Abstract
Objective:
To analyze the relationship between complement component 3 (C3) and the prevalence of cardiometabolic risk factors and disease activity in the rheumatic diseases having the highest rates of cardiovascular morbidity and mortality: rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
Methods:
This is a cross-sectional study including 200 RA, 80 PsA, 150 axSpA patients and 100 healthy donors. The prevalence of cardiometabolic risk factors [obesity, insulin resistance, type 2 diabetes mellitus, hyperlipidemia, apolipoprotein B/apolipoprotein A (apoB/apoA) and atherogenic risks and hypertension] was analyzed. Serum complement C3 levels, inflammatory markers and disease activity were evaluated. Cluster analysis was performed to identify different phenotypes. Receiver operating characteristic (ROC) curve analysis to assess the accuracy of complement C3 as biomarker of insulin resistance and disease activity was carried out.
Results:
Levels of complement C3, significantly elevated in RA, axSpA and PsA patients, were associated with the prevalence of cardiometabolic risk factors. Hard clustering analysis identified two distinctive phenotypes of patients depending on the complement C3 levels and insulin sensitivity state. Patients from cluster 1, characterized by high levels of complement C3 displayed increased prevalence of cardiometabolic risk factors and high disease activity. ROC curve analysis showed that non-obesity related complement C3 levels allowed to identify insulin resistant patients.
Conclusions:
Complement C3 is associated with the concomitant increased prevalence of cardiometabolic risk factors in rheumatoid arthritis and spondyloarthritis. Thus, complement C3 should be considered a useful marker of insulin resistance and disease activity in these rheumatic disorders.
Keywords: axial spondyloarthritis, cardiovascular risk, complement C3, disease activity, obesity, psoriatic arthritis, rheumatoid arthritis
Introduction
Patients with inflammatory rheumatic diseases are at increased risk of developing cardiometabolic comorbidities.1 Thus, rheumatoid arthritis and spondyloarthritis are characterized by an increased prevalence of certain cardiovascular risk factors including type 2 diabetes, obesity, hypertension and dyslipidemia compared to the general population.2,3 Cardiometabolic disease involves an alteration in metabolic organs and the cardiovascular (CV) system that significantly contribute to the development of cardiovascular disease (CVD).4 A detailed study of the whole cardiometabolic status might be very complex, expensive and time-consuming in the rheumatological outpatient setting. Thus, the identification of surrogate biomarkers that could be used as a screening strategy to select those patients requiring deeper evaluation is of great interest for clinicians.
In this sense, complement factors have recently drawn much attention due to their association with metabolic disorders including metabolic syndrome, obesity and other CV risk components.5 The complement system is a protein complex of the innate immune system that participates not only in inflammatory response, coagulation or fibrinolysis,6 but also in the development and progression of cardiometabolic disease.4 Thus, a recent study has proposed that complement component 3 (C3) and component 4 (C4) could be involved in the development of the metabolic syndrome due to their increased levels in patients with metabolic syndrome in cross-sectional and prospective analysis.7 In particular, large epidemiological studies have demonstrated the key role of complement C3 as a potential predictor of CV events.8 In addition, increased levels of complement C3 have been closely related with insulin resistance, abdominal obesity and hypertension.9 Apart from immune cells, human adipose tissue is an important source of complement factors. In fact, the activated form of complement C3 can act as a hormone involved in lipid storage and energy homeostasis.10 Despite this fact, complement C3 has been associated with insulin secretion independent of the adiposity, subclinical inflammation and insulin sensitivity in non-diabetic subjects.11 The mechanisms underlying the relationship between complement C3 and the cardiometabolic alterations have not been completely defined, but it has been described how increased complement C3 levels are associated with higher levels of dearginine (C3a-desArg), which might affect adipocytes and macrophages in the adipose tissue.12
There is accumulating evidence about the direct role of adipose tissue in the production and release of complement C3, so that a direct relationship between adiposity and levels of complement C3 has been described.13 In this sense, increased rates of obesity have been linked to rheumatic diseases, mainly due to the physical inactivity and the chronic treatments administered. So, it would be easy to hypothesize that levels of complement C3 are increased in those diseases due to the increased body mass index (BMI). In fact, several authors have recently described high levels of complement C3 in psoriatic and rheumatoid arthritis closely related to insulin resistance.14–16
We sought to analyze the relationship between levels of complement C3 and the prevalence of cardiometabolic risk factors and the disease activity in the rheumatic diseases having the highest rates of cardiovascular morbidity and mortality: psoriatic arthritis (PsA), rheumatoid arthritis (RA), and axial spondyloarthritis (axSpA), specifically excluding obesity.
Methods
Study design, setting and participants
A cross-sectional study in 200 patients with RA, 150 patients with axSpA, 80 patients with PsA and 100 healthy donors (HDs) was carried out after approval from the ethics committee of the Reina Sofia Hospital (code PI17/01316 and PI-0139-2017), Cordoba, Spain, and written informed consent was obtained from all participants. All participants were Caucasian and consecutively recruited during daily clinical routine at the rheumatology service of the Reina Sofia Hospital. Patients were examined by experienced rheumatologists and had to fulfil the classification criteria for rheumatoid arthritis (ACR2010),17 psoriatic arthritis (CASPAR)18 and axial spondyloarthritis (ASAS classification criteria).19 None of the HDs had a history of other autoimmune disease. HDs were divided into two groups according to the age and sex matching criteria. The HDs(1) group was age and sex-matched with rheumatoid arthritis patients and the HDs(2) group was age and sex-matched with psoriatic arthritis or ankylosing spondylitis cohorts. Clinical details of patients and HDs are shown in Table 1.
Table 1.
Clinical details of healthy donors and patients with rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis.
| Healthy donors (1) | Rheumatoid arthritis | Healthy donors (2) | Psoriatic arthritis | Axial spondyloarthritis | |
|---|---|---|---|---|---|
| Clinical parameters | |||||
| Women/men (n/n) | 39/11 (50) | 158/42 (200) | 17/33 (50) | 34/46 (80) | 45/105 (150) |
| Age (years) | 46.06 ± 10.08 | 46.94 ± 7.94 | 43.85 ± 11.15 | 45.82 ± 9.99 | 44.63 ± 11.93 |
| Disease duration (years) | – | 10.00 ± 9.43 | – | 8.43 ± 7.81 | 14.28 ± 12.86b,c |
| RF (n +/n–) | – | 140/60 | – | – | – |
| ACPAs (n +/n–) | – | 151/49 | – | – | – |
| DAS28 | – | 4.54 ± 1.57 | – | 3.87 ± 1.74 | – |
| BASDAI | – | – | – | – | 4.32 ± 2.56 |
| BASMI | – | – | – | – | 3.09 ± 1.73 |
| Smoking (yes/no) | 7/43 | 70/130a | 16/84 | 25/55 | 68/82a,b,c |
| BMI (kg/m²) | 24.66 ± 4.76 | 26.95 ± 5.13a | 25.06 ± 3.93 | 28.29 ± 3.87a | 26.51 ± 4.31c |
| Laboratory parameters | |||||
| Glucose (mg/dl) | 81.15 ± 8.40 | 85.07 ± 23.71 | 86.85 ± 15.08 | 91.72 ± 23.14 | 85.54 ± 16.09c |
| Insulin (mg/dl) | 6.30 ± 3.35 | 9.15 ± 6.26a | 7.56 ± 5.23 | 11.71 ± 7.80a,b | 7.83 ± 5.26b,c |
| Cholesterol (mg/dl) | 177.43 ± 32.44 | 198.34 ± 36.22 | 196.44 ± 28.68 | 188.76 ± 38.06 | 188.76 ± 38.06b |
| HDL-cholesterol (mg/dl) | 56.98 ± 14.41 | 57.24 ± 19.11 | 56.34 ± 15.05 | 50.00 ± 12.57a,b | 53.06 ± 15.70b |
| LDL-cholesterol (mg/dl) | 115.54 ± 29.04 | 119.83 ± 31.43 | 119.96 ± 25.45 | 130.64 ± 26.96a,b | 114.73 ± 30.90c |
| Apolipoprotein A (mg/dl) | 150.65 ± 23.32 | 146.64 ± 30.05 | 149.95 ± 25.11 | 148.00 ± 27.51 | 138.35 ± 23.43a,b,c |
| Apolipoprotein B (mg/dl) | 87.45 ± 22.88 | 84.76 ± 20.72 | 90.38 ± 24.87 | 97.97 ± 18.64b | 90.82 ± 25.02b,c |
| Triglycerides (mg/dl) | 89.64 ± 43.36 | 99.27 ± 47.67 | 97.54 ± 43.43 | 106.82 ± 46.32 | 104.24 ± 55.01 |
| ESR (mm/1 h) | 7.55 ± 4.74 | 22.94 ± 17.35a | 6.62 ± 5.12 | 19.07 ± 14.57a | 16.52 ± 17.57a |
| CRP (mg/dl) | 1.40 ± 1.75 | 12.70 ± 26.56a | 1.60 ± 2.04 | 14.13 ± 20.42a | 11.38 ± 18.53a |
| Treatments | |||||
| NSAIDs (yes/no) | – | 162/38 | – | 64/16 | 138/12b |
| Corticosteroids (yes/no) | – | 140/60 | – | 32/48b | 4/145b,c |
| Antimalarial (yes/no) | – | 33/167 | – | – | – |
| Methotrexate (yes/no) | – | 110/90 | – | 31/49b | 7/143b,c |
| Leflunomide (yes/no) | – | 81/119 | – | 17/63b | – |
Values are mean ± SD, unless stated otherwise.
HDs(1), healthy donors cohort, age and sex-matched with rheumatoid arthritis group; HDs(2), healthy donors cohort, age and sex-matched with psoriatic arthritis and ankylosing spondylitis cohorts.
Significant differences versus their corresponding HD group (p < 0.05).
Significant differences versus RA (p < 0.05).
Significant differences versus PsA (p < 0.05).
ACPAs, anti-citrullinated protein antibodies; BASDAI, Bath ankylosing spondylitis disease activity index; BMI, body mass index; CRP, C-reactive protein; DAS, disease activity score; ESR, erythrocyte sedimentation rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NSAIDs, non-steroidal anti-inflammatory drugs; RF, rheumatoid factor.
Variables and data measurement
Analytical measures including fasting serum glucose, insulin, lipid profile, acute phase reactants (C-reactive protein, CRP; erythrocyte sedimentation rate, ESR) and complement C3 were analyzed in all participants. In addition, traditional cardiovascular risk factors such as obesity (BMI > 30 kg/m2), type 2 diabetes (fasting blood glucose levels > 126 mg/dL, hemoglobin A1c level > 6.5% or antidiabetic treatment), hyperlipidemia (cholesterol > 200 mg/dL and triglycerides > 150 mg/dL), hypertension (blood pressure higher than 130 over 80 mmHg) were analyzed.
Non-traditional cardiovascular risk factors such as atherogenic index (AI), apolipoprotein B/apolipoprotein A (ApoB/ApoA) ratio and insulin resistance markers were also evaluated.
Atherogenic risk was calculated by AI based on the levels of total cholesterol (TC) (mg/dL) and high-density lipoprotein (HDL) (mg/dL): AI = TC/HDL. Atherogenic risk was established as >4.5 in women and >5 in men.20
Cardiovascular risk according to the levels of apolipoproteins was calculated by apolipoprotein ratio establishing relative CVD risk groups: low CVD risk (women: 0.3–0.59; men: 0.4–0.69), moderate CVD risk (women: 0.6–0.79; men: 0.7–0.89) and high CVD risk (women: 0.8–1; men: 0.9–1.1). In this study, to calculate the prevalence of CVD risk by ApoB/ApoA in the different rheumatic diseases, subjects were separated into two groups: low CVD risk and moderate to high CVD risk.21,22
Homeostatic model assessment (HOMA) was used to quantify insulin resistance (IR) and β-cell function from fasting glucose and insulin concentrations. HOMA is a standard of the relationship between glucose and insulin concentrations for different combinations of insulin resistance and β-cell function. The equation for insulin resistance uses a fasting blood sample, insulin and glucose divided by a constant: HOMA-IR = [glucose (mg/dL) × insulin (µU/mL)]/405. HOMA-IR values > 2.5 indicated IR.
Likewise, to determine insulin activity we used a marker of basal insulin secretion of pancreatic β-cells: HOMA-β = 360 × fasting insulin (µU/mL)/fasting glucose (mg/dL) – 63 (%).
Quantitative insulin sensitivity check index (QUICKI) was used to quantify insulin sensitivity by a mathematical transformation of plasma glucose and insulin levels, taking both the logarithm and the reciprocal of the glucose and insulin product: QUICKI = 1/log [insulin (µU/mL)) + log (glucose (mg/dL)].
Statistical methods
A test for normal distribution was performed. In addition, to compare two independent groups, we used Student’s t-test or alternatively a non-parametric test (Mann–Whitney rank sum test). For multiple comparisons, the one-way analysis of variance (ANOVA) test or Kruskall–Wallis test were performed. The chi-squared test was performed to analyze qualitative data. Receiver operating characteristic (ROC) curves, plotting the true positive rate (sensitivity) versus the false positive rate (1-specificity) at various threshold settings, and the areas under the curve (AUC) analysis were used to determine the sensitivity, specificity and corresponding cut-off values using GraphPad Prism 8.0.1. Furthermore, in order to establish different phenotypes of patients according to their cardiometabolic risk state we performed a cluster analysis with a hard clustering method. Variables included in the cluster analysis were complement C3 levels and HOMA-IR. Besides, a multiple linear regression model was applied to identify whether the treatments affect complement C3 levels. Treatment variables included were methotrexate, leflunomide, non-steroidal anti-inflammatory drugs (NSAIDs), hydroxychloroquine and corticosteroids. Correlations were assessed by Spearman’s rank correlation. p < 0.05 was considered statistically significant.
Results
Differential prevalence of cardiometabolic risk factors in patients with rheumatoid arthritis and spondyloarthritis
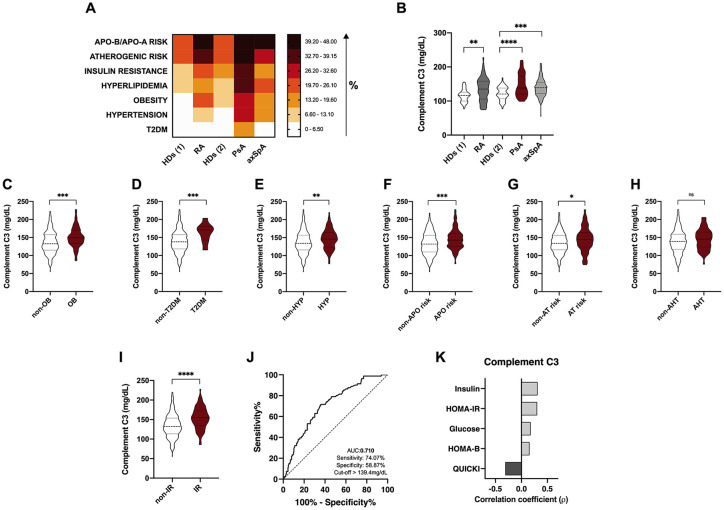
We first evaluated the prevalence of cardiometabolic risk factors in patients with rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis, including: ApoB/ApoA and atherogenic risks, insulin resistance, hyperlipidemia, obesity, hypertension and type 2 diabetes mellitus (T2DM). In our cohorts of three rheumatic diseases, psoriatic arthritis was the one showing the worst CVr risk profile with the highest accumulated number of CV risk factors, showing a significant increase in the prevalence of the seven CV risk factors studied compared to the aged-matched control group (HDs(2)) (Figure 1A). Rheumatoid arthritis was associated with an elevated frequency of ApoB/ApoA and atherogenic risks, insulin resistance, hyperlipidemia, obesity and hypertension compared to the aged-matched control group (HDs(1)). Finally, axSpA patients were characterized by a significantly increased prevalence of both ApoB/ApoA and atherogenic risks, hyperlipidemia, obesity and hypertension compared to healthy controls (HDs(2)) (Figure 1A).
Figure 1.
Association between complement C3 levels and cardiometabolic risk factors in rheumatoid arthritis and spondyloarthritis (PsA and axSpA). (A) Heat map showing the prevalence of cardiometabolic risk factors in RA, PsA, axSpA patients and healthy donors. (B) Serum levels of complement C3 in RA, PsA and axSpA patients. (C) Serum levels of complement C3 in non-obese and obese patients in the whole cohort of RA and spondyloarthritis. (D) Serum levels of complement C3 in non-T2DM and T2DM patients in the whole cohort of RA and spondyloarthritis. (E) Serum levels of complement C3 in patients with or without hyperlipidemia in the whole cohort of RA and spondyloarthritis. (F) Serum levels of complement C3 in patients with or without ApoB/ApoA risk in the whole cohort of RA and spondyloarthritis. (G) Serum levels of complement C3 in patients with or without atherogenic risk in the whole cohort of RA and spondyloarthritis. (H) Serum levels of complement C3 in patients with or without hypertension in the whole cohort of RA and spondyloarthritis. (I) Serum levels of complement C3 in patients with or without insulin resistance in the whole cohort of RA and spondyloarthritis. (J) ROC curve analysis of complement C3 to assess the accuracy of this parameter as a biomarker of insulin resistance in RA and spondyloarthritis. (K) Spearman correlation between levels of complement C3 and insulin resistance in the whole cohort of RA and spondyloarthritis.
HDs(1), healthy donors cohort, age and sex-matched with rheumatoid arthritis group.
HDs(2), healthy donors cohort, age and sex-matched with psoriatic arthritis and ankylosing spondylitis cohorts.
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
AHT, hypertension; APO risk, apoB/apoA risk; AT, atherogenic; axSpA, axial spondyloarthritis; C3, component C3; HDs, healthy donors; HOMA, homeostatic model assessment; HYP, hyperlipidemia; IR, insulin resistance; OB, obese; PsA, psoriatic arthritis; QUICKI, quantitative insulin-sensitivity check index; RA, rheumatoid arthritis; ROC, receiver operating characteristic; T2DM, type 2 diabetes mellitus.
ApoB/ApoA and atherogenic risks, hyperlipidemia, obesity and hypertension risk were the four cardiovascular risk factors commonly increased in the three rheumatic diseases included in this study (Figure 1A).
Complement C3 levels in rheumatoid arthritis and spondyloarthritis: association with obesity, insulin resistance, T2DM, hypertension, atherogenic and ApoB/ApoA risks
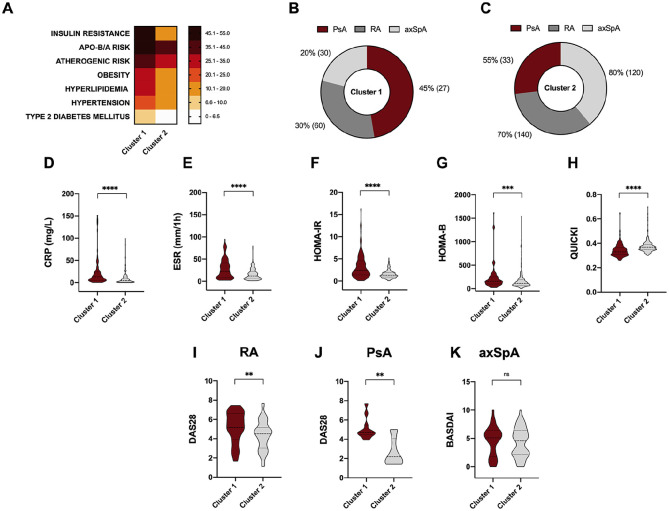
Serum levels of component C3 were significantly elevated in RA, PsA and axSpA patients compared to healthy donors (Figure 1B). In addition, those patients having obesity, T2DM, hyperlipidemia, hypertension, atherogenic and ApoB/ApoA risks and insulin resistance had elevated serum levels of complement C3 (Figure 1C–I). Of note, among all the CV risk factors, complement C3 levels were able to discriminate between insulin resistant and insulin sensitive patients, showed by the ROC analyses (Figure 1J). In addition, levels of complement C3 strongly correlated with serum levels of insulin and HOMA-IR or QUICKI indexes (Figure 1K). Due to the strong association observed between insulin resistance and complement C3, we aimed to know whether this association could differentiate a worse cardiometabolic profile. Thus, cluster analysis including HOMA-IR and complement C3 levels as variables distinguished two different phenotypes of patients according to their cardiometabolic risk factor prevalence. Thus, cluster 1 was characterized by a significant increase of cardiovascular risk compared to cluster 2, showing higher rates of insulin resistance, ApoB/ApoA and atherogenic risks, obesity, hyperlipidemia, hypertension and T2DM (Figure 2A). This group was mainly composed of a higher proportion of PsA patients, followed by RA and axSpA (Figure 2B). Conversely, cluster 2 had a higher proportion of axSpA patients, followed by RA and PsA patients (Figure 2C).
Figure 2.
Cluster analysis recognizes two different phenotypes of patients according to their cardiometabolic risk burden. (A) Cluster analysis including HOMA-IR and complement C3 levels as variables distinguished two different phenotypes of patients according to their cardiometabolic risk factor prevalence. (B) The proportion of RA, PSA and axSpA patients composing cluster 1. (C) The proportion of RA, PsA and axSpA patients composing cluster 2. (D) CRP levels in cluster 1 and cluster 2. (E) ESR levels in cluster 1 and cluster 2. (F) HOMA-IR levels in cluster 1 and cluster 2. (G) HOMA-B levels in cluster 1 and cluster 2. (H) QUICKI levels in cluster 1 and cluster 2. (I) DAS28 levels in RA patients included in cluster 1 and cluster 2. (J) DAS28 levels in PsA patients included in cluster 1 and cluster 2. (K) BASDAI levels in axSpA patients included in cluster 1 and cluster 2.
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
axSpA, axial spondyloarthritis; BASDAI, Bath ankylosing spondylitis disease activity index; CRP, C-reactive protein; C3, component C3; DAS, disease activity score; ERS, erythrocyte sedimentation rate; HOMA, homeostatic model assessment; PsA, psoriatic arthritis; QUICKI, quantitative insulin sensitivity check index; RA, rheumatoid arthritis; T2DM, type 2 diabetes mellitus.
Patients embedded in cluster 1 had significantly elevated levels of CRP, ESR and markers of insulin resistance such as HOMA-IR and HOMA-B compared to cluster 2 (Figure 2D–H).
Regarding disease activity, PsA and RA patients classified in cluster 1 according to complement C3 levels and insulin resistance had elevated disease activity (DAS28 index) (Figure 2I and J). In contrast, disease activity score of the axSpA patients was not different between the two clusters (Figure 2K).
Non-related obesity complement C3 levels and its association with CV risk factors and clinical parameters in rheumatoid arthritis and spondyloarthritis
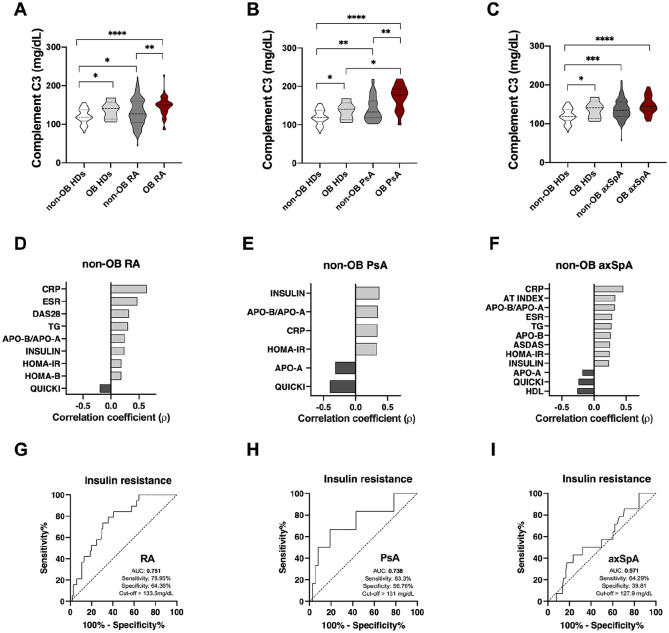
As elevation of the BMI has been strongly related to the levels of complement C3, and obesity is increased in our cohorts of patients with rheumatic diseases, we sought to investigate the role of complement C3 as a cardiovascular risk biomarker in these diseases independently of the obesity rates.
As expected, serum levels of complement C3 were significantly elevated in obese compared to non-obese subjects regardless of the presence or absence of any rheumatic disease (Figure 3A–C). Among non-obese subjects, patients suffering either of three rheumatic diseases (RA, PsA or axSpA) had significantly elevated levels of complement C3 compared to healthy donors (Figure 3A–C). Only obese PsA patients had significantly higher levels of complement C3 compared to obese controls (Figure 3B).
Figure 3.
Association between complement C3 levels and disease activity and cardiometabolic risk factors in non-obese patients with rheumatoid arthritis and spondyloarthritis (psoriatic arthritis and spondyloarthritis). (A) Serum levels of complement C3 in healthy donors and RA patients with or without obesity. (B) Serum levels of complement C3 in healthy donors and PsA patients with or without obesity. (C) Serum levels of complement C3 in healthy donors and axSpA patients with or without obesity. (D) Spearman correlation between levels of complement C3 and clinical and laboratory parameters in non-obese RA patients. (E) Spearman correlation between levels of complement C3 and clinical and laboratory parameters in non-obese PsA patients. (F) Spearman correlation between levels of complement C3 and clinical and laboratory parameters in non-obese axSpA patients. (G) ROC curve analysis of complement C3 to assess the accuracy of this parameter as a biomarker of insulin resistance in non-obese RA patients. (H) ROC curve analysis of complement C3 to assess the accuracy of this parameter as a biomarker of insulin resistance in non-obese PsA patients. (I) ROC curve analysis of complement C3 to assess the accuracy of this parameter as a biomarker of insulin resistance in non-obese axSpA patients.
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
ASDAS, ankylosing spondylitis disease activity score; axSpA, axial spondyloarthritis; CRP, C-reactive protein; C3, component C3; ESR, erythrocyte sedimentation rate; HDL, high-density lipoprotein; HOMA, homeostatic model assessment; OB, obese; PsA, psoriatic arthritis; QUICKI, quantitative insulin sensitivity check index; RA, rheumatoid arthritis; ROC, receiver operating characteristic; TG, triglycerides.
In the three cohorts of rheumatic diseases, excluding obese patients, levels of complement C3 correlated with insulin resistance, inflammatory markers, dyslipemia and activity of the disease (Figure 3D–F). In addition, ROC curve analyses showed that complement C3 levels in the absence of obesity could be used to discriminate between insulin resistant and insulin sensitive patients (Figure 3G–I), especially in RA and PsA (Figure 3G and H).
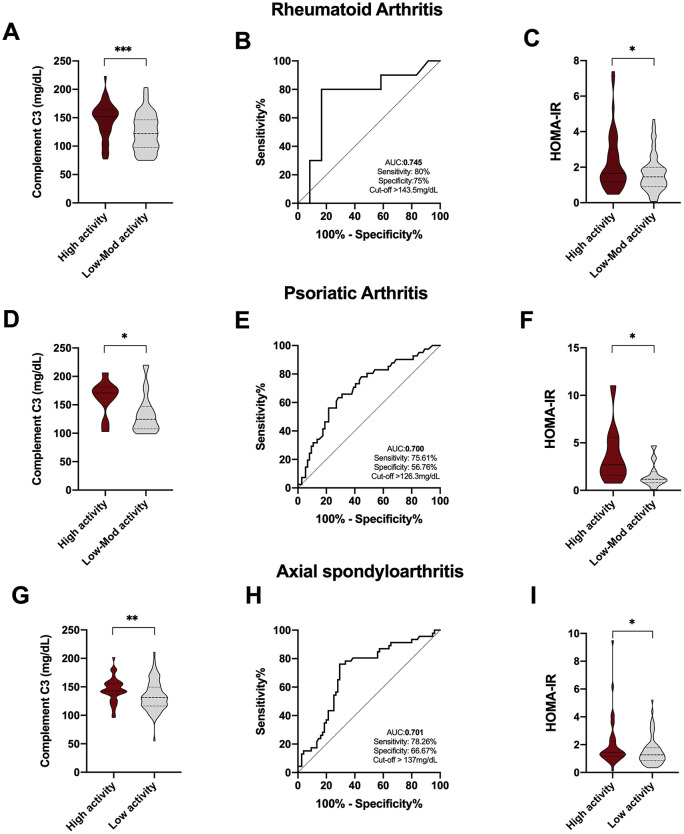
Complement C3 levels as a biomarker of disease activity in rheumatoid arthritis and spondyloarthritis
Serum levels of complement C3 were significantly elevated in those RA, PsA and axSpA patients having high disease activity [defined by a disease activity score 28 (DAS28) > 5.1 for RA and PsA and a Bath ankylosing spondylitis disease activity index (BASDAI) > 4/ESR > 15 for AS] (Figure 4A, D and G). Thus, ROC curve analysis, performed to assess the accuracy of complement C3 as a biomarker of disease activity, allowed us to discriminate among patients with high activity disease [DAS28 and ankylosing spondylitis disease activity score (ASDAS)] (Figure 4B, E and H). In addition, insulin resistance was strongly associated with the activity of the disease in the three rheumatic diseases studied. Thus, patients with high disease activity had higher levels of HOMA-IR, suggesting the strong link between the development of insulin resistance and systemic inflammation (Figure 4C, F and I).
Figure 4.
Complement C3 levels as a biomarker of disease activity in RA, PsA and axSpA. (A) Complement C3 levels in RA patients with high and low to moderate disease activity. (B) ROC curve analysis of complement C3 to assess the accuracy of this parameter as biomarker of disease activity in RA patients. (C) HOMA-IR levels in RA patients with high and low to moderate disease activity. (D) Complement C3 levels in PsA patients with high and low to moderate disease activity (E) ROC curve analysis of complement C3 to assess the accuracy of this parameter as biomarker of disease activity in PsA patients. (F) HOMA-IR levels in PsA patients with high and low to moderate disease activity. (G) Complement C3 levels in axSpA patients with high and low disease activity. (H) ROC curve analysis of complement C3 to assess the accuracy of this parameter as a biomarker of disease activity in axSpA patients. (I) HOMA-IR levels in axSpA patients with moderate to high and low disease activity.
*p < 0.05, **p < 0.01, ***p < 0.001.
axSpA, axial spondyloarthritis; C3, component C3; HOMA, homeostatic model assessment; IR, insulin resistance; PsA, psoriatic arthritis; QUICKI, quantitative insulin sensitivity check index; RA, rheumatoid arthritis; ROC, receiver operating characteristic.
Discussion
The major finding of this study is that complement C3 levels, significantly elevated not only in rheumatoid arthritis and psoriatic arthritis but also in axial spondyloarthritis, are associated with the concomitant presence of cardiometabolic risk factors, including atherogenic and ApoB/ApoA risks, obesity, insulin resistance, T2DM, hypertension and hyperlipidemia. This relationship was also noticed in a non-obesity setting. In addition, complement C3 not only could be considered a useful marker of CV risk in rheumatoid arthritis and spondylarthritis, but also a biomarker of disease activity.
Here we showed the presence of cardiometabolic risk factors in three rheumatic diseases, which are well known for their association with CV morbidity and mortality, RA, PsA and axSpA.23,24 Among them, PsA was the disease with the worst CV risk profile, shown by the higher prevalence of ApoB/ApoA and atherogenic risks, obesity, insulin resistance, hyperlipidemia, hypertension and T2DM compared to RA and axSpA. In all of them, levels of complement C3 were significantly elevated and strongly related to obesity, IR, T2DM, hyperlipidemia and atherogenic and ApoB/ApoA risks. Moreover, ROC curves showed that complement C3 levels could be used as a useful marker of insulin resistance in these diseases. To date, several reports have linked complement components, C3 or C4 and cardiovascular disease, although the mechanisms underlying this association are not completely understood yet.25
In our work, cluster analysis including complement C3 and HOMA-IR as variables, identified two phenotypes of patients characterized by high and low CV risk. Thus cluster 1, mainly composed of PsA patients, had a concomitant higher prevalence of cardiometabolic risk factors and high disease activity compared to cluster 2, mainly composed of axSpA patients, which displayed less CV risk and lower disease activity. These results suggest that inflammation and abnormal glucose metabolism are strongly linked to a defective CV system and an altered lipid profile, and thus the subsequent progression of these rheumatic diseases, especially psoriatic arthritis.
Increased systemic inflammation is a determinant in the development of adult cardiometabolic diseases such as insulin resistance, dyslipidemia, atherosclerosis, and hypertension. The complement system is a part of the innate immune system and plays a key role in the regulation of inflammation.25 In particular, complement C3 levels have been shown to be correlated with inflammatory markers such as CRP, lipid profile, BMI, fasting glucose levels and fat distribution in randomly selected elderly individuals, suggesting its contribution to inflammation and the metabolic syndrome.5 Complement C3 is produced primarily by the liver but is also generated in adipocytes, macrophages and endothelial cells, all of which are present in adipose tissues. Epidemiologically, patients with cardiometabolic disease (obese and non-obese) have increased complement levels.25 In our hands, serum complement C3 levels were significantly increased in obese subjects compared with non-obese subjects regardless of the presence or absence of rheumatic disease, confirming the strong link between fat mass and the production of complement C3. In addition, comparing non-obese subjects, patients with either of the three rheumatic diseases had elevated levels of complement C3. However, only obese PsA patients had significantly higher levels of complement C3 compared to obese ‘healthy donors’, suggesting that PsA might have more dysfunctional adipose tissue contributing to the higher cardiometabolic risk observed. Interestingly, in our hands, non-obesity-related complement C3 levels strongly correlated to inflammatory and insulin resistance markers, lipid profile and disease activity in rheumatoid arthritis and spondyloarthritis, suggesting the strong link of this immune component with clinical parameters of the disease and cardiovascular risk factors regardless of fat mass contribution. In addition, in this condition, complement C3 was able to act as a marker of insulin resistance in the three rheumatic diseases studied.
Recently, complement C3 has been suggested as a marker of insulin resistance in rheumatoid arthritis and psoriatic arthritis patients.14,16 In fact, these authors reported that complement C3 could predict the presence of T2DM26 and might be a surrogate biomarker of fatty liver disease in RA patients.27 Thus, the link of complement C3 with metabolic abnormalities has been studied deeply in rheumatoid arthritis. However, there is much less evidence in psoriatic arthritis and none in axial spondyloarthritis. This is the first study that gives an insight regarding the association of complement C3 and cardiometabolic alterations in axSpA.
On the other hand, the current search for serum biomarkers of disease activity highlights the need for having objective measures. In our work complement C3 levels were elevated in those patients having high disease activity, so that ROC curve analysis showed that complement C3 might be a useful biomarker of the assessment of disease activity in the clinical setting of rheumatoid arthritis and spondyloarthritis.
In addition, HOMA-IR levels were also significantly increased in those patients with high disease activity in rheumatoid arthritis and spondyloarthritis, suggesting the strong link between systemic inflammation and the development of insulin resistance in those diseases. In fact, inflammatory cytokines have directly been involved in the development of insulin resistance.28 Thus, we recently reported that metabolic disturbances associated with rheumatoid arthritis depend on the degree of inflammation and identified tumor necrosis factor (TNF)-α and interleukin (IL)-6 as the main actors causing insulin resistance in this disorder.29 In addition, insulin resistance has been strongly related to the DAS28 in rheumatoid arthritis.30
All in all, complement C3 could be considered as a surrogate biomarker taken into account in the cardiovascular risk and progression of the disease assessments in rheumatoid arthritis and spondyloarthritis. Thus, complement C3 may represent a new field for intervention in the prevention of cardiometabolic disorders associated with rheumatic diseases.
Acknowledgments
The authors would like to thank all of the patients who participated in the study. We also thank Roche Farma, S.A for its collaboration. In memory of Maria del Carmen Castro Villegas, co-author of this article, who passed away during the revision process, she will always be with us.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Instituto de Salud Carlos III (PI15/01316), cofinanciado por el Fondo Europeo de Desarrollo Regional de la Unión Europea ‘Una manera de hacer Europa’ and Rheumatic Diseases Network (RIER), Instituto de Salud Carlos III (RD16/0012/0015) and Junta de Andalucía (PI-0139-2017). C.L-P was supported by a contract from the Spanish Junta de Andalucía (‘Nicolas Monardes’ programme). AIC was supported by ‘Juan de la Cierva’ programme (FJCI-2016-30825).
ORCID iD: Nuria Barbarroja  https://orcid.org/0000-0002-0962-6072
https://orcid.org/0000-0002-0962-6072
Contributor Information
Iván Arias de la Rosa, Medicine Department, University of Cordoba, Maimonides Institute for Research in Biomedicine of Cordoba (IMIBIC), Reina Sofia University Hospital, Cordoba, Spain.
Pilar Font, Medicine Department, University of Cordoba, Maimonides Institute for Research in Biomedicine of Cordoba (IMIBIC), Reina Sofia University Hospital, Cordoba, Spain.
Alejandro Escudero-Contreras, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
María Dolores López-Montilla, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Carlos Pérez-Sánchez, Department of Medicine, University of Cambridge School of Clinical Medicine, Cambridge Biomedical Campus, Cambridge, UK.
María Carmen Ábalos-Aguilera, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Lourdes Ladehesa-Pineda, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Alejandro Ibáñez-Costa, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Carmen Torres-Granados, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Yolanda Jimenez-Gomez, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Alejandra Patiño-Trives, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
María Luque-Tévar, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
María Carmen Castro-Villegas, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Jerusalem Calvo-Gutiérrez, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Rafaela Ortega-Castro, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Chary López-Pedrera, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Eduardo Collantes-Estévez, Rheumatology service, IMIBIC, Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
Nuria Barbarroja, Medicine Department, University of Cordoba, Maimonides Institute for Research in Biomedicine of Cordoba (IMIBIC), Reina Sofia University Hospital; CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN), Instituto de Salud Carlos III, Madrid, Spain.
References
- 1. Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 2015; 11: 693–704. [DOI] [PubMed] [Google Scholar]
- 2. Castañeda S, Martín-Martínez MA, González-Juanatey C, et al. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: baseline data of the CARMA project. Semin Arthritis Rheum 2015; 44: 618–626. [DOI] [PubMed] [Google Scholar]
- 3. López-Medina C, Jiménez-Gómez Y, Moltó A, et al. Cardiovascular risk factors in patients with spondyloarthritis from Northern European and Mediterranean countries: an ancillary study of the ASAS-COMOSPA project. Joint Bone Spine 2018; 85: 447–453. [DOI] [PubMed] [Google Scholar]
- 4. Hertle E, Stehouwer CDA, van Grevenbroek MMJ. The complement system in human cardiometabolic disease. Mol Immunol 2014; 61: 135–148. [DOI] [PubMed] [Google Scholar]
- 5. Nilsson B, Hamad OA, Ahlström H, et al. C3 and C4 are strongly related to adipose tissue variables and cardiovascular risk factors. Eur J Clin Invest 2014; 44: 587–596. [DOI] [PubMed] [Google Scholar]
- 6. Oikonomopoulou K, Ricklin D, Ward PA, et al. Interactions between coagulation and complement – their role in inflammation. Semin Immunopathol 2012; 34: 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xin Y, Hertle E, van der Kallen CJH, et al. Complement C3 and C4, but not their regulators or activated products are associated with incident metabolic syndrome: the CODAM study. Endocrine 2018; 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engstrom G, Hedblad B, Janzon L, et al. Complement C3 and C4 in plasma and incidence of myocardial infarction and stroke: a population-based cohort study. Eur J Cardiovasc Prev Rehabil 2007; 14: 392–397. [DOI] [PubMed] [Google Scholar]
- 9. Philips CM, Kesse-Guyot E, Ahluwalia N, et al. Dietary fat, abdominal obesity and smoking modulate the relationship between plasma complement component 3 concentrations and metabolic syndrome risk. Atherosclerosis 2012; 220: 513–519. [DOI] [PubMed] [Google Scholar]
- 10. Barbu A, Hamad OA, Lind L, et al. The role of complement factor C3 in lipid metabolism. Mol Immunol 2015; 67: 101–107. [DOI] [PubMed] [Google Scholar]
- 11. Fiorentino TV, Hribal ML, Andreozzi F, et al. Plasma complement C3 levels are associated with insulin secretion independently of adiposity measures in non-diabetic individuals. Nutr Metab Cardiovasc Dis 2015; 25: 510–517. [DOI] [PubMed] [Google Scholar]
- 12. Vlaicu SI, Tatomir A, Boodhoo D, et al. The role of complement system in adipose tissue-related inflammation. Immunol Res 2016; 64: 653–664. [DOI] [PubMed] [Google Scholar]
- 13. Xin Y, Hertle E, van der Kallen CJH, et al. Longitudinal associations of the alternative and terminal pathways of complement activation with adiposity: the CODAM study. Obes Res Clin Pract 2018; 12: 286–292. [DOI] [PubMed] [Google Scholar]
- 14. Ursini F, Grembiale A, Naty S, et al. Serum complement C3 correlates with insulin resistance in never treated psoriatic arthritis patients. Clin Rheumatol 2014; 33: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 15. Ursini F, D’Angelo S, Russo E, et al. Complement C3 is the strongest predictor of whole-body insulin sensitivity in psoriatic arthritis. PLoS One 2016; 11: e0163464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ursini F, D’Angelo S, Russo E, et al. Serum complement C3 strongly correlates with whole-body insulin sensitivity in rheumatoid arthritis. Clin Exp Rheumatol 2017; 35: 18–23. [PubMed] [Google Scholar]
- 17. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 18. Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 19. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009; 68: 777–783. [DOI] [PubMed] [Google Scholar]
- 20. Millan J, Pintó X, Zúñiga M, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009; 5: 757–765. [PMC free article] [PubMed] [Google Scholar]
- 21. Lima LM, das Carvalho M, Sousa MO. Apo B/apo A-I ratio and cardiovascular risk prediction. Arq Bras Cardiol 2007; 88: e187–e190. [DOI] [PubMed] [Google Scholar]
- 22. Walldius G1, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy – a review of the evidence. J Intern Med 2006; 259: 493–519. [DOI] [PubMed] [Google Scholar]
- 23. Liew JW, Ramiro S, Gensler LS. Cardiovascular morbidity and mortality in ankylosing spondylitis and psoriatic arthritis. Best Pract Res Clin Rheumatol 2018; 32: 369–389. [DOI] [PubMed] [Google Scholar]
- 24. Lauper K, Gabay C. Cardiovascular risk in patients with rheumatoid arthritis. Semin Immunopathol 2017; 39: 447–459. [DOI] [PubMed] [Google Scholar]
- 25. Copenhaver M, Yu CY, Hoffman RP. Complement components, C3 and C4, and the metabolic syndrome. Curr Diabetes Rev 2019; 15: 44–48. [DOI] [PubMed] [Google Scholar]
- 26. Ursini F, Angelo SD, Russo E, et al. Serum complement C3 and type 2 diabetes in rheumatoid arthritis: a case–control study. Rev Recent Clin Trials 2018; 13: 215–221. [DOI] [PubMed] [Google Scholar]
- 27. Ursini F, Russo E, Mauro D, et al. Complement C3 and fatty liver disease in RA patients: a cross-sectional study. Eur J Clin Invest 2017; 47: 728–735. [DOI] [PubMed] [Google Scholar]
- 28. Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz) 2013; 61: 119–125. [DOI] [PubMed] [Google Scholar]
- 29. de la Rosa IA, Escudero-Contreras A, Rodriguez-Cuenca S, et al. Defective glucose and lipid metabolism in rheumatoid arthritis is determined by chronic inflammation in metabolic tissues. J Intern Med 2018; 284: 61–77. [DOI] [PubMed] [Google Scholar]
- 30. Costa NT, Iriyoda TMV, Kallaur AP, et al. Influence of insulin resistance and TNF-α on the inflammatory process, oxidative stress, and disease activity in patients with rheumatoid arthritis. Oxid Med Cell Longev 2016; 2016: 8962763. [DOI] [PMC free article] [PubMed] [Google Scholar]