Abstract
Background:
Cardiac glycosides (CGs) possess a chemical structure similar to steroids, and are inhibitors of the sodium potassium pump. An anti-tumor effect of CGs in breast and prostate cancers has been reported, but the effect of CGs on ovarian cancer is still unclear.
Aims:
In this study, the effects of CGs on proliferation, cytotoxicity and cell cycle of ovarian cancer cell line (SKOV-3) have been investigated.
Procedure:
The cell proliferation and cytotoxicity were detected by MTT assay and LDH activity assay, respectively. CGs, at concentrations higher than IC50, decreased cell proliferation and showed increased cytotoxicity toward SKOV-3 cells. The colony-formation ability was reduced after treatment with digoxin and digitoxin for 10 days. Furthermore, we explored the effect of digoxin and digitoxin on the distribution of cell cycle by flow cytometry.
Results:
Results revealed that both digoxin and digitoxin led to cell cycle arrest in G0/G1 phase with 24 or 48 hours, but the arrest of G0/G1 phase was not observed at 72 hours. We evaluated the percentage of hypodiploid cell population as an index of the cellular fragments through flow cytometry. The data indicated that cellular fragments were significantly increased by treating with digitoxin at the concentrations of IC50 and 10−6 M for 72 hours.
Conclusion:
Taken together, these data suggest that CGs decreased cell proliferation and increased cytotoxicity through cell cycle arrest at the G0/G1 phase. CGs have anti-tumor effect in SKOV-3 cells and might be a potential therapeutic drug for ovarian cancer. Since this study is a preliminary investigation of CGs on SKOV-3 cells, more experiments might be performed in the future. Furthermore, more ovarian cancer cell lines might also be employed in the future studies to confirm the effect of CGs in ovarian cancer.
Keywords: digoxin, digitoxin, ouabain, SKOV-3, cell growth
Introduction
Ovarian cancer is the deadliest disease in gynecologic malignancies. Different from breast cancer, ovarian cancer lacks early stage diagnostic markers and non-significant symptoms until cancer cells have metastasized, and then cause a poor survival rate.1 Clinical data point out that steroid hormones promote proliferation, metastasis and epithelial-mesenchymal transition (EMT).2 Progesterone is a potent hormone to inhibit ovarian cancer cell growth whereas estrogen may be a risk factor of ovarian cancer deterioration.3 Estrogens, one of steroid hormones, play an important role in proliferation and development of the ovary.4 The biofunctions of estrogen are through binding to estrogen receptor (ER)α and ERβ.5 Estradiol (E2) enhances cell proliferation through activating ERα-regulated gene (IGFBP3).6 On the contrary, ERβ agonists liquiritigenin and S-equol inhibit cell growth, migration and invasion and induce cell apoptosis.1
The chemical structure of cardiac glycosides is similar to steroids; they are also known as cardiac steroid glycosides.7 Cardiac glycosides (CG) including digoxin, digitoxin, and ouabain are inhibitors of the sodium potassium pump, and have been used in heart failure for a long time.8 CGs also inhibit testosterone production via the attenuation of cAMP accumulation, activities of adenylyl cyclase and cytochrome P450scc in rat testicular cells9-11 and progesterone release by rat granulosa cells via a Na+, K+-ATPase-independent mechanism involving the inhibition of post-cAMP pathway cytochrome P450scc and the protein function of steroidogenic acute regulatory (StAR) protein.12 CGs have been known to possess anticancer effects in endocrine-related cancers comprising lung cancer,13 prostate cancer,14-17 and breast cancer18 through inhibiting the cell migration19 and proliferation and inducing apoptosis.20,21 CG induces cell apoptosis in MCF-7 (estrogen receptor positive-breast cancer cell line) and MDA-MB-468 (triple-negative breast cancer) through arresting the G0/G1 phase.22 On the other hand, ingesting CG for a long time may increase the risk of breast cancer, which is caused by the similar structures of CG and estrogen. Digitoxin and bufalin are reported to decrease the cell migration or cell growth through changing the microenvironment and suppression of the ITGB2/FAK pathway in ovarian cancer.6,19 Comprehensively, there are 2 opinions for the inhibitory effect of CG on estrogen receptor in breast cancer, one is antitumor, the other one is tumor promotion. It has been known that ovarian cancer is an estrogenic hormone related cancer.23 However, the effect of CG on ovarian cancer is not clearly known.
In this study, the dual effects of CG, both stimulatory and suppressive, at low and high concentrations in the ovarian cancer have been investigated. We examined cell proliferation and cytotoxicity after administration of CG in ovarian cancer cells. Furthermore, the cell cycle distribution was detected following treatments with digoxin and digitoxin.
Meterials and Methods
Cell Line and Reagents
Human ovarian cancer cell line SKOV3 was purchased from American Type Culture Collection(ATCC®). Digoxin, digitoxin, ouabain, MTT(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and propidium iodide were purchased from Sigma Chemical Co (St. Louis, MO, USA).
Cell Culture
SKOV3 cells were cultured in RPMI1640 medium (Gibico Laboratories, Buffalo, Grand Island, NY, USA) containing 10% fetal bovin serum (Gibico Laboratories, Buffalo, Grand Island, NY, USA), 1.5 g/l sodium bicarbonate, 1% penicillin and streptomycin (Sigma, St. Louis, MO, USA) and refreshed media per 3 or 4 days. Cells were incubated in a 5% CO2 incubator at 37°C.
Cell Proliferation Assay
The effect of CGs (including digoxin, digitoxin, and ouabain) on cell proliferation was measured by MTT assay. Cells were seeded in 96-well plates (2.0 × 103 cells per well) and incubated in a 5% CO2 incubator at 37°C for 24 hours. After incubation, cells were treated with or without CGs in a dose dependent manner for 24 or 48 hours. Serum free media containing 1.0 mg/ml MTT solution was added to 96-well plates (100 μl per well) and incubated for 3 hours in a 5% CO2 incubator at 37°C. DMSO was used to solubilize formazan crystals and the optical density (O.D.) was detected by ELISA reader at O.D. 570 nm absorbance with 620 nm as the wavelength reference.
Cytotoxicity Assay
The cell cytotoxicity effect of CGs was detected through LDH-Cytotoxicity Assay Kit II (BioVision, Milpitas, CA, USA). Cells were seeded in 96-well plates (2.0 × 104 cells per well) and incubated in a 5% CO2 incubator at 37°C for 24 hours. After cells adhesion, cells were treated with 200 μl fresh medium with or without CGs for 24 hours. The administrations were descripted as the following:
(a) low control (medium only), high control (10% cell lysis solution)
(b) 200 μl fresh medium (without cells)
(c) 200 μl fresh medium (containing 0.1% DMSO)
(d) 200 μl fresh medium (containing 10−9-10−4 M digoxin)
(e) 200 μl fresh medium (containing 10−9-10−4 M digitoxin)
After incubation for 24 hours, 10 μl media obtained from (a) to (e) were mixed with 100 μl LDH after incubation in 96-well plates at room temperature under dark for 30 minutes. In the ELISA reader, the cytotoxicity was monitored at 450 nm absorbance with 630 nm as the wavelength reference.
Colony Forming Assay
Cells were seeded in 6-well plates with 5.0 × 102 cells per well. After cell adhesion, cells were treated with or without CGs for 10 days. Cells were washed with phosphate-buffered saline (PBS) and fixed by 100% methanol for 10 minutes at room temperature. Meanwhile, 0.5% crystal violet was used to stain cells for 15 minutes at room temperature and then cells were washed with ddH2O. The images of colonies were captured by camera and then dissolved crystal violet with 500 μl DMSO per well. The quantification of the number of colonies was measured by ELISA reader at 595 nm.
Flow Cytometry
Cells were seeded in 10 cm culture dish with 1.0 × 106 cells per dish and incubated overnight. After cell adhesion, cells were treated with or without CGs containing digoxin and digitoxin for 24, 48, and 72 hours. Cells were harvested and washed twice with PBS. Cells were fixed with 1 ml 70% ethanol at −20°C overnight. After cells were resuspended in 1.0 ml 0.1% PBS containing 0.05 mg/ml Triton X-100 and 0.25 mg/ml propidium iodide, cells were incubated at 37°C for 15 minutes. Cells were analyzed by flow cytometry at 488 nm excitation wavelength.
Statistical Analysis
All results were expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to assess the difference among all groups and then followed by Duncan’s multiple range test to analyze the difference between control and treatment groups.24 The P value <.05 was considered statistically significant.
Results
The Inhibitory Effect of CGs on Cell Proliferation in SKOV-3 Cells
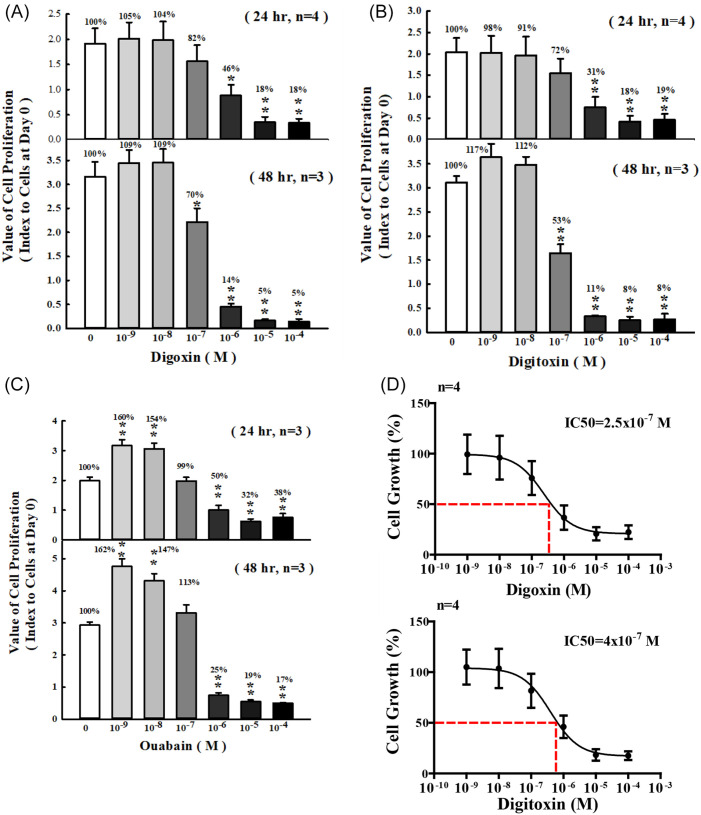
The cell proliferation effect of CGs containing digoxin, digitoxin and ouabain was determined by MTT assay. SKOV-3 cells were treated with a series of concentrations of CGs for 24 and 48 hours. Cell proliferation was slightly increased at 10−9 to 10−8 M digoxin for 24 and 48 hours compared with control group, respectively. The inhibitory effect of CGs on cell proliferation in SKOV-3 after 24 and 48 hours treatment with 10−7 to 10−4 M digoxin was comparable with control group. The inhibitory percentage of cell proliferation was decreased by 54% and 82% in 10−6 and 10−5 to 10−4 M digoxin for 24 hours, respectively. The inhibitory percentage of cell proliferation was decreased by 86% and 95% in 10−6 and 10−5 to 10−4 M digoxin for 48 hours, respectively (Figure 1A). The similar phenomenon was observed in digitoxin and ouabain treated groups. The cell proliferation was decreased significantly after treatment with 10−6 to 10−4 M and 10−7 to 10−4 M digitoxin for 24 and 48 hours, respectively (Figure 1B). In the ouabain-treated group, the data showed that cell proliferation was inhibited significantly from 10−6 M to 10−4 M for 24 and 48 hours. Different from the results of digoxin and digitoxin, the cell proliferation was siginificantly increased by ouabain at the concentration of 10−9 to 10−8 M (Figure 1C). Taken together, these results showed that the response of cell proliferation was in a time-dependent and dose-dependent manner (Figure 1A-C). The half maximal inhibitory concentration values (IC50) calculated from dose-response curves for digoxin and digitoxin were 2.5 × 10−7 M and 4.0 × 10−7 M, respectively, and these concentration values were used in follow-up experiments (Figure 1D).
Figure 1.
Effects of CGs on proliferation of SKOV-3 cells. SKOV-3 cells were incubated with (A) digoxin (B) digitoxin and (C) ouabain at different concentrations for 24 and 48 hours. (D) Half maximal inhibitory concentration (IC50) of digoxin and digitoxin.
Cell proliferation was analyzed by MTT assay. Each value represents mean ± SEM. These experiments were repeated 3 to 4 times.
Digoxin and Digitoxin Suppressed Colony Formation
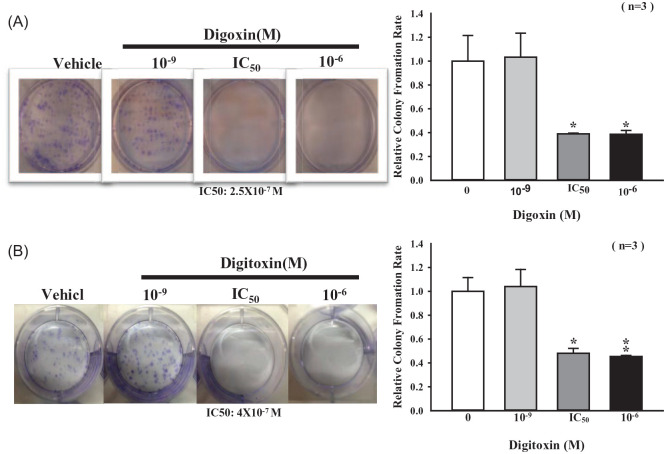
Accordding to the above observations, digoxin, and digitoxin have been demonstrated to play an important role in antitumor effect. We then investigated whether CGs affect the colony-forming abilities. The cell growth was observed after 10-day treatment with CGs. We found that CGs significantly decreased the colony-forming abilities by 50% to 60% when higher concentration (greater than IC50) of CGs was employed (Figure 2A and B).
Figure 2.
Effects of CGs on colonies formation of SKOV-3 cells. Cells were incubated with indicated concentrations of (A) digoxin and (B) digitoxin for 10 days. Colony-forming ability was compared with the control group.
Each value represents mean ± SEM of 3 independent experiments.
*P < .05, **P < .01 as compared with control group.
The Cytotoxicity of Digoxin and Digitoxin on SKOV-3 Cells
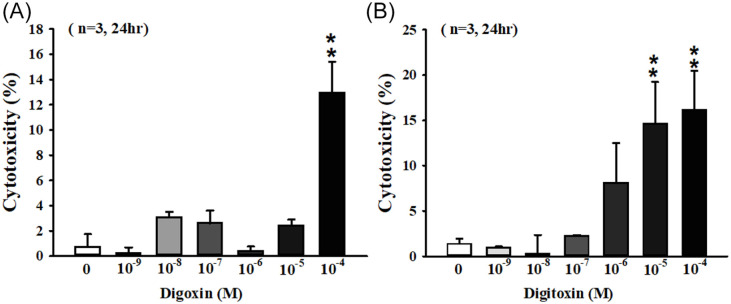
Our results showed that CGs had antitumor effect. To further investigate the antitumor effect of CGs on cell cytotoxicity, SKOV-3 cells were treated with different concentrations of digoxin and digitoxin for 24 hours and the quantity of LDH were measured as the reference of cell death rate. The cytotoxicity was significantly increased about 13 folds in 10−4 M digoxin-treated group compared with control group (Figure 3A). In 10−5 and 10−4 M digitoxin-treated groups, the cytotoxicity was significantly increased 15 and 16 folds, respectively (Figure 3B).
Figure 3.
CGs induced cytotoxicity in SKOV-3 cells. Cells were incubated in 96 well plate for 24 hours. After adhesion, cells were exposed to (A) digoxin and (B) digitoxin at indicated concentrations for 24 hours. The quantity of LDH in culture medium was measured by ELISA reader. The ratio of cytotoxicity was compared with control group.
Each value represents mean ± SEM of 3 independent experiments.
**P < .01 as compared with control group.
Digoxin and Digitoxin Arrest SKOV-3 Cells at G0/G1
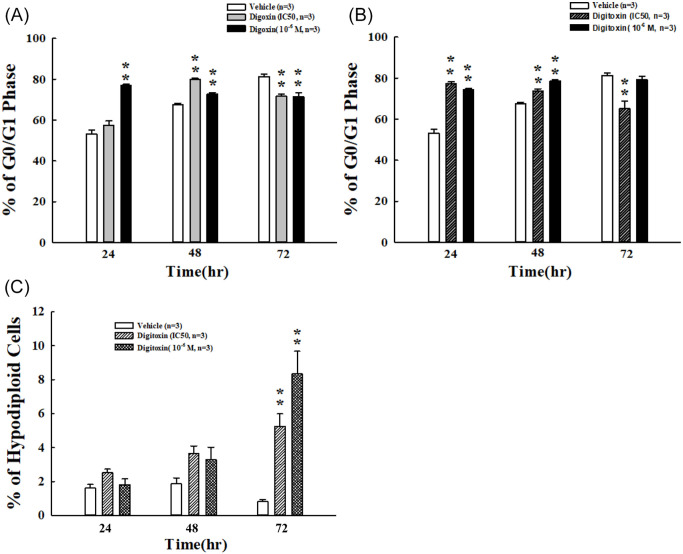
After exposure to digoxin and digitoxin with IC50 and 10−6 M for 24, 48, and 72 hours, SKOV-3 cells showed an arrest at the G0/G1 phase reaching 80% with digoxin (IC50) for 48 hours. Digoxin treatment with 10−6 M for 24 and 48 hours led the accumulation of SKOV-3 cells at the G0/G1 phase with 72% and 76%, respectively (Figure 4A). We found similar phenomenon in digitoxin-treated groups. The cell cycle arrested at the G0/G1 phase with 77% and 73% after administration of digitoxin at IC50 for 24 and 48 hours, respectively. Digitoxin treatment induced cells arrests at the G0/G1 phase with 74% and 78% at 10−6 M for 24 and 48 hours, respectively (Figure 4B). However, both digoxin- and digitoxin-induced cell cycle arrests in the G0/G1 phase were not observed after 72 hours. Therefore, we detected the distribution of hypodiploid cell population. We found that the percentage of hypodiploid was siginificantly increased by 5 and 8 folds after treatment with digitoxin at IC50 and 10−6 M for 72 hours, respectively (Figure 4C).
Figure 4.
Distribution of digoxin and digitoxin on cell cycle in SKOV-3 cells. Cells were exposed to (A) digoxin and (B) digitoxin at indicated concentrations for 24, 48, and 72 hours. Flow cytometric analysis was used to detect DNA contents. Percentages of cells in the G0/G1 phase were presented. (C) The percentage of hypodiploid cells incubated with digitoxin at indicated concentrations for 24, 48, and 72 hours were measured under the sub-G1 fraction.
Each value represents mean ± SEM of 3 independent experiments.
**P < .01 as compared with control group.
Discussion
Recent studies displayed that CGs have anti-tumor effect in several cancers,25 such as breast and prostate cancer. The core structure of CGs is similar to steroid hormones and it has been known that long-term ingestion of CGs induces higher risk of breast cancer, cervical cancer and some estrogen-sensitive cancers.26 However, this phenomenon is not significant in ovarian cancer, which is low in sensitivity to estrogen.27 CGs inhibit cell proliferation in many cancers through different mechanisms.28 For example, bufalin induced apoptosis by increasing the protein level of Fas and p53 in LNCaP cells, an androgen-dependent prostate cancer cell line, but not in DU145 and PC3 cells, the androgen-independent prostate cell lines. Both DU145 and PC3 cells are p53-mutation cell lines which is the reason why bufalin has no effect on these 2 prostate cancer cell lines.15
It has been known that digitalis has dual effects of cell proliferation and cell death in human fibroblasts and HeLa cells.7,29 Our results also showed that cell proliferation was increased slightly at 1 to 10 nM after treatment with digoxin or digitoxin in SKOV-3 cells. The cell proliferation was significantly increased when SKOV-3 cells exposed to ouabain at concentrations less than 10 nM for 24 and 48 hours. On the other hand, treatment of high concentration of CGs (eg, 100 nM) significantly decreased cell proliferation in SKOV-3 cells. Similar results have been reported in breast cancer.30 Some reports advocated a stimulatory effect of CGs at low concentrations on cell proliferation in vascular smooth muscle and prostatic smooth muscle cells.31,32 The stimulatory effect of CGs on cells growth might through activating the mitogen-activated protein kinase (MAPK) pathway. The protein level of phospho-Erk1/2 was higher after administration of 30 nM digoxin and ouabain as compared to the control.33 Taken together, we demonstrated that digoxin, digitoxin, and oubain play a dual role on cell growth in SKOV-3 cells.
Treatment with CGs at high concentration inhibited Na/K-ATPase, but increased the intracellular calcium concentration. In cancer cells, high intracellular calcium concentration induced cytochrome c release and caspase 3 activation to induce cell death.34 The anti-cancer effect of CGs in ovarian cancer was through inhibiting cell migration and angiogenesis.19 In this study, we demonstrated that CGs induced cell death and inhibited cell proliferation through cell cycle arrest at the G0/G1 phase.
Our results first demonstrated that digoxin treatment led to cell cycle arrest at the G0/G1 phase in SKOV-3 cells. It has been reported that CGs arrest the cell cycle in many cancers including glioblastoma,35 breast,22 ovarian,28 and bladder cancers.36 Digoxin treatment causes cell cycle arrest at the G0/G1 and G2/M phase for Raji and NAMALWA cells, and Burkitt’s lymphoma cell lines, respectively. These results suggested that the same administration for the same type of cancer but different cell lines might lead to cells arrested at different phases of the cell cycle.37 In ovarian cancer cells, it has been reported that bufalin and digitoxin arrest cell cycle at the G0/G1 and G2/M phases, respectively.28,38 Recently, it has been shown that digoxin and digitoxin create anti-cancer effects on hepatocellular carcinoma by inhibiting the cell viability and migration.39 It is apparent that not only reproduction-related cancers, but also the cancers of other systems are affected by digoxin and digitoxin. Taken together, these reports point out that the different drugs or chemicals and different exposure time led to a specific effect on ovarian cancer. Therefore, we investigated the distribution of cell cycle after treatment with digoxin for 24, 48, and 72 hours. We found that digoxin and digitoxin induced G0/G1 phase arrest at 24 and 48 hours. When the cell cycle was arrested at the G0/G1 phase longer than 72 hours, cell debris was significantly increased. These results indicated that digoxin- and digitoxin-induced cell death in SKOV-3 cells was positively related to exposure time.
In summary, the present study demonstrated that digoxin and digitoxin inhibited cell growth through arresting the cell cycle at the G0/G1 phase and increasing cytotoxicity (Figure 5). The results also showed the dual effect on cell growth after treatment with oubain for 24 and 48 hours. To confirm the anti-cancer effect of CGs on ovarian cancer cells, more ovarian cancer cell lines may be administered with CGs. A study of cell apoptosis measured by flow cytometry is suggested to explore the inhibitiory mechanisms of CGs on ovarian cancer cells.
Figure 5.
Summary of the effect of digoxin and digitoxin on SKOV-3 cells. Digoxin and digitoxin treatment inhibited the ability of colonies formation and cell proliferation. The cytotoxicity was increased after administration with digoxin or digitoxin for 24 hours. CGs led to cell cycle arrest at G0/G1 phase.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Paulus S. Wang  https://orcid.org/0000-0003-0241-6440
https://orcid.org/0000-0003-0241-6440
References
- 1. Benhadjeba S, Edjekouane L, Sauve K, Carmona E, Tremblay A. Feedback control of the CXCR7/CXCL11 chemokine axis by estrogen receptor alpha in ovarian cancer. Mol Oncol. 2018;12:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo Y, Li B, Yan X, et al. Bisphenol A and polychlorinated biphenyls enhance the cancer stem cell properties of human ovarian cancer cells by activating the WNT signaling pathway. Chemosphere. 2020;246:125775. [DOI] [PubMed] [Google Scholar]
- 3. Jeon SY, Hwang KA, Choi KC. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J Steroid Biochem Mol Biol. 2016;158:1-8. [DOI] [PubMed] [Google Scholar]
- 4. Cunat S, Rabenoelina F, Daures JP, et al. Aromatase expression in ovarian epithelial cancers. J Steroid Biochem Mol Biol. 2005;93:15-24. [DOI] [PubMed] [Google Scholar]
- 5. Dey P, Barros RP, Warner M, Strom A, Gustafsson JA. Insight into the mechanisms of action of estrogen receptor beta in the breast, prostate, colon, and CNS. J Mol Endocrinol. 2013;51:T61-T74. [DOI] [PubMed] [Google Scholar]
- 6. Andersen CL, Sikora MJ, Boisen MM, et al. Active estrogen receptor-alpha signaling in ovarian cancer models and clinical specimens. Clin Cancer Res. 2017;23:3802-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorp RH, Watson TR. A survey of the occurrence of cardio-active constituents in plants growing wild in Australia. I. families apocynaceae and asclepiadaceae. Aust J Exp Biol Med Sci. 1953;31:529-532. [DOI] [PubMed] [Google Scholar]
- 8. Godfraind T. The biphasic action of cardiac glycosides on the Na+, K+-pump and its relevance in the treatment of heart failure. Eur Heart J. 1982;3(suppl D):53-57. [PubMed] [Google Scholar]
- 9. Wang SW, Chiao YC, Tsai SC, et al. Inhibition of bufalin on pituitary and testicular function in rats. J Pharmacol Exp Ther. 1997;283:528-532. [PubMed] [Google Scholar]
- 10. Lin H, Wang SW, Tsai SC, et al. Inhibitory effect of digoxin on testosterone secretion through mechanisms involving decreases of cyclic AMP production and cytochrome P450scc activity in rat testicular interstitial cells. Br J Pharmacol. 1998;125:1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang SW, Lin H, Hwang JJ, Wang PS. Inhibition of testosterone secretion by digitoxin in rat testicular interstitial cells. J Cell Biochem. 1999;74:74-80. [PubMed] [Google Scholar]
- 12. Chen JJ, Wang PS, Chien EJ, Wang SW. Direct inhibitory effect of digitalis on progesterone release from rat granulosa cells. Br J Pharmacol. 2001;132:1761-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boff L, Persich L, Brambila P, et al. Investigation of the cytotoxic activity of two novel digitoxigenin analogues on H460 lung cancer cells. Anticancer Drugs. 2020;31:452-462. [DOI] [PubMed] [Google Scholar]
- 14. Lin H, Juang JL, Wang PS. Involvement of Cdk5/p25 in digoxin-triggered prostate cancer cell apoptosis. J Biol Chem. 2004;279:29302-29307. [DOI] [PubMed] [Google Scholar]
- 15. Yu CH, Kan SF, Pu HF, Jea Chien E, Wang PS. Apoptotic signaling in bufalin- and cinobufagin-treated androgen-dependent and -independent human prostate cancer cells. Cancer Sci. 2008;99:2467-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeh JY, Huang WJ, Kan SF, Wang PS. Inhibitory effects of digitalis on the proliferation of androgen dependent and independent prostate cancer cells. J Urol. 2001;166:1937-1942. [PubMed] [Google Scholar]
- 17. Yeh JY, Huang WJ, Kan SF, Wang PS. Effects of bufalin and cinobufagin on the proliferation of androgen dependent and independent prostate cancer cells. Prostate. 2003;54:112-124. [DOI] [PubMed] [Google Scholar]
- 18. Bielawski K, Winnicka K, Bielawska A. Inhibition of DNA topoisomerases I and II, and growth inhibition of breast cancer MCF-7 cells by ouabain, digoxin and proscillaridin A. Biol Pharm Bull. 2006;29:1493-1497. [DOI] [PubMed] [Google Scholar]
- 19. Trenti A, Boscaro C, Tedesco S, et al. Effects of digitoxin on cell migration in ovarian cancer inflammatory microenvironment. Biochem Pharmacol. 2018;154:414-423. [DOI] [PubMed] [Google Scholar]
- 20. Pessoa MTC, Valadares JMM, Rocha SC, et al. 21-Benzylidene digoxin decreases proliferation by inhibiting the EGFR/ERK signaling pathway and induces apoptosis in HeLa cells. Steroids. 2020;155:108551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren Y, Ribas HT, Heath K, et al. Na+/K+-ATPase-targeted cytotoxicity of (+)-Digoxin and several semisynthetic derivatives. J Nat Prod. 2020;83:638-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kulkarni YM, Yakisich JS, Azad N, et al. Anti-tumorigenic effects of a novel digitoxin derivative on both estrogen receptor-positive and triple-negative breast cancer cells. Tumour Biol. 2017;39:1010428317705331. [DOI] [PubMed] [Google Scholar]
- 23. Mungenast F, Thalhammer T. Estrogen biosynthesis and action in ovarian cancer. Front Endocrinol (Lausanne). 2014; 5:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steel RGD, Torrie JH. Principles and Procedures of Statistics. 2nd ed. McGraw-Hill Book Company, Inc.; 1980. [Google Scholar]
- 25. Ren Y, Chen WL, Lantvit DD, et al. Cardiac glycoside constituents of streblus asper with potential antineoplastic activity. J Nat Prod. 2017;80:648-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biggar RJ, Wohlfahrt J, Melbye M. Digoxin use and the risk of cancers of the corpus uteri, ovary and cervix. Int J Cancer. 2012;131:716-721. [DOI] [PubMed] [Google Scholar]
- 27. Biggar RJ. Molecular pathways: digoxin use and estrogen-sensitive cancers–risks and possible therapeutic implications. Clin Cancer Res. 2012;18:2133-2137. [DOI] [PubMed] [Google Scholar]
- 28. Chen WL, Ren Y, Ren J, et al. (+)-Strebloside-induced cytotoxicity in ovarian cancer cells is mediated through cardiac glycoside signaling networks. J Nat Prod. 2017;80:659-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramirez-Ortega M, Maldonado-Lagunas V, Melendez-Zajgla J, et al. Proliferation and apoptosis of HeLa cells induced by in vitro stimulation with digitalis. Eur J Pharmacol. 2006; 534: 71-76. [DOI] [PubMed] [Google Scholar]
- 30. Biggar RJ, Wohlfahrt J, Oudin A, Hjuler T, Melbye M. Digoxin use and the risk of breast cancer in women. J Clin Oncol. 2011;29:2165-2170. [DOI] [PubMed] [Google Scholar]
- 31. Abramowitz J, Dai C, Hirschi KK, et al. Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell line, A7r5. Circulation. 2003;108:3048-3053. [DOI] [PubMed] [Google Scholar]
- 32. Chueh SC, Guh JH, Chen J, Lai MK, Teng CM. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol. 2001;166:347-353. [PubMed] [Google Scholar]
- 33. Winnicka K, Bielawski K, Bielawska A, Miltyk W. Dual effects of ouabain, digoxin and proscillaridin A on the regulation of apoptosis in human fibroblasts. Nat Prod Res. 2010; 24:274-285. [DOI] [PubMed] [Google Scholar]
- 34. Varghese E, Samuel SM, Sadiq Z, et al. Anti-cancer agents in proliferation and cell death: the calcium connection. Int J Mol Sci. 2019;20(12):3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li S, Dai Z, Yang D, et al. Targeting β2 subunit of Na+)/K+-ATPase induces glioblastoma cell apoptosis through elevation of intracellular Ca2. Am J Cancer Res. 2019;9:1293-1308. [PMC free article] [PubMed] [Google Scholar]
- 36. Hong SH, Choi YH. Bufalin induces apoptosis through activation of both the intrinsic and extrinsic pathways in human bladder cancer cells. Oncol Rep. 2012;27:114-120. [DOI] [PubMed] [Google Scholar]
- 37. Wang T, Xu P, Wang F, et al. Effects of digoxin on cell cycle, apoptosis and NF-кB pathway in Burkitt’s lymphoma cells and animal model. Leuk Lymphoma. 2017;58:1673-1685. [DOI] [PubMed] [Google Scholar]
- 38. Takai N, Ueda T, Nishida M, Nasu K, Narahara H. Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int J Mol Med. 2008;21:637-643. [PubMed] [Google Scholar]
- 39. Huang WJ, Hsu HP, Jeng YM. Effects of digoxin and digitoxin on cell viability and migration in hepatocellular carcinoma. Adapt Med. 2020;12: 89-94. [Google Scholar]