Gonzalez-Roibon N, et al. (1)(8)F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56(7):1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal R, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naive and castration-resistant metastatic prostate cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53(12):1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nuclear Med Off Publ Soc Nuclear Med. 2007;48(3):471–80. [PubMed] [Google Scholar]
- 15.Mease RC, Dusich CL, Foss CA, Ravert HT, Dannals RF, Seidel J, et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer Res. 2008; 14( 10):3036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection-histopathologic correlation. Radiology. 2010;255(1): 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 18.Choo R. Salvage radiotherapy for patients with PSA relapse following radical prostatectomy: issues and challenges. Cancer Res Treat. 2010;42(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42(8J: 1284–94. [DOI] [PubMed] [Google Scholar]
- 20.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(2): 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nuclear Med Off Publ Soc Nuclear Med. 2015;56(5):668–74. [DOI] [PubMed] [Google Scholar]
- 22.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Einspieler I, Rauscher I, Duwel C, Kronke M, Rischpler C, Habl G, et al. Detection efficacy of hybrid 68Ga-PSMA ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by Phoenix criteria. Journal of nuclear medicine : official publication. Society of Nuclear Medicine. 2017. [DOI] [PubMed] [Google Scholar]
- 24.Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015; 17(4):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomacker K, et al. Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga-PSMAHBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17(4):575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical impact of 68 Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2016. [DOI] [PubMed] [Google Scholar]
- 27.Sterzing F, Kratochwil C, Fiedler H, Katayama S, Habl G, Kopka K, et al. (68)Ga-PSMA-ll PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43( 1 ):34–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


