Abstract
The blood–brain barrier (BBB) limits entry of most chemotherapeutic agents into the CNS, resulting in inadequate exposure within CNS tumor tissue. Intranasal administration is a proposed means of delivery that can bypass the BBB, potentially resulting in more effective chemotherapeutic exposure at the tumor site. The objective of this study was to evaluate the feasibility and pharmacokinetics (plasma and CSF) of intranasal delivery using select chemotherapeutic agents in a non-human primate (NHP) model. Three chemotherapeutic agents with known differences in CNS penetration were selected for intranasal administration in a NHP model to determine proof of principle of CNS delivery, assess tolerability and feasibility, and to evaluate whether certain drug characteristics were associated with increased CNS exposure. Intravenous (IV) temozolomide (TMZ), oral (PO) valproic acid, and PO perifosine were administered to adult male rhesus macaques. The animals received a single dose of each agent systemically and intranasally in separate experiments, with each animal acting as his own control. The dose of the agents administered systemically was the human equivalent of a clinically appropriate dose, while the intranasal dose was the maximum achievable dose based on the volume limitation of 1 mL. Multiple serial paired plasma and CSF samples were collected and quantified using a validated uHPLC/tandem mass spectrometry assay after each drug administration. Pharmacokinetic parameters were estimated using non-compartmental analysis. CSF penetration was calculated from the ratio of areas under the concentration–time curves for CSF and plasma ( AUCCSF:plasma). Intranasal administration was feasible and tolerable for all agents with no significant toxicities observed. For TMZ, the degrees of CSF drug penetration after intranasal and IV administration were 36 (32–57) and 22 (20–41)%, respectively. Although maximum TMZ drug concentration in the CSF (Cmax) was lower after intranasal delivery compared to IV administration due to the lower dose administered, clinically significant exposure was achieved in the CSF after intranasal administration with the lower doses. This was associated with lower systemic exposure, suggesting increased efficiency and potentially lower toxicities of TMZ after intranasal delivery. For valproic acid and perifosine, CSF penetration after intranasal delivery was similar to systemic administration. Although this study demonstrates feasibility and safety of intranasal drug administration, further agent-specific studies are necessary to optimize agent selection and dosing to achieve clinically-relevant CSF exposures.
Keywords: Intranasal, Chemotherapy, Non-human primate, Temozolomide, Valproic acid, Perifosine, Pharmacokinetics, DIPG
Introduction
A major challenge to successful treatment of CNS tumors is overcoming the blood–brain barrier (BBB) in order to achieve adequate chemotherapeutic exposure at the tumor site. The BBB is essential to protect the CNS from toxins and maintain a homeostatic environment [1, 2]. The specialized tight junctions and active efflux transporters which form a neurovascular unit comprising the BBB limit entry of systemically administered chemotherapeutic agents, especially those that are large or hydrophilic, resulting in inadequate delivery of agents to the tumor site. While the BBB and blood:tumor barriers may be disrupted in some tumors, as evidenced by contrast enhancement on MRI, this disruption is incomplete and heterogeneous. Invasive cells at the leading edge of infiltrative tumors are frequently in areas where the BBB is intact. The BBB of some tumors such as diffuse intrinsic pontine glioma (DIPG) [3] and low grade glioma, are frequently intact, restricting drug delivery. Attempts to overcome this barrier have included systemic administration of higher chemotherapeutic doses, intra-arterial delivery, and BBB disruption, each of which are associated with significant toxicities [4, 5].
An alternate route of drug delivery to the CNS is intranasal administration. Intranasal delivery is non-invasive, may bypass the BBB, and has been successfully used for administration of drugs for other neurologic diseases [6–10]. Its utility for delivery of chemotherapeutic agents has yet to be clinically established. Intranasal drug delivery may overcome issues with systemic drug delivery including poor bioavailability, slow absorption, drug degradation, adverse gastrointestinal tract side effects, and first-pass hepatic metabolism [11, 12]. The exact mechanism of CNS delivery after intranasal drug administration is not completely understood, although animal studies have shown evidence of direct extracellular and intracellular transport pathways between the epithelial layer of the nasal cavity and the CNS. Drugs administered by the intranasal route can enter the CNS directly, as well as via transport along the olfactory and trigeminal nerve branches, predominantly by following the respective perivascular and neuronal channels [1, 10, 13–17].
Animal models have previously shown the potential for intranasal delivery of anti-tumor agents [18–22], such as the oligonucleotide telomerase inhibitor GRN163 [21, 22]. A limited number of clinical trials involving a small number of patients with refractory CNS tumors have been performed [23]. A recent Phase I/II study of intranasal administration of perillyl alcohol, an agent that affects the TGF-β and/or Ras signaling pathways, was well tolerated in adult patients with recurrent malignant gliomas when administered via inhalation. The authors report modest activity with radiographic evidence of tumor regression and longterm stability [24]. No clinical trials of intranasally delivered chemotherapeutic agents have been performed in children.
The primary objectives of this study were to determine if intranasal chemotherapeutic drug delivery is safe and feasible, and to establish a proof-of-principle that clinically meaningful exposure could be achieved in the CSF after intranasal delivery. The agents selected for this study, TMZ, valproic acid, and perifosine, were chosen because of their varying degrees of CSF penetration and potential anti-tumor activity. The alkylating agent TMZ is small (MW 194.15 Da) and hydrophilic with a CSF penetration of approximately 33% [25]. Valproic acid is also small (MW 144.20 Da) and hydrophilic, but with a lower CSF penetration of approximately 13% [26]. Commonly used as an antiepileptic, it has an epigenetic role as an HDAC inhibitor in CNS malignancies, although clinically relevant anti-tumor exposures are difficult to achieve because of toxicity. The Akt inhibitor, perifosine, is a large (MW 461.66 Da) and lipophilic agent, with a CSF penetration of only approximately 0.16% [27].
Materials and methods
NHPs
The National Cancer Institute Animal Care and Use Committee approved this study. Adult male rhesus macaques (Macaca mulata) weighing 9.5–14.2 kg were used. Each animal had an indwelling, subcutaneous central venous catheter in the femoral or saphenous vein for blood sampling, and a fourth or lateral ventricular reservoir for CSF sampling. The NHPs were socially housed, fed chow with water ad libitum, and cared for in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals [28].
Agents
Temodar® (temozolomide; Merck, Whitehouse Station, NJ) was supplied as lyophilized powder in 100 mg-vials. Valproic acid was obtained as Valproate Sodium in solution for injection (APP, Fresenius Kabi, USA LLC, Lake Zurich, Il). Perifosine was supplied as lyophilized powder (IV administration: Cancer Therapy Evaluation Program and IN administration: Sigma-Aldrich, St. Louis, MO). All agents were stored at 4 °C. Prior to intranasal administration, the drugs were prepared at room temperature and dissolved either in sterile water (TMZ) or normal saline (perifosine).
Study design and procedure
Systemic administration technique and dosing
The NHP acted as its own control. Each animal received an individual agent systemically at the following doses: TMZ: 7.5 mg/kg (human equivalent dosing (HED) of 150 mg/m2) infused IV over 1 h, valproic acid: 18.5 mg/kg (HED of 370 mg/m2) PO, perifosine: 7 mg/kg (HED of 140 mg/m2) PO. Serial paired whole blood (1 mL) and CSF samples (0.3 mL) were collected prior to all experiments and at multiple set time points within the sampling period depending on the half-life of each agent. Collection times were TMZ: 0–24 h, valproic acid: 0–48 h, perifosine: 0–8 weeks (Supplementary Table 1). After collection, plasma was immediately separated by centrifugation. For TMZ, CSF and plasma samples were placed in tubes prepared with 30 and 100 μL of 1 N hydrochloric acid (10% by volume), respectively, as a preservative. Plasma and CSF were stored at −20 °C then transferred to storage at −80 °C until analysis.
Intranasal administration technique and dosing
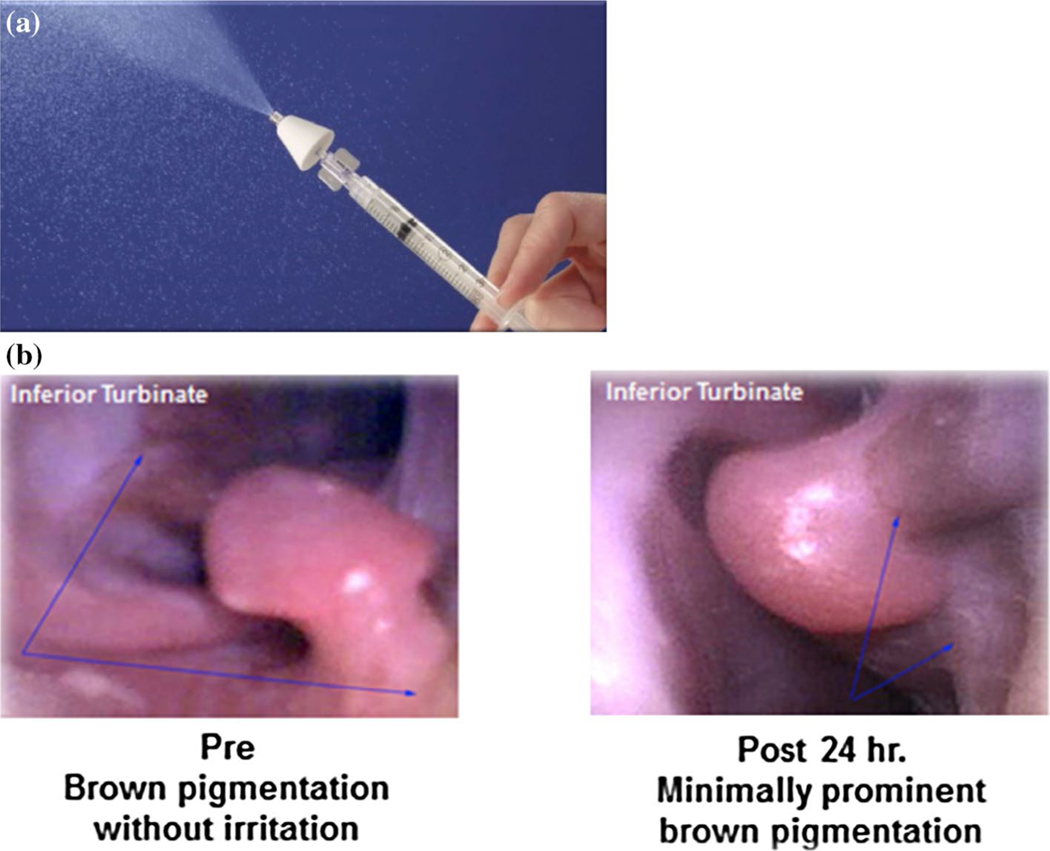
Intranasal dosing was limited to the maximum concentration achievable within a total volume of 1 mL (0.5 mL/nare), as this volume was determined to be within safe limits to administer to the animals. Resulting mean doses and numbers of animals receiving each agent were: TMZ (n = 4), intranasal (1.8 mg/kg) and IV (7.5 mg/kg); valproic acid (n = 4), intranasal (9.2 mg/kg) and PO (11.2 mg/kg); and perifosine (n = 3), intranasal (6.8 mg/kg) and PO (7.1 mg/kg). Animals were sedated with intramuscular ketamine (10 mg/kg) and dexmedetomidine (1000 mcg/m2), intubated to prevent aspiration during intranasal administration, and placed in the Mygind position, a dorsal recumbency with the head angled at 30°–45°. This position is optimal for intranasal administration of agents to gain maximum exposure to the olfactory epithelium [29]. Intranasal administration was performed using a mucosal atomization device (MAD, LMA MAD Nasal™, Fig. 1a). Each agent (0.5 mL) was sprayed in each nare followed by 0.2 mL sterile saline flushes. The primates remained in the Mygind position for 15 min after dosing followed by further recovery in the upright position. Blood and CSF sample collection and sampling time points were similar to those used for systemic administration.
Fig. 1.
a Mucosal atomization device (MAD) and b Image of NHP nasal turbinates prior to and 24 h after administration of intranasal agents. Incidental finding of benign epithelial pigmentation (blue arrows) were observed without any identified acute changes directly correlated with intranasal administration of all chemotherapeutic agents
Safety monitoring
Vital signs were monitored during the procedure and throughout recovery. The nasal cavity of each animal was visually evaluated using a WiFi flexible boroscope endoscope for iPhone/iPAD/Android (Vividia), 3.9 mm scope prior to, and at least 24 h following intranasal delivery. (Fig. 1b). They were monitored through clinical chemistries and complete blood counts, which remained within normal limits. Care of the animals was performed in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals [28].
Sample analysis
Drug concentrations in plasma and CSF were quantitatively measured using validated ultra-high performance liquid chromatography-tandem mass spectrometry (uHPLC-MS/MS) assays with a lower limit of quantitation (LLQ) of 5, 25, and 0.5 ng/mL, for TMZ [30], valproic acid, and perifosine, respectively.
Pharmacokinetic analysis
Non-compartmental analyses of plasma and CSF pharmacokinetic parameters were calculated using Phoenix® WinNonlin 6.4 (Certara, Cary, NC). Any plasma concentration measured below the LLQ was excluded from analyses. The maximum concentration (Cmax) and time to Cmax (Tmax) were recorded as observed values. The area under the plasma concentration versus time curve was calculated using the linear up/log down trapezoidal rule, both to the last quantifiable time point (AUClast) and to time infinity (AUCINF). The elimination rate (Kel) was calculated as the slope of the best-fit line drawn through at least three terminal concentration points. Half-life (t1/2) was calculated as ln2/Kel. The mean and sample standard deviation of each of the above parameters was calculated using Microsoft Excel 2011 for Mac, version 14.4.2.
Results
Feasibility
Total planned drug volumes were successfully delivered intranasally to each animal using the MAD. All animals tolerated the procedure well; there were no systemic effects following drug administration; no changes in the nasal cavity were detected by borescope visualization after administration of any agent with the exception of a minimal increase in brown pigmentation around the nasal turbinates (see Fig. 1). This phenomenon was seen with all agents.
Pharmacokinetics
Intranasal and IV TMZ
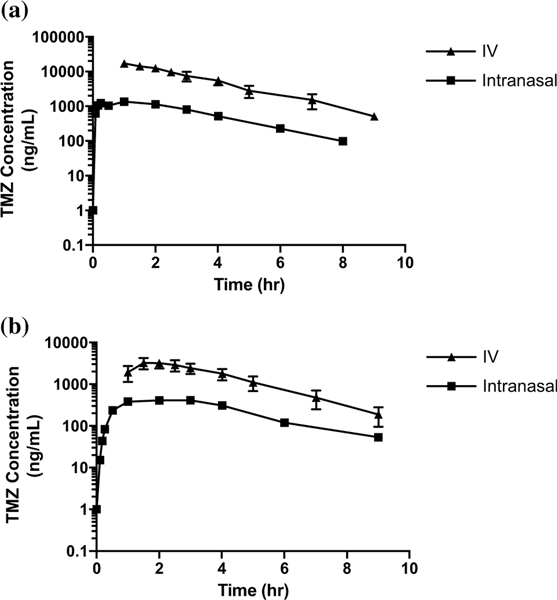
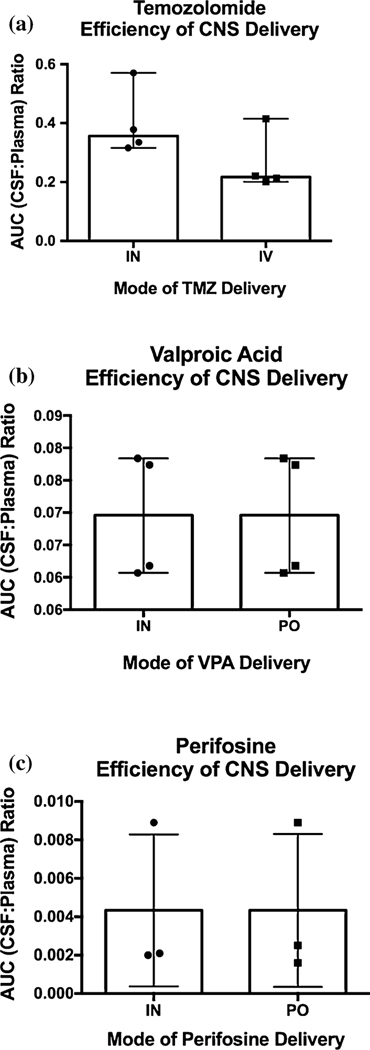
Logarithmic plots of mean plasma and CSF concentrations versus time by method of administration for TMZ are shown in Fig. 2. Comparisons between the mean (range) Cmax, time to Cmax (Tmax) and half-life (t½) are shown in Supplementary Table 2. After intranasal administration, median (range) CSF penetration of TMZ (as measured by the AUCCSF:plasma ratio) was 36 (32–57)%. After IV TMZ administration, CSF penetration was 22 (20–41)% (Fig. 5a). TMZ CSF exposures after IN administration were 40% that in the plasma, compared to 24% after systemic administration.
Fig. 2.
Plasma (a) and CSF (b) concentration (ng/mL) × time (h) logarithmic curves of TMZ (intranasal and IV) up to approximately 8 h
Fig. 5.
AUCCSF:Plasma comparison between systemic and intranasal (IN) routes for a temozolomide, b valproic acid, and c perifosine
Intranasal and PO valproic acid
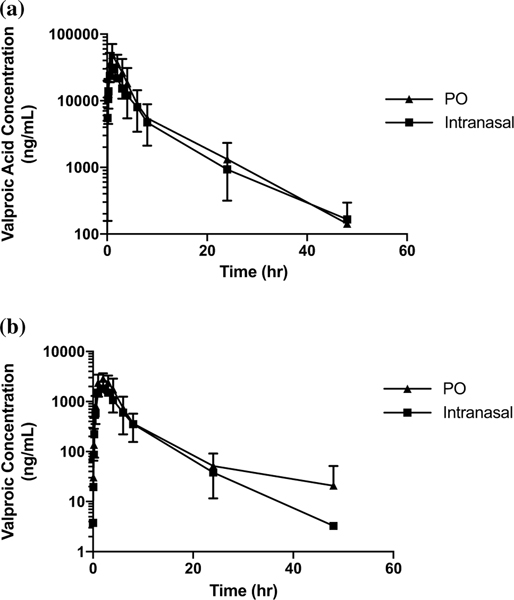
The logarithmic plots of the mean of all plasma and CSF concentrations versus time between intranasal and PO valproic acid are shown in Fig. 3. Comparisons between the mean (range) Cmax, Tmax and t½ are shown in Supplementary Table 3. For intranasal valproic acid, CSF penetration was 7 (6–9)%; for PO valproic acid, CSF penetration was also 7 (6–8)%. (Fig. 5b).
Fig. 3.
Plasma (a) and CSF (b) concentration (ng/mL) × time (h) logarithmic curves of valproic acid (intranasal and PO) up to 48 h
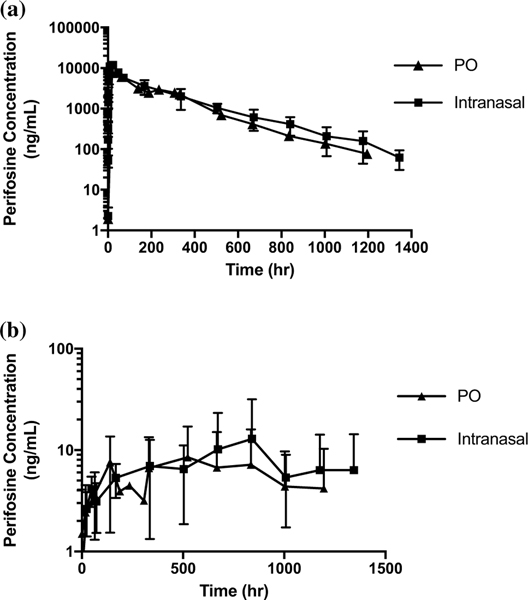
Intranasal and PO perifosine
The logarithmic plots of the mean of all plasma and CSF concentrations versus time between intranasal and PO perifosine are shown in Fig. 4. Comparisons between the mean (range) Cmax and time to Cmax (Tmax) are shown in Supplementary Table 4. For intranasal perifosine, CSF penetration was 0.4 (0.2–0.9)%; for PO perifosine, CSF penetration was also 0.4 (0.2–0.9)%. (Fig. 5c).
Fig. 4.
Plasma (a) and CSF (b) concentration (ng/mL) × time (h) logarithmic curves of perifosine (intranasal (IN) and PO) up to approximately 1400 h
Discussion
This study demonstrates the safety and feasibility of intranasal administration of chemotherapeutic agents in a non-human primate model. It also demonstrates the proof-of-principle that agents delivered intranasally can reach the CSF. However, the potential pharmacokinetic benefit of intranasal delivery over systemic delivery varies by drug, and may be limited by maximum drug concentration in a deliverable volume.
As expected from the smaller dose administered intranasally, the maximum concentration of TMZ in plasma and CSF was decreased compared to IV administration. However, when comparing the degree of CSF penetration as defined by the AUCCSF:plasma, a greater percentage of TMZ entered the CSF after intranasal administration compared to IV administration, and exposures achieved in the CSF were in the effective exposure range of TMZ. This suggests that intranasal delivery of TMZ may provide equivalent CNS exposure with less systemic exposure (and hence, less systemic toxicity) compared to IV administration. This may be due to its high CSF penetration, which would allow rapid entry into the CSF and avoidance of first pass effect. In contrast, plasma and CSF exposures for valproic acid and perifosine were similar for both methods of delivery.
This study is the first to use a non-human primate model to investigate the intranasal pharmacokinetics of clinically available chemotherapeutic agents. Several limitations were encountered in the process. Although this study was designed as a proof-of-principle approach, a major limitation was the maximum volume placed on intranasal dosing, precluding direct comparison of CSF penetration by each route of administration. While the study could be designed to administer lower doses systemically, our goal was to evaluate clinically relevant doses, and to determine if effective CSF exposures could be achieved safely after intranasal administration. In addition, our study was limited by the animal model itself. Although prior studies have demonstrated that the NHP model is predictive of pharmacokinetics in children after systemic administration, this species has a greater percentage of olfactory:respiratory epithelium in the nares compared to humans. However, this model is closer to humans than the prior studies evaluating intranasal administration in a rodent species, which has an even greater olfactory:respiratory epithelium ratio [31]. Another consideration in this model is the absence of a closed loop system. Although the Mygind position is the optimal positioning to direct drug towards the olfactory region, some drug is likely absorbed across mucosal membranes or inhaled into the pulmonary system. However, this is a challenge that would also be present in patients. Finally, in this NHP model, due to limitations with CNS tissue access, CSF drug concentrations are utilized as a surrogate for CNS tissue delivery, with the assumption that there is a direct, although not absolute, correlation of CSF and CNS tissue penetration. Studies examining CNS extracellular fluid concentrations after IN delivery will therefore better define drug penetration in the tissue.
In summary, intranasal delivery of chemotherapeutic agents appears safe and feasible. For certain agents, it may offer a pharmacokinetic advantage, potentially improving the efficiency of CNS delivery and limiting toxicities by reducing systemic exposure. The pharmacokinetics of select agents for intranasal delivery should be studied individually to determine the potential benefit over systemic delivery.
Supplementary Material
Acknowledgements
This work was presented in part at the 2015 Society of Neuro-Oncology and the Society of CNS Interstitial Delivery of Therapeutics (SNO-SCIDOT) Joint Conference on Therapeutic Delivery to the CNS in San Antonio, TX. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclaimer The views expressed in this article are those of the author(s) and do not reflect the official policy or views of the National Cancer Institute, the National Institutes of Health, the U.S. Department of Health and Human Services, the Department of the Army/Navy/Air Force, Department of Defense, or any other agency of the U.S. Government.
Compliance with ethical standards
Research involving human and animal rights The National Cancer Institute Animal Care and Use Committee approved this study. All applicable institutional guidelines for the care and use of animals were followed. This study does not contain any studies with human participants.
Electronic supplementary material The online version of this article (doi:10.1007/s11060-017-2388-x) contains supplementary material, which is available to authorized users.
Conflict of interest Authors James C. League-Pascual, Cynthia M. Lester-McCully, Shaefali Shandilya, Lukas Ronner, Louis Rodgers, Rafael Cruz, Cody J. Peer, William D. Figg, and Katherine E. Warren all individually declare no conflict of interest.
References
- 1.Abbott NJ (2013) Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36(3):437–449 [DOI] [PubMed] [Google Scholar]
- 2.Pasha S, Gupta K (2010) Various drug delivery approaches to the central nervous system. Expert Opin Drug Deliv 7(1):113–135 [DOI] [PubMed] [Google Scholar]
- 3.Vanan MI, Eisenstat DD (2015) DiPG in children–what can we learn from the past? Front Oncol 5:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren KE (2013) Novel therapeutic delivery approaches in development for pediatric gliomas. CNS Oncol 2(5):427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyake MM, Bleier BS(2015) The blood–brain barrier and nasal drug delivery to the central nervous system. Am J Rhinol Allergy 29(2):124–127 [DOI] [PubMed] [Google Scholar]
- 6.Pires A et al. (2009) Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci 12(3):288–311 [DOI] [PubMed] [Google Scholar]
- 7.Costantino HR et al. (2007) Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm 337(1–2):1–24 [DOI] [PubMed] [Google Scholar]
- 8.Panagiotou I, Mystakidou K (2010) Intranasal fentanyl: from pharmacokinetics and bioavailability to current treatment applications. Expert Rev Anticancer Ther 10(7):1009–1021 [DOI] [PubMed] [Google Scholar]
- 9.Bitter C, Suter-Zimmermann K, Surber C (2011) Nasal drug delivery in humans. Curr Probl Dermatol 40:20–35 [DOI] [PubMed] [Google Scholar]
- 10.Mittal D et al. (2014) Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv 21(2):75–86 [DOI] [PubMed] [Google Scholar]
- 11.Djupesland PG (2013) Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res 3(1):42–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attkins NJ et al. (2009) Predictability of intranasal pharmacokinetics in man using pre-clinical pharmacokinetic data with a dopamine 3 receptor agonist, PF-219061. Xenobiotica 39(7):523–533 [DOI] [PubMed] [Google Scholar]
- 13.Dhuria SV, Hanson LR, Frey WH 2nd (2010) Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99(4):1654–1673 [DOI] [PubMed] [Google Scholar]
- 14.Lochhead JJ, Thorne RG (2012) Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 64(7):614–628 [DOI] [PubMed] [Google Scholar]
- 15.Illum L (2000) Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci 11(1):1–18 [DOI] [PubMed] [Google Scholar]
- 16.Johnson NJ, Hanson LR, Frey WH (2010) Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm 7(3):884–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkus FW, van den Berg MP (2007) Can nasal drug delivery bypass the blood–brain barrier?: questioning the direct transport theory. Drugs R D 8(3):133–144 [DOI] [PubMed] [Google Scholar]
- 18.Thorne RG et al. (2008) Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience 152(3):785–797 [DOI] [PubMed] [Google Scholar]
- 19.van Woensel M et al. (2013) Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers (Basel) 5(3):1020–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shingaki T et al. (2010) Transnasal delivery of methotrexate to brain tumors in rats: a new strategy for brain tumor chemotherapy. Mol Pharm 7(5):1561–1568 [DOI] [PubMed] [Google Scholar]
- 21.Djupesland PG, Messina JC, Mahmoud RA (2014) The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv 5(6):709–733 [DOI] [PubMed] [Google Scholar]
- 22.Hashizume R et al. (2008) New therapeutic approach for brain tumors: intranasal delivery of telomerase inhibitor GRN163. Neuro Oncol 10(2):112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson A et al. (2014) A systematic review of inhaled intranasal therapy for central nervous system neoplasms: an emerging therapeutic option. J Neurooncol 116(3):437–446 [DOI] [PubMed] [Google Scholar]
- 24.CO DAF et al. (2013) Long-term outcome in patients with recurrent malignant glioma treated with Perillyl alcohol inhalation. Anticancer Res 33(12):5625–5631 [PubMed] [Google Scholar]
- 25.Patel M et al. (2003) Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J Neurooncol 61(3):203–207 [DOI] [PubMed] [Google Scholar]
- 26.Stapleton SL et al. (2008) Plasma and cerebrospinal fluid pharmacokinetics of valproic acid after oral administration in non-human primates. Cancer Chemother Pharmacol 61(4):647–652 [DOI] [PubMed] [Google Scholar]
- 27.Cole DE et al. (2015) Plasma and cerebrospinal fluid pharmacokinetics of the Akt inhibitor, perifosine, in a non-human primate model. Cancer Chemother Pharmacol 75(5):923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albus U (2012) Guide for the care and use of laboratory animals (8th edn). Lab Anim 46(3):267–268 [Google Scholar]
- 29.Mygind N, Upper airway: structure, function and therapy, in in aerosol in medicine. Princples, diagnoses, and therapy. 1985, Elsevier, Amsterdam, pp 1–20 [Google Scholar]
- 30.Peer CJ et al. (2016) Quantification of temozolomide in non-human primate fluids by isocratic ultra-high performance liquid chromatography-tandem mass spectrometry to study brain tissue penetration following intranasal or intravenous delivery. Separations 3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harkema JR (1990) Comparative pathology of the nasal mucosa in laboratory animals exposed to inhaled irritants. Environ Health Perspect 85:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.