Abstract
Introduction:
Glioblastoma multiforme (GBM) is the most aggressive glioma, and its diffuse nature makes resection of it difficult. Moreover, even with the administration of postoperative radiotherapy and chemotherapy, prolonged remission is often not achieved. Hence, innovative or alternative treatments for GBM are urgently required. Traditional Chinese herbs and their functional components have long been used in the treatment of various cancers, including GBM. The current study investigated the antitumor activity of Wedelia chinensis and its major functional components, luteolin and apigenin, on GBM.
Materials and Methods:
MTT assay, Transwell migration assay, and flow cytometry analysis were adopted to assess the cell viability, invasive capability, and cell cycle. Immunofluorescence staining and Western blotting were used to detect the expressions of apoptotic and autophagy-related signaling molecules.
Results:
The W. chinensis extract (WCE) significantly inhibited the proliferation and invasive ability of both GBM8401 and U-87MG cells in a dose-dependent manner. Moreover, differential effects of WCE on GBM8401 and U-87MG cells were observed: WCE induced apoptosis in GBM8401 cells and autophagy in U-87MG cells. Notably, WCE had significant effects in reducing the cell survival and invasive capability of both GBM8401 and U-87MG cells than the combination of luteolin and apigenin.
Conclusions:
Taken together, these findings indicate the potential of using WCE and the combination of luteolin and apigenin for GBM treatment. However, further investigations are warranted before considering recommending the clinical use of WCE or the combination of luteolin and apigenin as the standard for GBM treatment.
Keywords: glioblastoma multiforme, traditional Chinese medicine, Wedelia chinensis extract, apoptosis, autophagy
Introduction
The incidence of glioblastoma multiforme (GBM) is approximately 3.19 cases per 100 000 persons, and its 5-year survival rate is 4% to 5%.1 GBM, as a grade 4 brain tumor arising from glial cells, is the most aggressive glioma and accounts for approximately 15% of all primary brain tumors.2 The symptoms of brain tumors vary depending on tumor location and may include decreased appetite, sustained headaches, blurred vision, vomiting, and changes in psychological status such as new-onset seizures and speech difficulty.3 GBMs are primarily treated by surgical resection, followed by radiotherapy and chemotherapy. They are surrounded by an area of migrating and infiltrating tumor cells, making complete resection difficult. Moreover, the current recommended treatment does not yield a prolonged remission period.4 Hence, innovative or alternative treatments for GBM are urgently required.
Various complementary and alternative medicine therapies, including music, massage, yoga, acupuncture, and herbal medicines, have long been used for cancer treatment,5 with additional therapies frequently being recommended.6 Notably, certain traditional Chinese herbs or their functional components such as Danggui, triptolide, and Withania somnifera have been shown to be effective against brain tumors.7-9 Danggui was reported to inhibit the proliferation of GBM8401 cells and microvessel formation in xenografted tumors in nude mice.7 A study reported that triptolide extracted from Tripterygium wilfordii induces apoptosis in the glioma cell lines U251MG and U87MG.10
Wedelia chinensis is a traditional hepatoprotective herbal medicine.11,12 Recently, W. chinensis has been used in the treatment of various types of cancers. W. chinensis extract (WCE) and its major functional components, luteolin, and apigenin, have anticancer properties effective against prostate, lung, breast, glioblastoma, colon, and pancreatic cancer cells.8,13-15 However, information regarding their effects on different GBM cell lines is limited. Therefore, this study examined whether WCE, luteolin, and apigenin are effective against GBM.
Materials and Methods
Preparation of WCE
The whole plant of W. chinensis was purchased from a reputable Chinese medicinal herb farm (Organic Wucun Farm) in Taichung, Taiwan. Its total extract (WCE) and its major functional components, luteolin and apigenin, were prepared as previously reported.8 Briefly, 100 g of air-dried W. chinensis plant was ground and homogenized in ethanol. To increase the content of aglycone flavonoids, the extracted solution was acid-hydrolyzed with HCl at pH 2.0 and 80°C for 35 min, neutralized with NaOH, and separated into fractions by using a C18 column (Biotage, Uppsala, Sweden). WCE was dried as a powder and stored in a freezer at −80°C until use. Luteolin (13.96 mg in 372 mg of WCE) and apigenin (2.32 mg in 372 mg of WCE) were purified from the subfractions by using semi-preparative HPLC. The yields of luteolin and apigenin were 0.0375% and 0.00624%, respectively.
Cell Culture
Human brain astrocyte (HBA) cells and human malignant glioma cell lines, GBM8401 and U-87MG, were purchased from ScienCell (CA, USA) and ATCC (Rockville, MD, USA), respectively. HBA, GBM8401, and U-87MG cells were maintained in 2% fetal bovine serum (FBS)-containing astrocyte medium (ScienCell, CA, USA), 10% FBS-containing RPMI 1640 medium, and 10% FBS-containing Eagle’s Minimum Essential Medium (ATCC® 30-2003™), respectively.
Cell Viability
Briefly, cells were cultured in a 96-well plate at a density of 5 × 103 cells per well overnight in a CO2 incubator. The cells were then treated with various doses of WCE, luteolin, or apigenin for 1 or 2 days. Next, the medium was replaced with 0.2 mL of 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent (0.5 mg/mL) and incubated for another 4 hours. Finally, dimethyl sulfoxide was added (0.2 mL/well) to dissolve the formazan crystals, and absorbance was detected at 570 nm (SpectraMax M5, USA).
Transwell Migration Assay
A 24-well Millicell Hanging Cell Culture inserts with 8 µm PET membranes (EMD Millipore Corporation, Billerica, Massachusetts, USA) was adopted to detect the invasive capability. First, serum-free medium containing WCE, luteolin, apigenin, or combination of luteolin and apigenin were added to the upper chamber and the medium containing 10% fetal bovine serum (FBS) was added to the lower chamber. Subsequently, neutral-buffered formalin (10%) was adopted to fix the migrated cells after incubation for 24 hours and the migrated cells were stained with 0.5% crystal violet. The migrated cells were then counted in 6 randomly selected microscopic fields at 200× magnification per filter.
Flow Cytometric Analysis
For flow cytometry analysis, cells were incubated with various concentrations of WCE for 24 hours. The cells were then harvested, washed with phosphate-buffered saline (PBS), fixed with 70% alcohol for 12 hours at 4°C, and washed with PBS. Next, 10 μL of propidium iodide staining solution was added, and the resultant mixture was mixed and incubated in an ice bath in the dark. Finally, the cells were filtered through a 40-μm nylon screen and analyzed with a FACS Calibur analyzer (Becton Dickinson, Bedford, MA, USA).
Monitoring Autophagy-Immunofluorescence Staining
To analyze autophagy flux, we followed the Guidelines for the use and interpretation of assays for monitoring autophagy.16 LC3B Antibody Kit for Autophagy (Invitrogen, MA, USA) was adopted for analysis and 25 µM chloroquine diphosphate (CQ) was used as positive control for artificially generating autophagosomes. A 2 × 105 U-87MG cells per well was cultured in Millicell EZ SLIDE 8-well glass slides and maintained with fresh medium containing different doses of WCE or CQ for 24 hours. Next, cells were washed with 1× PBS and fixed with 4% paraformaldehyde. The cells were then incubated in blocking solution and hybridized with antibodies against LC3B (LC3-II) (Invitrogen, MA, USA) after permeabilization in 0.3% Triton X-100 for 6 minutes. Slides were mounted with ProLong™ Gold Antifade Mountant with DAPI (Thermo Fisher Scientific Inc., MA, USA) after the incubation with Alexa Fluor® 488 goat anti-rabbit IgG (H+L) antibodies (Invitrogen, MA, USA). Finally, the cells were observed under a ZEISS AXioskop2 fluorescence microscope (Carl Zeiss Microscopy, LLC, NY, USA).
Immunoblotting
Immunoblotting was adopted to investigate the expression of apoptotic and autophagic proteins. Briefly, cells were treated with different doses of WCE for 24 hours in culture medium and cell lysates were made in lysis buffer (PRO-PREP™, iNtRON, USA). Protein samples were then separated on a 12% SDS-PAGE gel and were transferred to nitrocellulose membranes (Bio-Rad, USA). Antibodies against Bcl-2, Bax, cytochrome c, Apaf-1, caspase-3, caspase-9 (Santa Cruz Biotechnology, CA, USA), ATG-7, ATG-5, p62, Beclin-1 (R&D Systems, MN, USA), LC3 (Abcam, Cambridge, UK), and β-actin (Upstates, Charlottesville, Virginia, USA) were diluted in PBS (1/1000) with 2.5% BSA and reacted with gentle agitation at 4°C overnight. After washing with 1× PBS 4 times, the secondary antibody conjugated with horseradish peroxidase was added, and incubation continued for another 1 hour. Finally, Immobilon Western HRP Chemiluminescent Substrate (Millipore, USA) and a chemiluminescence imaging instrument (GE ImageQuant TL 8.1; GE Healthcare Life Sciences, PA, USA) were used to measure the presence of antigen-antibody complexes. The blots were scanned and quantified using densitometry (Appraise, Beckman-Coulter, Brea, CA, USA).
Statistical Analysis
All statistical analyses were performed using SAS JMP 7.0 (JMP, NC, USA) to perform 1-way analysis of variance. The Tukey multiple comparison test was used to calculate statistical significance. All values are depicted as mean ± standard error of the mean. All experiments were repeated at least 3 times. P < .05 was set as statistically significant.
Results
Effects of WCE on GBM8401 and U-87MG cells
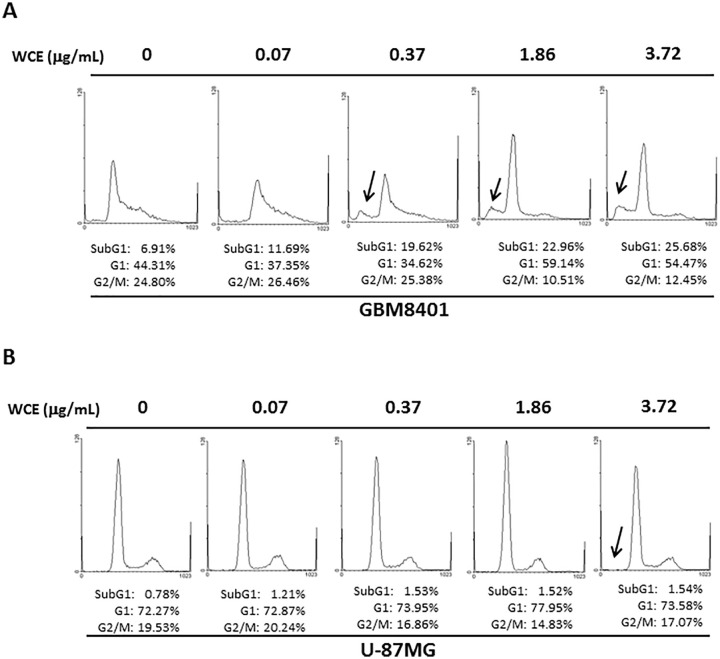
To investigate the effects of WCE on human malignant glioma cells, 2 human malignant glioma cell lines, GBM8401 and U-87MG, and a human normal brain astrocyte cell line, HBA, were used for the MTT assay and Transwell migration assay. The survival ratio of GBM8401 and U-87MG cells was significantly lower in the presence of 0.37, 1.86, and 3.72 μg/mL WCE than in the presence of 0 μg/mL WCE or HBA cells (Figure 1). Notably, a significantly higher survival ratio of U-87MG cells was observed in the presence of 1.86 μg/mL WCE for 24 hours compared with that of GBM8401 cells, whereas a significantly lower survival ratio of U-87MG cells was observed in the presence of 3.72 μg/mL WCE (Figure 1). Correspondingly, significantly decreased invaded GBM8401 and U-87MG cells were observed in the presence of WCE in a dose-dependent manner than those in the presence of 0 μg/mL WCE (Figure 2). A markedly increased sub-G1 proportion was detected in GBM8401 cells in the presence of WCE in a dose-dependent manner (Figure 3A). No significant difference in sub-G1 proportion was observed in U-87MG cells with any concentration of WCE (Figure 3B).
Figure 1.
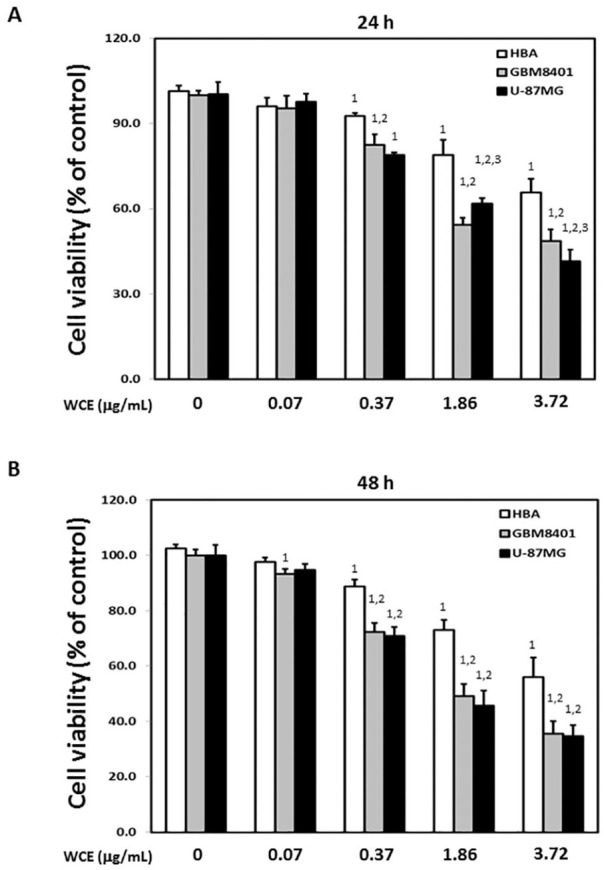
Effects of Wedelia chinensis extract (WCE) on the viability of GBM8401, U-87MG, and human brain astrocyte (HBA) cells. Relative cell survival of GBM8401, U-87MG, and HBA cells after treatment with various concentrations of WCE for (A) 24 and (B) 48 hours. Similar results were observed in 3 repeated experiments.
The numbers 1, 2, and 3 indicate a significant difference (P < .05) compared with control (0 μg/mL), HBA, and GBM8401 cells, respectively.
Figure 2.
Effects of Wedelia chinensis extract (WCE) on the invasive ability of GBM8401 and U-87MG cells. The representative photos of invaded (A) GBM8401 and (B) U-87MG cells treated with different concentrations of WCE for 24 hours. Quantified results of invaded percentage for both cells were shown in the lower panel, respectively. Similar results were observed in 3 repeated experiments.
The symbol * indicates significant differences (P < .05) compared with control.
Figure 3.
Representative results of flow cytometry in GBM8401 and U-87MG cells in the presence of various concentrations of WCE for 24 hours. The arrow indicates the sub-G1 proportion. Similar results were observed in 3 repeated experiments.
WCE Induces Apoptosis in GBM8401 Cells and Autophagy in U-87MG Cells
To verify the possible mechanisms involved in WCE-induced cell death in GBM8401 and U-87MG cells, immunoblotting was performed to detect the expression of apoptotic and autophagic molecules. Significantly increased levels of Bax, cytochrome c, Apaf-1, cleaved caspase-9, and cleaved caspase-3 and a significantly decreased Bcl-2 level were detected in GBM8401 cells in the presence of WCE in a dose-dependent manner (Figure 4A and B). Since no significantly increased apoptotic molecules were detected in U-87MG cells that were treated with WCE, we further analyzed the autophagic flux in U-87MG cells by using immunofluorescence and Western blot. Notably, apparent LC3B puncta was observed in U-87MG cells in the presence of 1.86 and 3.72 μg/mL WCE than controls (0 μg/mL) (Figure 5). Correspondingly, a significantly increased LC3-II/I ratio and expressions of Atg-7, Atg-5, and Beclin-1 level were observed in U-87MG cells in the presence of WCE in a dose-dependent manner whereas a significantly decreased P62 level was detected (Figure 6). Furthermore, we used CQ, an autophagy inhibitor, to confirm the contribution of an autophagic mechanism to WCE-induced death in U-87MG cells (Figure 7). U-87MG cells were pre-treated with CQ (25 µM) for 1 hour and then incubated with 3.72 μg/mL WCE for 24 hours. In agreement, decreased P62 and increased LC3-II was observed in U-87MG cells that were treated with 3.72 μg/mL WCE. Blocking lysosomal degradation by pre-treatment with CQ rescued LC3-II and P62 break down that result in LC3-II and P62 accumulation.
Figure 4.
Expression of apoptotic proteins. (A) The expression of Bcl-2, Bax, cytochrome c (Cyt-C), Apaf-1, cleaved caspase-9, and cleaved caspase-3 proteins in GBM8401 cells treated with various concentrations of WCE for 24 hours. (B) Bars represent protein quantification relative to β-actin. Similar results were observed in 3 repeated experiments.
The symbol * indicates P < .05 compared with control (0 μg/mL).
Figure 5.

Detection of LC3B puncta in U-87MG cells. Representative images of immunofluorescence staining with specific antibodies against LC3B proteins in U-87MG cells in the presence of different concentrations of WCE for 24 hours. Middle panel shows the images U-87MG cells stained with DAPI and right panel presents the merged images of DAPI and LC3B. Arrows indicate the expression of LC3B puncta. Similar results were observed in 3-repeated experiments.
Scale bars = 15 μm.
Figure 6.
Expression of autophagy-related proteins. (A) The expression of LC3, Atg-7, Atg-5, Beclin-1, and P62 proteins in U-87MG cells treated with different concentrations of WCE for 24 hours. Bars represent the ratio of (B) LC3-II/LC3-I and the protein quantification of (C) Atg-7, (D) Atg-5, (E) Beclin-1, (F) P62 relative to β-actin. Similar results were observed in 3 repeated experiments.
*Indicates P < .05 compared with control (0 μg/mL).
Figure 7.

Involvement of autophagy in the response of U-87MG cells on WCE. U-87MG cells were pre-treated (1 hour) with 25 µM CQ (autophagy inhibitor) before WCE treatment (3.72 μg/mL). (A) Cell lysates were collected after 24 hours and expressions of P62 and LC3-II were detected by western blot. Bars represent protein quantification of (B) P62 and (C) LC3-II relative to β-actin. Similar results were observed in 3 repeated experiments.
The numbers 1, 2, and 3 indicate a significant difference (P < .05) compared with control (0 μg/mL), CQ (25 µM), and WCE (3.72 μg/mL), respectively.
Differential Effects of WCE, Luteolin, Apigenin, and Combination of Luteolin and Apigenin on GBM8401 and U-87MG Cells
To compare the differential effects of luteolin, apigenin, and WCE on the proliferation of human malignant glioma cells, the viability of GBM8401 and U-87MG cells was evaluated in the presence of luteolin, apigenin, their combination, and WCE. Significantly lower viability of GBM8401 and U-87MG cells was detected in the presence of luteolin, apigenin, and WCE in a dose-dependent manner (Figure 8A and B), with the LD50 values of GBM8401 cells being 4.1, 7.4, and 43.7 μg/mL, respectively (Figure 8A), and those of U-87MG cells being 3.6, 6.2, and 41.6 μg/mL, respectively (Figure 8B). Because luteolin and apigenin accounted for 3.75% and 0.63% of WCE used in this study, 0.19 μg/mL luteolin and 0.03 μg/mL apigenin were compared with 5 μg/mL WCE. Significantly lower viability of both GBM8401 and U-87MG cells was observed in the presence of luteolin but not apigenin. Significantly lower viability of both GBM8401 and U-87MG cells was detected in the presence of the combination of luteolin and apigenin than in the presence of luteolin or apigenin alone (Figure 8C). Notably, significantly lower viability of both GBM8401 and U-87MG cells was detected in the presence of WCE compared with those treated with the combination of luteolin and apigenin (Figure 8C). Correspondingly, significantly lower invasive capability of both GBM8401 and U-87MG cells was detected in the presence of WCE compared with those treated with the combination of luteolin and apigenin (Figure 9A and B).
Figure 8.
Effects of WCE, luteolin, apigenin, and combination of luteolin and apigenin on the viability of GBM8401 and U-87MG cells. Relative cell survival of (A) GBM8401 and (B) U-87MG in the presence of various concentrations of luteolin, apigenin, and WCE for 24 hours. (C) Relative cell survival of GBM8401 and U-87MG in the presence of luteolin, apigenin, the combination of luteolin and apigenin, and WCE for 24 hours. Similar results were obtained in 3-repeated experiments.
The numbers 1, 2, 3, and 4 indicate a significant difference (P < .05) compared with control (0 μg/mL), luteolin, apigenin, and the combination of luteolin and apigenin, respectively.
Figure 9.
Effects of WCE, luteolin, apigenin, and combination of luteolin and apigenin on the invasive ability of GBM8401 and U-87MG cells. The representative photos of invaded (A) GBM8401 and (B) U-87MG cells treated with different concentrations of WCE for 24 hours. Quantified results of invaded percentage for both cells are shown in the lower panel, respectively. Similar results were observed in 3-repeated experiments.
The numbers 1, 2, 3, and 4 indicate a significant difference (P < .05) compared with control (0 μg/mL), luteolin, apigenin, and the combination of luteolin and apigenin, respectively.
Discussion
Traditional Chinese herbs exhibit multiple biological effects, and several Chinese herbal extracts have been used in the treatment of various cancers for thousands of years.17 W. chinensis, a Formosan plant-derived drug with antihepatotoxic actions,18 has been demonstrated to be cytotoxic to various cancer cells, such as prostate cancer cells (LNCaP, PC-3, and 22Rv1 cell lines), nasopharyngeal carcinoma cells (CNE-1), and melanoma cells (B16F-10).8,19,20 In the current study, we found that WCE significantly inhibited the proliferation of both GBM8401 and U-87MG cells. Moreover, WCE induced apoptosis in GBM8401 cells and autophagy in U-87MG cells. These results suggest that WCE-induced GBM8401 cell apoptosis may be caused through P53-dependent pathways and that WCE-induced U-87MG cell autophagy may be caused through P53-independent pathways. Some major differences between GBM8401 and U-87MG cells are attributable to the p53 gene and their glial nature. U-87MG cells express a wild-type p53 gene, whereas GBM8401 cells express a mutated p53 gene.21 Moreover, compared with GBM8401 cells, U-87MG cells exhibit significantly higher levels of astroglial differentiation and glial fibrillary acidic protein mRNA expression and lower expression levels of nestin and vimentin mRNA.21 The differences in biological properties between these 2 cell lines may contribute to different responses to WCE or signaling pathways, which may explain the differential effects of WCE on GBM8401 and U-87MG cells. Further studies are necessary to elucidate the precise mechanism of action of WCE on GBM8401 and U-87MG cells.
Chemotherapy is one of the most common methods of cancer therapy,22 but single-agent chemotherapy has disadvantages, including inadequate efficacy, drug resistance, systemic toxicity with high doses, and weakness of the immune system.23-25 Therefore, combination therapy is highly valued for cancer treatment. Luteolin and apigenin are well-known flavonoid phytochemicals present in various types of fruits, vegetables, and traditional Chinese herbs;14 they are also the 2 major functional components of W. chinensis that exhibit anticancer activity.8,13 In the present study, the doses of luteolin and apigenin used for the experiments were 0.19 and 0.03 µg/mL, respectively, reflecting their content in WCE. The cytotoxic effect of the combination of luteolin and apigenin on GBM8401 and U-87MG cells was significantly higher than that of luteolin or apigenin alone, implying that the combination exerts a more inhibitory effect. Moreover, the cytotoxic effect of WCE on GBM8401 and U-87MG cells was significantly higher than even that of the combination of luteolin and apigenin, suggesting higher inhibitory effects of WCE than the combination of luteolin and apigenin. However, future studies should adopt a xenograft animal model to assess the effects of both WCE and the combinational use of luteolin and apigenin in vivo.
Luteolin and apigenin have long been used in cancer therapy;26,27 however, the precise mechanisms of their activity against GBM cells and other cancers remain unclear. Many studies have reported the diverse routes by which flavonoids (such as luteolin and apigenin) inhibit cancer cells. Flavonoids, including apigenin, luteolin, kaempferol, and quercetin, have been reported to exert pro-oxidant cytotoxicity against cancer cells through a reactive oxygen species-triggered mitochondrial apoptotic pathway.28 Flavonoids, such as quercetin, apigenin, and luteolin, have been reported to inhibit cytokine expression and secretion, suggesting their possible roles in cancer treatment as cytokines modulators.29 Luteolin and apigenin are known modulators of oncogenic transcription factors, resulting in reduced NRF2 expression in cancer cells and increased chemosensitivity of cancer cells to cytostatic drugs.30,31 Furthermore, apigenin and luteolin can directly bind to mRNA splicing-related proteins to induce a widespread change in splicing patterns in tumorigenic cells, resulting in the nuclear accumulation of poly(A)+ RNAs and cancer cell death.32 A very recent study reported that luteolin can inhibit the proliferation of different cancer cells by modulating various pathways, including JAK-STAT, Wnt/β-catenin, and NOTCH signaling,33 and by mediating the regulation of noncoding RNAs.33 Taken together, these diverse anticancer functions of luteolin and apigenin explain the significantly higher inhibitory effects of WCE or the combination of luteolin and apigenin.
Several concerns in this study should be noted. First, the in vivo effects of WCE, luteolin, apigenin and the combinational use of luteolin and apigenin on GBM cancer cells are still unclear. Therefore, an animal study should be considered to verify the effects of WCE, luteolin, apigenin and the combinational use of luteolin and apigenin. Second, the presence of blood brain barrier (BBB) can cause efflux machinery or hindrance for brain-entrance of medicine and is known as a major extreme difficulty in brain tumor treatment.34 Hence, an orthotopic intra-brain model should be adopted to verify whether WCE, luteolin, apigenin and combinational use of luteolin and apigenin has anti-brain tumor activity. Third, a comparison between main chemotherapy drugs used in GBM clinical practice and WCE, luteolin, apigenin, or the combinational use of luteolin and apigenin should be performed to verify the potentials of WCE or its components on clinical practice. Forth, we did not find WCE-induced apoptosis in U-87MG cells and autophagy in GBM8401 cells in this study, suggesting diverse roles of WCE on inducing different GBM cell lines death. Therefore, further investigations are needed to verify the precise mechanism of WCE or its components on inducing apoptosis in GBM8401 cells and autophagy in U-87MG cells. Finally, the overall components of WCE that display anti-GBM activity are still unclear. Since the concentrations of luteolin and apigenin are about 0.19 µg/mL and apigenin 0.03 in 5 µg/mL WCE, we therefore tested the combination of luteolin (0.19 µg/mL) and apigenin (0.03 µg/mL) and revealed the more significantly cytotoxic effect of WCE on GBM cell lines than the combinational use of luteolin and apigenin in this study. Hence, the other anti-GBM components of WCE should be discovered and investigated to explain the more aggravated cytotoxic effects of WCE than the combinational use of luteolin and apigenin. The other anti-GBM components of WCE should be verified and investigated to explain whether synergistic effects exist.
Footnotes
Author Contributions: Conceptualization, Bor-Show Tzang and Cheng-Hui Lin; Data curation and Formal analysis, Li-Jeng Chen, Tsai-Ching Hsu, Pei-Jung Yeh, Jia Le Yow, Chia-Ling Chang, and Bor-Show Tzang; Funding acquisition, Tsai-Ching Hsu, Bor-Show Tzang, and Cheng-Hui Lin; Writing—original draft, Tsai-Ching Hsu and Bor-Show Tzang; Writing—review and editing, Li-Jeng Chen, Tsai-Ching Hsu, Bor-Show Tzang, and Cheng-Hui Lin. All authors have read and agreed to the published version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by clinical research grant from Kaohsiung Armed Force General Hospital, Kaohsiung, Taiwan (No. 106-04) and the experimental supplies in cell study were partially supported by Ministry of Science and Technology (MOST-107-2314-B040-004 and MOST-108-2320-B-040-024-MY3).
ORCID iDs: Li-Jeng Chen  https://orcid.org/0000-0002-0043-6248
https://orcid.org/0000-0002-0043-6248
Bor-Show Tzang  https://orcid.org/0000-0003-0140-9943
https://orcid.org/0000-0003-0140-9943
References
- 1. Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 2017;24:3002-3009. [DOI] [PubMed] [Google Scholar]
- 2. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Politsky JM. Brain tumor-related epilepsy: a current review of the etiologic basis and diagnostic and treatment approaches. Curr Neurol Neurosci Rep. 2017;17:70. [DOI] [PubMed] [Google Scholar]
- 4. Olin MR, Pluhar GE, Andersen BM, et al. Victory and defeat in the induction of a therapeutic response through vaccine therapy for human and canine brain tumors: a review of the state of the art. Crit Rev Immunol. 2014;34:399-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cassileth BR. Evaluating complementary and alternative therapies for cancer patients. CA Cancer J Clin. 1999;49:362-375. [DOI] [PubMed] [Google Scholar]
- 6. Brooks SL, Rowan G, Michael M. Potential issues with complementary medicines commonly used in the cancer population: a retrospective review of a tertiary cancer center’s experience. Asia Pac J Clin Oncol. 2018;14:e535-e542. [DOI] [PubMed] [Google Scholar]
- 7. Lee WH, Jin JS, Tsai WC, et al. Biological inhibitory effects of the Chinese herb danggui on brain astrocytoma. Pathobiology. 2006;73:141-148. [DOI] [PubMed] [Google Scholar]
- 8. Lin FM, Chen LR, Lin EH, et al. Compounds from Wedelia chinensis synergistically suppress androgen activity and growth in prostate cancer cells. Carcinogenesis. 2007;28:2521-2529. [DOI] [PubMed] [Google Scholar]
- 9. Dhami J, Chang E, Gambhir SS. Withaferin A and its potential role in glioblastoma (GBM). J Neurooncol. 2017;131:201-211. [DOI] [PubMed] [Google Scholar]
- 10. Lin J, Chen LY, Lin ZX, Zhao ML. The effect of triptolide on apoptosis of glioblastoma multiforme (GBM) cells. J Int Med Res. 2007;35:637-643. [DOI] [PubMed] [Google Scholar]
- 11. Lin SC, Lin CC, Lin YH, Shyuu SJ. Hepatoprotective effects of Taiwan folk medicine: Wedelia chinensis on three hepatotoxin-induced hepatotoxicity. Am J Chin Med. 1994;22:155-168. [DOI] [PubMed] [Google Scholar]
- 12. Lu Y, Hu D, Ma S, et al. Protective effect of wedelolactone against CCl4-induced acute liver injury in mice. Int Immunopharmacol. 2016;34:44-52. [DOI] [PubMed] [Google Scholar]
- 13. Tsai CH, Lin FM, Yang YC, et al. Herbal extract of Wedelia chinensis attenuates androgen receptor activity and orthotopic growth of prostate cancer in nude mice. Clin Cancer Res. 2009;15:5435-5444. [DOI] [PubMed] [Google Scholar]
- 14. Imran M, Rauf A, Abu-Izneid T, et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612. [DOI] [PubMed] [Google Scholar]
- 15. Xu L, Zaky MY, Yousuf W, et al. The anticancer potential of apigenin via immunoregulation. Curr Pharm Des. Published online July 13, 2020. doi: 10.2174/1381612826666200713171137 [DOI] [PubMed] [Google Scholar]
- 16. Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiao L, Bi L, Lu Y, et al. Cancer chemoprevention and therapy using chinese herbal medicine. Biol Proced Online. 2018;20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang LL, Yen KY, Konno C, Oshima Y, Kiso Y, Hikino H. Antihepatotoxic principles of Wedelia chinensis herbs. Planta Med. 1986;6:499-500. [PubMed] [Google Scholar]
- 19. Manjamalai A, Berlin Grace VM. Antioxidant activity of essential oils from Wedelia chinensis (Osbeck) in vitro and in vivo lung cancer bearing C57BL/6 mice. Asian Pac J Cancer Prev. 2012;13:3065-3071. [DOI] [PubMed] [Google Scholar]
- 20. Liu M, Wang W, Li X, et al. Wedelia chinensis inhibits nasopharyngeal carcinoma CNE-1 cell growth by inducing G2/M arrest in a Chk1-dependent pathway. Am J Chin Med. 2013;41:1153-1168. [DOI] [PubMed] [Google Scholar]
- 21. Chu CW, Ko HJ, Chou CH, et al. Thioridazine enhances P62-mediated autophagy and apoptosis through wnt/beta-catenin signaling pathway in glioma cells. Int J Mol Sci. 2019;20:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [DOI] [PubMed] [Google Scholar]
- 23. Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review). Int J Oncol. 2007;30:233-245. [PubMed] [Google Scholar]
- 24. DeVita VT, Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643-8653. [DOI] [PubMed] [Google Scholar]
- 25. Schally AV, Engel JB, Emons G, Block NL, Pinski J. Use of analogs of peptide hormones conjugated to cytotoxic radicals for chemotherapy targeted to receptors on tumors. Curr Drug Deliv. 2011;8:11-25. [DOI] [PubMed] [Google Scholar]
- 26. Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8:634-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Q, Cheng G, Qiu H, et al. The p53-inducible gene 3 involved in flavonoid-induced cytotoxicity through the reactive oxygen species-mediated mitochondrial apoptotic pathway in human hepatoma cells. Food Funct. 2015;6:1518-1525. [DOI] [PubMed] [Google Scholar]
- 29. Leyva-Lopez N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB. Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int J Mol Sci. 2016;17:921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stepanic V, Gasparovic AC, Troselj KG, Amic D, Zarkovic N. Selected attributes of polyphenols in targeting oxidative stress in cancer. Curr Top Med Chem. 2015;15:496-509. [DOI] [PubMed] [Google Scholar]
- 31. Rajagopal C, Lankadasari MB, Aranjani JM, Harikumar KB. Targeting oncogenic transcription factors by polyphenols: a novel approach for cancer therapy. Pharmacol Res. 2018;130:273-291. [DOI] [PubMed] [Google Scholar]
- 32. Kurata M, Fujiwara N, Fujita KI, et al. Food-derived compounds apigenin and luteolin modulate mRNA splicing of introns with weak splice sites. iScience. 2019;22:336-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farooqi AA, Butt G, El-Zahaby SA, et al. Luteolin mediated targeting of protein network and microRNAs in different cancers: focus on JAK-STAT, NOTCH, mTOR and TRAIL-mediated signaling pathways. Pharmacol Res. 2020;160:105188. [DOI] [PubMed] [Google Scholar]
- 34. Grimm SA. Treatment of brain metastases: chemotherapy. Curr Oncol Rep. 2012;14:85-90. [DOI] [PubMed] [Google Scholar]