Abstract
Introduction
Myelodysplastic syndromes (MDS) are a group of heterogeneous bone marrow clonal diseases characterized by the abnormal differentiation and development of bone marrow cells. Src homology region 2 domain-containing phosphatase (SHP)-1 is an important tumor suppressor gene that regulates the signal transducer and activator of transcription (STAT) pathway.
Methods
Survival analysis was performed to evaluate the function of decitabine (5-Aza) in treating MDS patients with and without SHP-1 methylation. The effects of 5-Aza treatment on SHP-1 expression and methylation and STAT3 phosphorylation were investigated in MDS cells by methylation-specific PCR, reverse transcription PCR, and western blotting. Cell viability and apoptosis were similarly evaluated by MTT assay and flow cytometry.
Results
High-risk MDS patients showed significant SHP-1 hypermethylation compared with low-risk patients, and patients with no SHP-1 methylation had longer overall survival. SHP-1 expression was significantly increased at mRNA and protein levels following 5-Aza treatment, while the phosphorylation of STAT3 protein was significantly decreased. Apoptosis increased significantly in MDS cells treated with higher doses of 5-Aza while cell viability decreased significantly.
Conclusion
SHP-1 hypermethylation was associated with poor prognosis in HR patients with MDS, suggesting it could be used as a prognostic indicator.
Keywords: Myelodysplastic syndromes, Src homology region 2 domain-containing phosphatase-1 methylation, signal transducer and activator of transcription 3 phosphorylation, decitabine treatment, skm-1 cells, prognostic indicator
Introduction
Myelodysplastic syndromes (MDS) are a group of heterogeneous myeloid clonal diseases originating from hematopoietic stem cells, which are characterized by the abnormal differentiation and development of myeloid cells. Patients present with ineffective hematopoiesis, refractory cytopenia, hematopoietic failure, and an increased risk of developing acute myeloid leukemia (AML). According to the International Prognostic Scoring System (IPSS), MDS patients can be divided into four groups: low-risk (LR), intermediate (Int)-1-risk, Int-2-risk, or high-risk (HR).1 However, the molecular differences between higher and lower risk groups are unclear.
It was previously demonstrated that the mRNA expression of Src homology region 2 (SH2) domain-containing phosphatase-1 (SHP-1) was negatively correlated with an increased risk of transformation, and that its expression was significantly lower in HR MDS patients than LR patients (P < 0.01).2SHP-1 is an important tumor suppressor that regulates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway. Aberrant SHP-1 expression increases STAT protein phosphorylation which promotes cell proliferation and represses apoptosis.3–5 The most likely cause for the observed decrease in SHP-1 expression in leukemia and several hematopoietic cell lines is hypermethylation in the promoter region.6,7 However, this mechanism is yet to be validated in patients with MDS.
Decitabine [also known as 5-aza-2′-deoxycytidine (5-Aza)], a type of hypomethylating agent, has been widely used to treat HR patients with MDS. It incorporates itself into DNA molecules during synthesis and irreversibly binds to DNA methyltransferases to promote several cellular processes such as apoptosis.8–10 However, treatment outcomes differ markedly between patients. SHP-1 expression was previously reported to be useful as an indicator to predict the efficacy of vincristine, doxorubicin, and dexamethasone treatment in patients with multiple myeloma; the group with high mRNA levels had a significantly improved drug response compared with the low mRNA level group (P < 0.01).11 However, it has not been determined whether SHP-1 expression or its methylation status could be used as a prognostic marker for decitabine treatment in HR patients with MDS.
In the current study, SHP-1 methylation in LR patients was compared with that in HR patients to identify the cause of decreased gene expression. SHP-1 methylation was also investigated to determine whether it affected decitabine treatment outcome, and its potential value as a prognostic indicator for HR patients with MDS was evaluated. Three different concentrations of decitabine were used to treat the MDS skm-1 cell line, and variations in SHP-1 mRNA and protein expression were assessed to determine its effect on STAT3 protein phosphorylation and to investigate why SHP-1 methylation influences the efficacy of decitabine treatment.
Materials and methods
Patient information
The current study was approved by the Ethics Committee of Tianjin Medical University (Tianjin, China) and was conducted according to the protocol of the Ethics Committee for the Conduct of Human Research. Ninety-three patients with MDS and 20 healthy controls who attended Tianjin Medical University General Hospital were enrolled between 2013 and 2018. All patients provided written informed consent. MDS was diagnosed based on World Health Organization classification, including cell morphology analysis, cytogenetic evaluation, and cytochemistry analysis. Of the 93 cases, 22 had refractory anemia (RA), 37 had RA with excess blasts, 19 had ringed sideroblasts, and 15 had refractory cytopenia with multiple dysplasia. Patients with IPSS scores of <1.5 were grouped into the LR group, and patients with scores of ≥1.5 were classified as the HR group. Patient characteristics are presented in Table 1.
Table 1.
Clinical characteristics of patients with MDS and control subjects.
| Characteristic | Low-risk group (n = 48) | High-risk group (n = 45) | Control (n = 20) |
|---|---|---|---|
| Sex, male/female, n | 28/20 | 26/19 | 12/8 |
| Median age (range), years | 47 (11–75) | 45 (12–77) | 45 (14–72) |
| Median WBC (range), count ×109 cells/L | 3.4 (2.2–9.8) | 2.8 (1.6–13.9) | 5.9 (4.2–10.0) |
| Median PLT count (range), ×109 cells/L | 103.5 (16–351) | 57 (6–155) | 244 (112–308) |
| Median HB level (range) g/L | 80 (45–120) | 77.5 (40–115) | 112 (105–127) |
| Median IPSS score (range) | 0.5 (0–1.0) | 2.5 (1.5–3.0) | N/A |
| WHO classification | N/A | ||
| RA | 23 | 0 | |
| RARS | 17 | 0 | |
| RCMD | 8 | 7 | |
| RAEB | 0 | 38 |
MDS, myelodysplastic syndromes; WBC, white blood cell; PLT, platelet; HB, hemoglobin; IPSS, International Prognostic Scoring System; N/A, not applicable; WHO, World Health Organization; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RCMD, refractory cytopenia with multiple dysplasia; RAEB, refractory anemia with excess blasts.
Measurement of decitabine efficacy
The 45 patients in the HR group were separated into two sub-groups on the basis of their SHP-1 promoter methylation status. Methylation-specific polymerase chain reaction (MSP) was used to identify methylation as described below. Twenty-nine patients were placed in the methylated (Me) group and the remaining 16 were categorized into the unmethylated (UnMe) group. All patients were treated with 20 mg/m2 decitabine once daily for 5 consecutive days via intravenous injection. The conditions of all patients were determined following each course of treatment, and objective response rates (ORR) were calculated and compared between the two groups.
Survival analysis in patients in the HR group
A 3-year survival curve was created using Kaplan–Meier analysis to assess whether SHP-1 methylation could be used as a prognostic indicator for HR patients with MDS. The primary endpoint was the development of AML or mortality.
Cell culture
The MDS skm-1 cell line was purchased from the Cell Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were maintained in RPMI-1640 medium with 10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and incubated at 37°C with 5% CO2. Cells were only used for experiments when they had reached the exponential growth phase.
5-Aza treatment
5-Aza was purchased from Sigma-Aldrich Merck KGaA (Darmstadt, Germany) and three concentrations (0.5, 2, and 5 μmol/L) were prepared using deionized water. After 1 day of direct treatment with 5-Aza, 5 × 106 skm-1 cells were collected and washed prior to SHP-1, STAT3, and phosphorylated STAT3 (p-STAT3) expression analysis, MTT colorimetric assay, and flow cytometry analysis as described below. Untreated cells were used as controls.
SHP-1 promoter methylation analysis
The genomic DNA of all 113 enrolled individuals was extracted from blood samples using a DNeasy Blood & Tissue kit (Qiagen GmbH, Hilden, Germany), then treated using an EpiTect Bisulfite kit (Qiagen GmbH) according to the manufacturer’s protocol. The SHP-1 methylation status was determined using MSP with the following primers amplifying the methylated allele: forward, 5′-GAACGTTATTATAGTATAGCGTTC-3′ and reverse, 5′-TCACGCATACGAACCCAAACG-3′. Primers for unmethylated allele amplification were: forward, 5′-TCACGCATACGAACCCAAACG-3′ and reverse, 5′-TTCACACATACAAACCCAAACAAT-3′.12 PCR conditions were an initial denaturation at 95°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 45 s, and primer extension at 72°C for 1 minute, followed by a final extension at 72°C for 10 minutes. PCR products were separated by electrophoresis on a 5% agarose gel with ethidium bromide staining, and were 158 bp for both methylated and unmethylated products. .
Real-time reverse transcription quantitative PCR (RT-qPCR)
Total RNA of the skm-1 cell line was extracted using TRIzol® RNA Isolation reagent (Invitrogen, Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. RNA was then reverse-transcribed using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) and the cDNA was amplified three times using a three-step method with the CFX96 Touch™ Real-Time PCR Detection system (Bio-Rad Laboratories Inc., Hercules, CA, USA) and SYBR® Green qPCR Master mix. qPCR conditions were a hot-start DNA polymerase activation at 95°C for 5 minutes, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 56°C for 30 s, and extension at 72°C for 1 minute, with a final extension at 72°C for 10 minutes. Glyceraldehyde 3-phosphate dehydrogenase (GADPH) was amplified as the control and the Cq readings of SHP-1 amplification were normalized to those of GADPH to calculate the ΔCq. Finally, the relative expression of SHP-1 was obtained by relating ΔCq to that of the control gene using the 2-ΔΔCq method. The primers used for this analysis were as follows: SHP-1 forward, 5′-GCGGCAGTACTATGC-3′ and reverse, 5′-CAGTTCCAACACTCGGTTCTCA-3′; and GADPH forward, 5′-CGGGAAGCTTGTCATCAATGG-3′ and reverse, 5′-GGCAGTGATGGCATGGACTG-3′.
Western blot analysis
Antibodies against SHP-1, phosphorylated (p)-STAT3, STAT3, and GAPDH were purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA) and western blot analysis was performed as previously described.12 In brief, cells were collected and lysed with sodium dodecyl sulfate (SDS) lysis buffer, and centrifuged for 15 minutes at 12,000 × g. Following denaturation, proteins were separated by 8% to 12% SDS-pulsed field gel electrophoresis and electrotransferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked overnight with 10% bovine serum albumin at 4°C, then incubated with anti-SHP-1, anti-STAT3, anti-p-STAT3, and anti-GAPDH antibodies at 1:1,000 dilution for 40 minutes at room temperature. Horseradish peroxidase-labeled immunoglobulin secondary antibody (Santa Cruz Biotechnology, Inc.) was diluted 1:2,000 and incubated with the membranes for 30 minutes. Enhanced Chemiluminescence Western Blotting Detection reagent (GE Healthcare, Chicago, IL, USA) was used for visualization and the membranes were exposed to an X-ray film (Kodak, Rochester, NY, USA). ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for the densitometric analysis of SHP-1, STAT3, and p-STAT3 protein expression, which was normalized against that of GAPDH protein. Three biological repeats were performed for all samples.
Evaluation of apoptosis using flow cytometry
The apoptosis rate of treated skm-1 cells was evaluated using an annexin V/propidium iodide (PI) apoptosis kit from MultiSciences Biotech (Zhejiang, China) according to the manufacturer’s protocol. For each drug concentration, 1 to 3 × 106 cells were used on the BD FACSCanto II platform (BD Biosciences, San Jose, CA, USA). Intact cells were marked as annexin V–/PI–, whereas early apoptotic cells and late apoptotic/necrotic cells were marked as annexin V+/PI– and annexin V+/PI+, respectively.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay
Skm-1 cells (5 × 104) were plated in 96-well plates for 24 hours and treated with different concentrations of 5-Aza for an additional 24 hours. Cell viability was assessed using the MTT assay. In brief, 0.5 mg MTT was added to each well 4 hours before the end of 5-Aza treatment. Following removal of the supernatant, 100 μL of dimethyl sulfoxide was added to each well and incubated for 10 minutes. The optical density of each well was determined at 490 nm using a Polarstar Optima microplate reader (BMG Labtech GmbH, Ortenberg, Germany).
Statistical analysis
Data were assessed for normal distribution before being compared by two-tailed Student’s t-tests. The χ2 test was used to compare the decitabine response rate between HR and LR patients. The relationship between methylation levels and STAT3 protein phosphorylation was analyzed by Pearson’s correlation coefficient. One-way analysis of variance with the least significant difference post-hoc test was performed on the data of skm-1 cell model-based experiments following the homogeneity of variance test and linear correlation analysis, which assessed the correlation between STAT3 protein phosphorylation and SHP-1 methylation. Statistical analysis was performed using SPSS Software (version 19.0; IBM Corp., Armonk, NY, USA) and P < 0.05 was considered to indicate a statistically significant difference. Survival analysis was performed using the Kaplan–Meier method and hazard ratios. The 95% confidence interval (CI) and P-values were also calculated to assess differences between two groups using GraphPad Prism Software (version 7.0; GraphPad Software, Inc., La Jolla, CA, USA).
Results
SHP-1 promoter methylation status of HR and LR patients with MDS
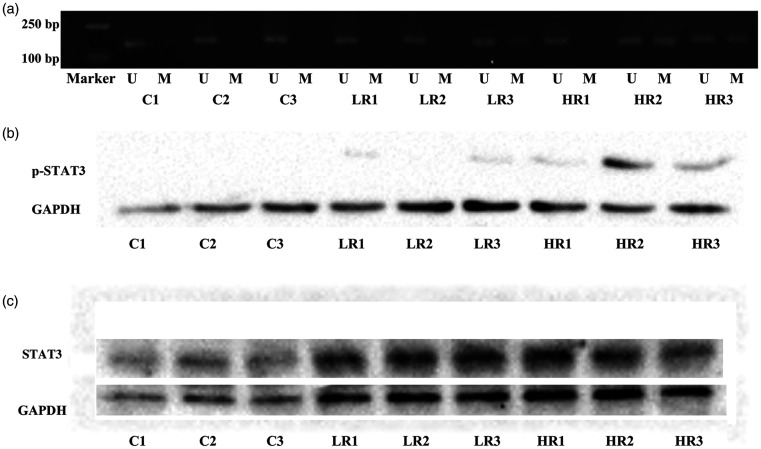
MSP revealed that the SHP-1 promoter region was methylated in 22.9% of samples (11/48) in the LR group compared with 64.4% in the HR group (29/45). The control group exhibited no methylation (P < 0.001; Figure 1a). Western blot analysis of p-STAT3 protein expression also revealed the same significant differences (P < 0.001), with 30/45 (66.7%) of patients in the HR group having detectable p-STAT3 protein, compared with 10/48 in the LR group (20.8%; Figure 1b). Correlation analysis showed that the phosphorylation level of STAT3 protein was positively associated with SHP-1 methylation (r = 0.57; P < 0.001), whereas STAT3 protein expression was indistinguishable between samples in different groups (Figure 1c). Pearson’s correlation coefficient was calculated based on the entire cohort of 93 cases; Pearson’s R index was >0.5, indicating a significant difference. SHP-1 gene expression was not measured as samples had been stored for >5 years, making the quantity and quality of the extracted total RNA unsuitable for RT-qPCR analysis.
Figure 1.
(a) Methylation-specific polymerase chain reaction of the methylation status in the SHP-1 promoter region in LR, HR, and healthy control samples. (b) Western blot analysis of p-STAT3 protein expression in LR, HR, and control groups. (c) Western blot analysis of STAT3 protein expression in LR, HR, and control groups. Lanes LR1–LR3, LR patient samples; lanes HR1–HR3, HR patient samples; lanes C1–C3, healthy control samples.
U, unmethylated; M, methylated; SHP-1, Src homology region 2 domain-containing phosphatase-1; LR, low-risk; HR, high-risk; p-STAT3, phosphorylated signal transducer and activator of transcription 3; STAT3, signal transducer and activator of transcription 3.
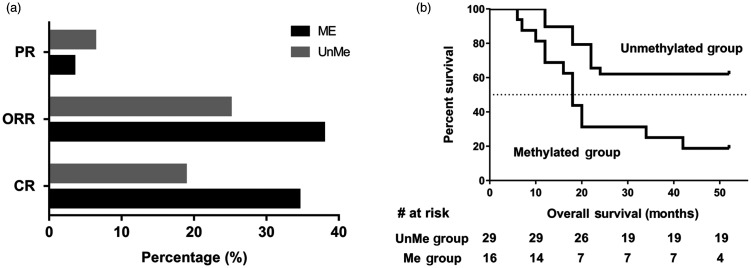
MDS HR patients with SHP-1 methylation showed significantly improved decitabine response rates compared with those without methylation. In the Me group of HR patients with MDS, 34.5% (10/29) had a complete response (CR) following 1 to 6 courses of treatment and 3.4% (1/29) had a partial response (PR). The ORR for this group was therefore 37.9% (CR + PR). By comparison, the ORR was only 25.0% (4/16) in the UnMe group, with 18.8% having a CR (3/16) and 6.3% a PR (1/16). The significant difference between groups (P < 0.05; Figure 2a) indicates that a lack of SHP-1 methylation is one factor that impaired the effect of decitabine in MDS patients.
Figure 2.
Comparison of decitabine treatment efficacy and survival analysis between methylated and unmethylated groups. (a) CR, PR, and ORR of patients. (b) Kaplan–Meier analysis of overall survival for 45 HR patients with MDS. Continuous lines represent the event-free fraction; the broken line represents the 50% survival mark.
ME, methylated group; UnMe, unmethylated group; CR, complete response; PR, partial response; ORR, objective response rate; HR, high-risk; MDS, myelodysplastic syndromes.
Kaplan–Meier analysis revealed that the time taken to develop AML or mortality was significantly shorter in the Me group compared with the UnMe group. The median overall survival time for the Me group was 18 months compared with the UnMe group, which had not yet reached 50% survival. The hazard ratio for overall survival was 4.85 (Me/UnMe) with a 95% CI of 1.332 to 8.195 and P < 0.01 (Figure 2b).
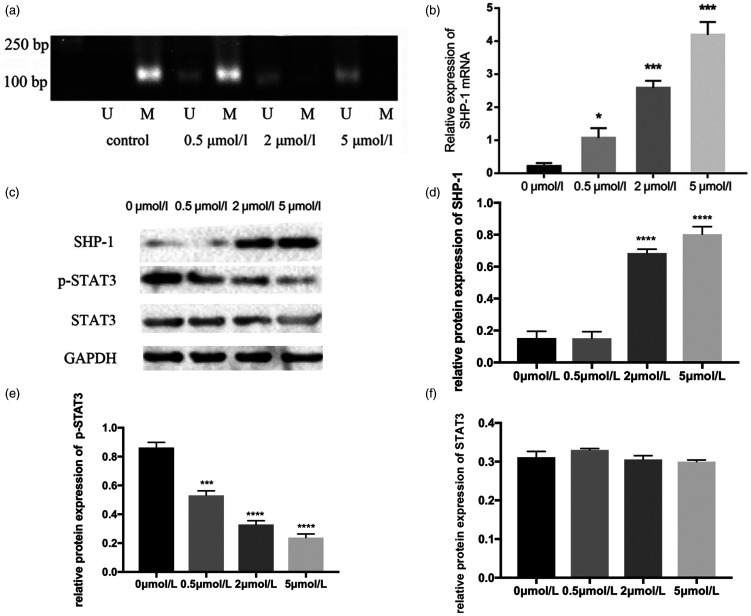
Effects of 5-Aza on SHP-1 and p-STAT3 expression in MDS SKM-1 cells
SHP-1 methylation in skm-1 cells decreased in a dose-dependent manner following treatment with 0.5, 2, and 5 µmol/L 5-Aza (Figure 3a). No SHP-1 mRNA expression was detected in the 5-Aza-untreated control group, but its expression was positively associated with the 5-Aza concentration (Figure 3b). SHP-1 expression in 0.5-, 2-, and 5 µmol/L 5-Aza-treated cells was significantly higher than that of untreated cells (P < 0.05, P < 0.001, and P < 0.001, respectively). Additionally, significant differences were detected in 2- and 5 µmol/L 5-Aza-treated cells compared with 0.5 µmol/L 5-Aza-treated cells (P < 0.05), and between 2- and 5 µmol/L 5-Aza-treated cells (P < 0.05). These data indicate that 5-Aza markedly influences SHP-1 re-expression, possibly by regulating the methylation status in the promoter region.
Figure 3.
SHP-1 methylation and SHP-1, p-STAT3, and STAT3 expression in 5-Aza-treated skm-1 cells. (a) Changes in SHP-1 methylation in skm-1 cells treated with 0.5, 2, and 5 µmol/L 5-Aza. Control cells were untreated. (b) Relative expression levels of SHP-1 mRNA as assessed by real-time reverse transcription quantitative PCR. Horizontal lines represent the relative expression of SHP-1 in various 5-Aza concentration groups. Significant differences in SHP-1 mRNA expression in 2 and 5 µmol/L 5-Aza-treated cells are seen compared with 0.5 µmol/L 5-Aza-treated cells (P < 0.05 and P < 0.01, respectively). (c) Protein expression of SHP-1, p-STAT3, and STAT3 in three 5-Aza concentration groups and the untreated group. (d) Relative expression of SHP-1 protein normalized to GAPDH. SHP-1 expression is positively correlated with 5-Aza concentration. (e) Relative expression of p-STAT3 protein normalized to GAPDH. p-STAT3 expression is positively correlated with 5-Aza concentration. (f) Relative expression of STAT3 protein normalized to GAPDH. STAT3 expression is negatively correlated with 5-Aza concentration. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
U, unmethylated; M, methylated; SHP-1, Src homology region 2 domain-containing phosphatase-1; p-STAT3, phosphorylated signal transducer and activator of transcription 3; STAT3, signal transducer and activator of transcription; 5-Aza, 5-aza-2′-deoxycytidine.
Western blot analysis produced similar results. No SHP-1 protein expression was detected in untreated skm-1 cells, but it was re-expressed following the addition of 5-Aza to culture medium (Figure 3c and d). SHP-1 expression gradually increased in line with the drug concentration, with significant differences observed between the different concentration groups (all P < 0.05). However, the opposite was observed for p-STAT3 expression. STAT3 protein levels were undetectable in cells across all 5-Aza-treated groups (Figure 3c and f), while untreated cells had the highest p-STAT3 expression, and phosphorylation decreased significantly with rising SHP-1 expression (P < 0.001, Figure 3c and e). This suggested that STAT3 phosphorylation was negatively regulated by SHP-1 protein in MDS skm-1 cells.
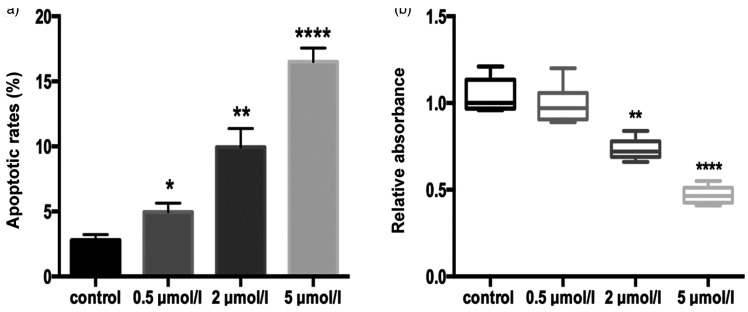
Biological response of skm-1 cells following 5-Aza treatment
Cell viability and apoptosis were measured using an MTT colorimetric assay and flow cytometric analysis, respectively. Flow cytometry revealed the lowest apoptosis rate of 3.2% in the control group, which was significantly different from the 4.54% seen in the 0.5 µmol/L 5-Aza group (P < 0.05). In cells treated with 2 and 5 µmol/L 5-Aza, apoptosis increased significantly to 9.31% and 16.58%, respectively (P < 0.01 and P < 0.0001, Figure 4a). In comparison, cell viability was shown to be negatively associated with drug concentration (Figure 4b). The difference in relative absorbance between the 0.5 µmol/L group and control group was not substantial (median absorbance values 1.04 vs. 0.96, respectively). However, significant differences were detected between 2 µmol/L and 5 µmol/L groups and the control (P < 0.01 and P < 0.0001, respectively).
Figure 4.
Cell viability and apoptosis rate in 5-Aza-treated skm-1 cells. (a) Apoptosis rate as measured by flow cytometry in control, 0.5, 2, and 5 µmol/L groups. Significant differences are seen in the apoptosis rate of 2 and 5 µmol/L 5-Aza-treated cells compared with the control (P < 0.01 and P < 0.0001, respectively). (b) Cell viability of skm-1-treated cells assessed via the MTT assay. Significant differences are seen in the relative absorbance of 2 and 5 µmol/L 5-Aza-treated cells compared with the control (P < 0.01 and P < 0.0001, respectively). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
5-Aza, 5-aza-2′-deoxycytidine; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Discussion
MDS consist of a number of types of cancer involving the abnormal differentiation and development of myeloid cells in the bone marrow. Approximately 30% of patients have a high risk of developing AML. Each year, MDS affects 7 in every 100,000 people, and is newly acquired in 4 in every 100,000.13 The prognosis of MDS is poor, and the survival rate following diagnosis is typically 2.5 years. Aberrant DNA methylation has been intensively studied in MDS14,15 and the hypomethylating agent decitabine used regularly outside of chemotherapy. However, although this has demonstrated promising effects compared with chemotherapy, the CR rate in several clinical trials was typically <30%.16–18
Previous studies have demonstrated that the anti-oncogene SHP-1 is downregulated in several hematological diseases, such as acute or chronic leukemia, lymphoma, multiple myeloma, and MDS.2,6,19–21 In the current study, reduced expression of SHP-1 was caused by abnormal methylation in its promoter region, and the proportion of patients with methylated SHP-1 differed among LR and HR groups. This indicates that SHP-1 methylation could be a factor that increases the risk of mortality or of MDS developing into AML. This was corroborated when patients were divided into subgroups depending on the presence or absence of methylation, with an observed 18-month overall survival for the SHP-1 methylated group. Therefore, the SHP-1 methylation status should be considered as a potential prognostic factor for HR patients with MDS. It can also be used as an indicator to predict the efficacy of decitabine treatment. Although the CR rate in the present study had not reached optimal results (trial ID-03-0180; CR 39%) compared with previously reported clinical trials,22 the methylated group had a better than average CR value of 34.5%16–18 compared with the unmethylated group with a CR of 18.8%. As shown in Figure 2, SHP-1 hypermethylation patients in the HR group had a worse prognosis than controls even though they had higher decitabine sensitivity.
To investigate the efficiency of removing DNA methylation with decitabine and the effect this had on the downstream protein regulation of SHP-1, the MDS cell line skm-1 was used as a cell model. Following only 1 day of treatment, 0.5 µmol/L 5-Aza-treated skm-1 cells showed a significant increase in SHP-1 expression at mRNA and protein levels, which increased with drug dose. This demonstrated that decitabine use is both efficient and dose-dependent for SHP-1 methylation removal.
SHP-1 has been shown to be associated with regulation of the JAK/STAT pathway in hematopoietic malignancies and other types of cancer. The two SH2 domains at the N-terminus of the protein together with the phosphatase domain allows for the negative regulation of JAKs via dephosphorylation.3,23 STAT3 hyperphosphorylation was reported to downregulate SHP-1 expression in several cancer types, including multiple myeloma and lymphoma.24,25 However, little is known about its role in MDS. The present study revealed that the level of p-STAT3 was closely correlated with the expression of SHP-1. With increased expression of SHP-1 protein, phosphorylated STAT3 levels were significantly decreased in decitabine-treated (2 and 5 µmol/L) cells, suggesting that SHP-1 protein also dephosphorylates STAT3 in MDS skm-1 cells, similar to other types of cancer.26,27
To investigate the effect of STAT3 dephosphorylation on the skm-1 cell biological response, possible changes in cell viability and apoptosis were assessed. A higher concentration of 5-Aza was associated with decreased cell viability and increased apoptosis, which is consistent with other reports.12,28 This suggests that one possible mechanism underlying the decitabine response in patients with MDS is the regulation of STAT3 phosphorylation via SHP-1 protein. Once decitabine enters an abnormal cell in patients with MDS, it reduces methylation at the SHP-1 promoter region to initiate SHP-1 protein expression. Subsequently, SHP-1 regulates the JAK/STAT signaling pathway by dephosphorylating p-STAT3 and p-JAKs. In the presence of sufficient 5-Aza, abnormal cells begin to lose viability and increase their apoptosis rate. However, because 5-Aza is not a specific DNA methyltransferase inhibitor, its treatment may promote the expression of other genes. Thus, additional influences that may be important for STAT3 signaling pathways and subsequent biological responses caused by the increased expression of different genes cannot be ruled out. Further investigation is required to evaluate the precise role of SHP-1 in the STAT3 pathway by SHP-1 knockdown in 5-Aza-treated skm-1 cells.
In conclusion, the present study indicated that methylation of the SHP-1 promoter region is associated with a higher risk of developing AML in patients with MDS, and that it could be used to classify MDS prognosis. It also demonstrated that decitabine affected SHP-1 re-expression and showed that patients with methylated SHP-1 had an improved CR rate compared with the unmethylated group. MDS skm-1 cells were used as a model to demonstrate that 5-Aza could remove SHP-1 methylation and increase SHP-1 protein expression in a dose-dependent manner. The STAT3 phosphorylation level was shown to be negatively correlated with SHP-1 expression, and cell viability and apoptosis rates were significantly altered in skm-1 cells treated with higher 5-Aza concentrations.
Acknowledgements
We would like to acknowledge Affiliated Hospital of Hebei University for partial funding for this research,and expression of gratitude to Ms. Sapna Aswal.
Footnotes
Ethics approval and consent to participate: The present study was approved by the Ethics Committee of the Tianjin Medical University (Tianjin, China). All patients included in the study provided written informed consent.
Availability of data and materials: The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The present study was supported by the National Natural Science Foundation of China (No. 81570111) and Program of Hebei Health Committee (No. 20160041).
ORCID iD: Ying Han https://orcid.org/0000-0001-7583-3697
References
- 1.Greenberg P, Cox C, LeBeau MM, et al . International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89: 2079–2088. [PubMed] [Google Scholar]
- 2.Han Y, Hua LM, Fan LX, et al. Expression and interaction of SHP-1 and c-kit genes in patients with myelodysplastic syndrome. Hebei Medical Journal 2012; 34: 226–227. [Google Scholar]
- 3.Wu C, Sun M, Liu L, et al. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene 2003; 306: 1–12. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg J. . Stat proteins and oncogenesis. J Clin Invest 2002; 109: 1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman T, Garcia R, Turkson J, et al. STATs in oncogenesis. Oncogene 2000; 19: 2474–2488. [DOI] [PubMed] [Google Scholar]
- 6.Oka T, Ouchida M, Koyama M, et al. Gene silencing of the tyrosine phosphastase SHP1 by aberrant methylation in leukemias/lymphomas. Cancer Res 2002; 62: 6390–6394. [PubMed] [Google Scholar]
- 7.Chim CS, Fung TK, Cheung WC, et al. SOCS1 and SHP1 hypermethylation in multiple myeloma: implications for epigenetic activation of the Jak/STAT pathway. Blood 2004; 103: 4630–4635. [DOI] [PubMed] [Google Scholar]
- 8.Schmelz K, Wagner M, Dorken B, et al. 5-Aza-2′-deoxycytidine induces p21WAF expression by demethylation of p73 leading to p53-independent apoptosis in myeloid leukemia. Int J Cancer 2005; 114: 683–695. [DOI] [PubMed] [Google Scholar]
- 9.Covey JM, D'Incalci M, Tilchen EJ, et al. Differences in DNA damage produced by incorporation of 5-aza-2′-deoxycytidine or 5,6-dihydro-5-azacytidine into DNA of mammalian cells. Cancer Res 1986; 46: 5511–5517. [PubMed] [Google Scholar]
- 10.Schermelleh L, Haemmer A, Spada F, et al. Dynamics of Dnmt1 interaction with the replication machinery and its role in postreplicative maintenance of DNA methylation. Nucleic Acids Res 2007; 35: 4301–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Luo JM, Yuan J, et al. Expression and significance of SHP-1 in multiple myeloma. Chinese Journal of Gerontology 2014; 34: 1207–1208. [Google Scholar]
- 12.Li Y, Yang L, Pan Y, et al. Methylation and decreased expression of SHP-1 are related to disease progression in chronic myelogenous leukemia. Oncol Rep 2014; 31: 2438–2446. [DOI] [PubMed] [Google Scholar]
- 13.Germing U, Kobbe G, Haas R, et al. Myelodysplastic syndromes: diagnosis, prognosis, and treatment. Dtsch Arztebl Int 2013; 110: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang H, Wang X, Gao L, et al. Clinical implications of the quantitative detection of ID4 gene methylation in myelodysplastic syndrome. Eur J Med Res 2015; 20: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra D, Tyagi S, Singh J, et al. Utility of 5-methylcytosine immunohistochemical staining to assess global DNA methylation and its prognostic impact in MDS patients. Asian Pac J Cancer Prev 2017; 18: 3307–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Jang JH, Park J, et al. A prospective multicenter observational study of decitabine treatment in Korean patients with myelodysplastic syndrome. Haematologica 2011; 96: 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol 2009; 27: 3842–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Zheng Y, Gao J, et al. Expression of SHP-1 and SOCS6 in patients with acute leukemia and their clinical implication. Onco Targets Ther 2017; 10: 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulou V, Kontandreopoulou E, Panayiotidis P, et al. Expression, prognostic significance and mutational analysis of protein tyrosine phosphatase SHP-1 in chronic myeloid leukemia. Leuk Lymphoma 2016; 57: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Baños D, Sánchez-Hernández B, Jiménez G, et al. Global methylation and promoter-specific methylation of the P16, SOCS-1, E-cadherin, P73 and SHP-1 genes and their expression in patients with multiple myeloma during active disease and remission. Exp Ther Med 2017; 13: 2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007; 109: 52–57. [DOI] [PubMed] [Google Scholar]
- 22.Yasukawa H Sasaki A andYoshimura A.. Negative regulation of cytokine signaling pathways. Annu Rev Immunol 2000; 18: 143–164. [DOI] [PubMed] [Google Scholar]
- 23.Beldi-Ferchiou A, Skouri N, Ben Ali C, et al. Abnormal repression of SHP-1, SHP-2 and SOCS-1 transcription sustains the activation of the JAK/STAT3 pathway and the progression of the disease in multiple myeloma. PLoS One 2017; 12: e0174835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honorat JF, Ragab A, Lamant L, et al. SHP1 tyrosine phosphatase negatively regulates NPM-ALK tyrosine kinase signaling. Blood 2006; 107: 4130–4138. [DOI] [PubMed] [Google Scholar]
- 25.Al-Jamal HA, Mat Jusoh SA, Hassan R, et al. Enhancing SHP-1 expression with 5-azacytidine may inhibit STAT3 activation and confer sensitivity in lestaurtinib (CEP-701)-resistant FLT3-ITD positive acute myeloid leukemia. BMC Cancer 2015; 15: 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh JS, Joo MK, Park JJ, et al. Inhibition of STAT3 in gastric cancer: role of pantoprazole as SHP-1 inducer. Cell Biosci 2018; 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu MH, Chen LJ, Chen YL, et al. Targeting SHP-1-STAT3 signaling: A promising therapeutic approach for the treatment of cholangiocarcinoma. Oncotarget 2017; 8: 65077–65089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia WQ, Wang ZT, Zou MM, et al. Verbascoside inhibits glioblastoma cell proliferation, migration and invasion while promoting apoptosis through upregulation of protein tyrosine phosphatase SHP-1 and inhibition of STAT3 phosphorylation. Cell Physiol Biochem 2018; 47: 1871–1882. [DOI] [PubMed] [Google Scholar]