Abstract
Objective
The relationships among sleep, circadian rhythm, and intensive care unit (ICU)-acquired delirium are complex and remain unclear. This study aimed to examine the pathophysiological mechanisms of sleep and circadian rhythm disturbances in patients with ICU-acquired delirium.
Methods
This study included critical adult patients aged 18 to 75 years who were treated in the ICU. Twenty-four-hour polysomnography was performed and serum melatonin and cortisol levels were measured six times during polysomnography. Receiver operating characteristic curves and binomial logistic regression were used to evaluate the potential of sleep, melatonin, and cortisol as indicators of delirium in the ICU.
Results
Patients with delirium (n = 24) showed less rapid eye movement (REM) sleep compared with patients without delirium (n = 24, controls). Melatonin levels were lower and cortisol levels were higher in the delirium group than in the control group. REM sleep, melatonin, and cortisol were significantly associated with delirium. The optimal cutoff values of REM sleep and mean melatonin and cortisol levels that predicted delirium were ≤1.05%, ≤422.09 pg/mL, and ≥212.14 ng/mL, respectively.
Conclusions
REM sleep, and melatonin and cortisol levels are significantly associated with the risk of ICU-acquired delirium. Improved sleep and readjustment of circadian rhythmicity may be therapeutic targets of ICU-acquired delirium.
Keywords: Delirium, critical care, polysomnography, sleep, melatonin, cortisol
Introduction
Delirium is an acute disorder of cognition and marked cerebral dysfunction that results in various symptoms. Delirium is common among patients treated in the intensive care unit (ICU) and is associated with poor outcomes, including increased mortality, increased length of hospital stay, cognitive decline, and long-term cognitive impairment.1–3
A core domain of delirium is represented by disruption of sleep. Sleep disturbances impair cognition, resulting in apathy and confusion.4–6 Therefore, we hypothesized that sleep disturbances represent a high risk factor for delirium. However, the relationship between sleep disturbances and delirium is unclear.7 Patients in the ICU are affected by noise, pain experienced, discomfort, medication, and the mode of mechanical ventilation.8 Therefore, assessment of sleep in the ICU setting is difficult. Polysomnography (PSG) is a standard sleep monitoring method that allows for assessment of sleep architecture. Some PSG studies that only monitored nighttime sleep reported that patients in the ICU showed sleep fragmentation,9 a severe decrease in rapid eye movement (REM) sleep, and circadian misalignment.10 Because sleep patterns of patients in the ICU do not follow a normal profile, they may sleep all day and night. Therefore, 24-hour continuous PSG is appropriate and necessary to monitor sleep. However, few studies have used 24-hour continuous PSG to examine sleep disturbances among patients in the ICU.11,12
Melatonin and cortisol levels follow a circadian rhythm, which is believed to be associated with sleep. Our previous study showed that patients with delirium in the ICU had reduced melatonin secretion.12 Other studies have suggested that increased cortisol levels are associated with a risk of brain dysfunction and postoperative delirium.13 However, whether abnormal secretion of melatonin and cortisol can be used as an indicator of delirium among patients in the ICU remains unclear.
We investigated the proposed pathophysiological mechanisms of disrupted sleep and circadian dysrhythmia as possible contributing factors of delirium in the ICU. To verify these hypotheses, we examined 24-hour continuous PSG, and serum melatonin and cortisol levels in patients with and without delirium in the ICU.
Methods
Participants
In this single-center, prospective case–control study, we recruited patients who were treated in the ICU of an educational hospital with 1903 beds and 22 ICU beds. The study was approved by the Human Research Ethics Committee of Binzhou Medical University Hospital (approval number: 2016-011-01) and was conducted in accordance with the ethical standards stated in the Helsinki Declaration. The study was registered in the Clinical Trials Register (ChiCTR-ROC-16010274). All patients or guardians provided written informed consent on the day of admission. The inclusion criteria were patients aged 18 to 75 years staying in the ICU during the period of the study (24 hours). The exclusion criteria were as follows: presence of delirium before ICU admission, psychiatric illness or dementia, pregnancy, nervous system disease, auditory or visual impairment, severe liver dysfunction, brain disease, advanced malignancy, and a history of sleep disorders.
All patients were routinely assessed in accordance with the confusion assessment method-ICU once per shift (one shift = 8 hours).14 Patients showing signs of delirium during the assessment were assigned to the delirium group. At the same time, Patients without delirium who were admitted into the ICU with a similar age ( < a 5-year age gap) and the same diseases were selected for the control group at a ratio of 1:1.
Clinical data collection
Clinical and laboratory data were recorded on admission. The Acute Physiology and Chronic Health Evaluation (APACHE) II was used to assess the severity of illness.15 All patients were monitored in accordance with standard ICU principles. Sedatives and analgesics were prescribed by attending physicians in accordance with the guidelines outlined by the Society of Critical Care Medicine.16
Peripheral venous blood samples (3 mL) were obtained six times during the 24-hour PSG monitoring period (02:00, 06:00, 10:00, 14:00, 18:00, and 22:00 hours). Each blood sample was poured into a serum separator tube and centrifuged for 10 minutes at 2000×g. The separated serum was collected and stored at −80°C until analysis. Cortisol and melatonin levels were assayed using the Immulite system (KGE008; R&D systems, Minneapolis, MN, USA and RE 54021; IBL International, Hamburg, Germany).
PSG procedure and analysis
Patients in both groups were monitored for 24 hours using a portable PSG device (Siesta 802; Compumedics, Melbourne, Australia). During sleep monitoring, patients underwent two-lead frontal electroencephalography (EEG [F3/M2, F4/M1]), two-lead electrooculography, one-chin electromyography, and one-lead electrocardiography. Standard skin preparation techniques were used. Gold cup EEG electrodes were placed in accordance with the International System.17 Electrodes were applied by trained technicians. Channels were calibrated before each recording and electrode impedance was kept below 5000 Ω. A notch filter was applied to reject 50-Hz electrical noise. We used a bedside laptop to display real-time monitoring signals, and between recordings, the nurse and technicians viewed the PSG signals and rechecked the electrodes.
Sleep recordings were manually scored by trained sleep technicians in 30-s epochs. PSG data were analyzed by technicians, who were blinded to the patient groups, in accordance with the standards outlined by the American Academy of Sleep Medicine (AASM).17,18 Total sleep time was defined as the sum of the total time spent in all sleep stages during the overall monitoring period. Total sleep time was further separated into daytime (06:00–22:00 hours) and nighttime (22:00–06:00 hours the next day) sleep periods. Arousals were defined on the basis of the AASM criteria.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 22.0 software (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean ± standard deviation or median (interquartile range), and categorical variables are expressed as numbers and percentages. Continuous variables were compared using the Student’s t-test or the Mann–Whitney U-test, as appropriate, and categorical variables were analyzed using the chi-square test. The different time points of excretion levels were assessed by repeated-measures analysis of variance. Peak and mean values of melatonin and cortisol excretion were compared between patients with and without delirium. Receiver operating characteristic curves were generated and the area under the curve was calculated. Odds ratios (ORs) with 95% confidence intervals (CIs) were computed using a binomial logistic regression with delirium as the dependent variable. For all tests, a P value of < 0.05 was considered significant.
Results
Characteristics of the patients
Sixty patients were enrolled from December 2017 to November 2018. Four patients with delirium removed PSG electrodes after recording began because of dysphoria. Therefore, these patients were excluded. Two more PSG records of delirium that were unable to be analyzed were excluded. Six patients in the corresponding control group (patients without delirium) were excluded at the same time. Finally, data from 48 patients, 24 who had delirium and 24 who did not have delirium, comprised the final PSG data set for analysis.
The basic clinical characteristics of the two groups are shown in Table 1. There was no significant difference in age or sex between the two groups. The mean APACHE II score was significantly higher in the delirium group than in the control group (P = 0.03). Lactic acid levels at the time of enrolment were significantly higher in the delirium group than in the control group (P = 0.009).
Table 1.
Patients’ baseline characteristics.
| Characteristic | Delirium group | Control group | P value |
|---|---|---|---|
| (n = 24) | (n = 24) | ||
| Age (years) | 55.29 ± 14.30 | 51.88 ± 15.08 | 0.425 |
| Male/female | 18/6 | 12/12 | 0.074 |
| APACHE II score | 18.42 ± 6.94 | 14.21 ± 6.08 | 0.03* |
| Lactic acid | 4.00 (2.93–5.20) | 2.25 (1.13–4.15) | 0.009# |
Values are shown as mean ± standard deviation, number, or median (interquartile range). *t-test; #Mann–Whitney U-test.
APACHE II, acute physiology and chronic health evaluation II.
PSG
There were substantial variations in the predominant background EEG activity in individual patients. Some patients showed suppressed EEG activity or diffuse slow waves. In some patients, PSG recordings showed high levels of alpha activity. Patients in both groups showed abnormal sleep architecture compared with standard normative values, as indicated by an abnormal distribution of sleep stage.19 Sleep stages 1 and 2 were predominant (23.50% and 53.12%, respectively) (Table 2). All patients showed a low rate of stage 3 and REM sleep, and were accompanied by a high rate of arousal. REM sleep in the delirium group was significantly lower than that in the control group (P = 0.002). Eighteen of the patients had no REM sleep. There were no significant differences in other sleep stages between the two groups.
Table 2.
Sleep characteristics.
| Variable | Normal values | All patients | Delirium group | Control group | P value |
|---|---|---|---|---|---|
| (n = 48) | (n = 24) | (n = 24) | |||
| Total sleep time (minutes) | 480 | 383.50 (241.5–605.0) | 369.00 (220.75–894.00) | 392.5 (298.00–564.00) | 0.837 |
| Total sleep time daytime (minutes) | 142.50 (47.25–265.25) | 143.00 (62.00–460.13) | 125.00 (34.87–194.00) | 0.124 | |
| Stage 1 (% total sleep time) | 4.4 | 23.50 (15.25–41.25) | 27.85 (17.48–53.55) | 18.85 (14.2–37.75) | 0.161 |
| Stage 2 (% total sleep time) | 56.5 | 53.12±17.54 | 52.95±19.40 | 53.30±15.89 | 0.945 |
| Stage 3 (% total sleep time) | 17.7 | 10.65 (3.60–17.35) | 7.1 (3.6–12.93) | 13.7 (4.85–22.83) | 0.119 |
| REM (% total sleep time) | 20.8 | 1.0 (0.23–4.58) | 0.45 (0.005–1.00) | 3.15 (0.88–7.15) | 0.002# |
| Arousals per hour | 16.84±10.14 | 14.80±9.91 | 18.88±10.15 | 0.165 |
Values are shown as mean ± standard deviation or median (interquartile range). #Mann–Whitney U-test.
REM, rapid eye movement.
Serum melatonin and cortisol levels
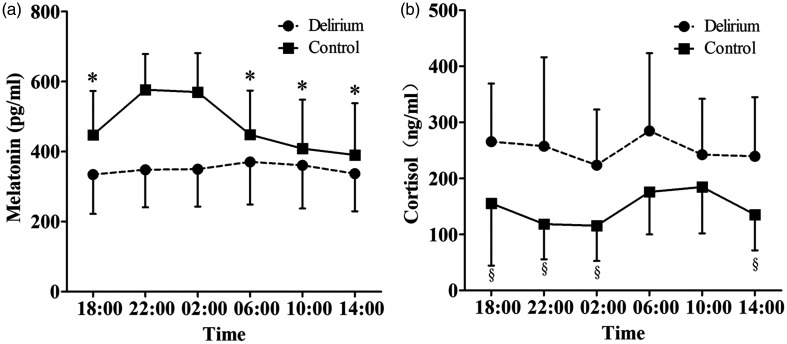
Serum melatonin and cortisol levels over the 24-hour monitoring period are shown in Figure 1. In the delirium group, peak values of melatonin and cortisol were observed at 06:00 hours. However, in the control group, peak melatonin and cortisol values were observed at 22:00 and 10:00 hours, respectively. There were significant differences between peak melatonin and cortisol values and values at other time points (except for 02:00 hours for melatonin and 06:00 hours for cortisol) in the control group (all P < 0.001), but these differences were absent in the delirium group.
Figure 1.
Melatonin (a) and cortisol (b) excretion in patients with or without delirium at different time points within 24 hours. Values are shown as means and error bars indicate standard deviations. *P<0.001, compared with the peak melatonin value at 22:00 hours in the control group (one-way analysis of variance). §P<0.001, compared with the peak cortisol value at 10:00 hours in the control group (one-way analysis of variance)
The peak and mean levels of melatonin were significantly lower in the delirium group than in the control group (both P < 0.0010) (Table 3). In contrast, the peak and mean levels of cortisol were significantly higher in the delirium group than in the control group (both P < 0.001). Additionally, the mean melatonin level was significantly lower and the mean cortisol level was significantly higher in the daytime (06:00–14:00 hours) than in the nighttime (18:00–02:00 hours) in the control group (P < 0.001 and P = 0.006, respectively). However, diurnal and nocturnal differences were absent in the delirium group (Table 3).
Table 3.
Serum melatonin and cortisol levels and circadian rhythm.
| Variables | Delirium group | Control group | P value |
|---|---|---|---|
| (n = 24) | (n = 24) | ||
| Melatonin | |||
| Peak value (pg/mL) | 461.88 ± 85.39 | 629.01 ± 93.41 | <0.001* |
| Mean value (pg/mL) | 349.97 ± 71.91 | 473.53 ± 90.94 | <0.001* |
| Mean daytime value | 356.03 ± 89.23 | 415.74 ± 118.57▲ | 0.070 |
| Mean nighttime value | 343.91 ± 74.94 | 531.32 ± 83.96 | <0.001* |
| Cortisol | |||
| Peak value (ng/mL) | 371.48 (276.11–425.52) | 239.66 (192.23–281.31) | <0.001# |
| Mean value (ng/mL) | 252.14 ± 82.22 | 144.16 ± 43.05 | <0.001* |
| Mean daytime value | 255.46 ± 96.17 | 165.22 ± 55.40▲ | <0.001* |
| Mean nighttime value | 248.82 ± 98.56 | 122.25 ± 47.79 | <0.001* |
Values are shown as mean ± standard deviation or median (interquartile range). *t-test; #Mann–Whitney U-test; ▲P<0.01, compared with the mean nighttime value in the control group.
Risk factors of delirium
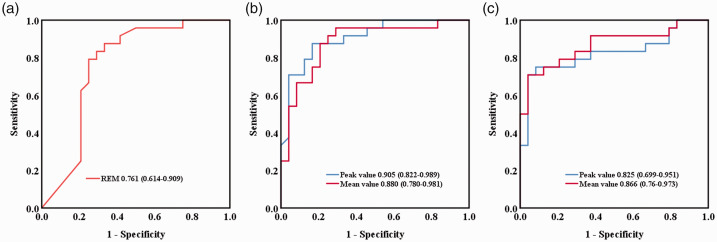
The areas under the curves for REM sleep and mean melatonin and cortisol levels in the delirium group were 0.761 (0.614–0.909), 0.880 (0.780–0.981), and 0.866 (0.760–0.973), respectively (Figure 2). Receiver operating characteristic analysis showed that the optimal cutoff values that predicted ICU-acquired delirium were as follows. For REM sleep, the optimal cutoff was ≤1.05%, with a sensitivity of 79.2% and a specificity of 75.0%. For melatonin levels, the optimal cutoff was ≤422.09 pg/mL, with a sensitivity of 91.7% and a specificity of 75.0%. For cortisol levels, the optimal cutoff was ≥212.14 ng/mL, with a sensitivity of 70.8% and specificity of 95.8%.
Figure 2.
Receiver operating characteristic curves for prediction of delirium. REM sleep (a), melatonin levels (b), and cortisol levels (c). The areas under the receiver operating characteristic curve values (95% CIs) are shown.
REM, rapid eye movement.
After adjustment for age, sex, the APACHE II score, and lactic acid levels, REM sleep (OR = 0.44; 95% CI, 0.21–0.88; P = 0.021), the mean melatonin value (OR = 0.972; 95% CI, 0.945–1.00; P = 0.039), and the mean cortisol value (OR = 1.05; 95% CI, 1.01–1.09; P = 0.014) were independently associated with delirium.
Discussion
Delirium is highly prevalent among critically ill patients and is associated with increased morbidity and mortality rates. The relationships among sleep, circadian rhythm, and delirium in the ICU are complex and likely bidirectional. To the best of our knowledge, this is the first prospective study to examine continuous 24-hour PSG data and the circadian rhythms of melatonin and cortisol secretion among patients in the ICU. We found that REM sleep and melatonin and cortisol levels were associated with delirium.
Research has suggested that memory consolidation is impaired by disruption of the natural slow-wave sleep and REM sleep cycle.20 Notably, REM sleep enhances procedural and emotional memory, and previous studies have shown that sleep disturbances can qualitatively and quantitatively alter memory representations.21,22 Loss of sleep on the first night post-surgery may be an early predictor of subsequent delirium.23 Taken together, these studies highlight the close relationship between sleep and delirium. In accordance with the findings of previous studies,10 we observed decreased REM sleep in patients with delirium. Therefore, disruption of sleep may play an important role in the occurrence and development of delirium among patients in the ICU.
In mammals, the melatonin rhythm is generated by an endogenous circadian master clock. Melatonin secretion is normally low during the daytime, increases soon after the onset of darkness, and peaks in the middle of the night.24 The melatonin circadian rhythm is severely disrupted in critically ill patients.25,26 Boyko et al.27 found that melatonin secretion in critically ill patients still followed a diurnal curve with the acrophase at 04:30 hours. Our study showed that serum melatonin rhythms were altered in patients with delirium. We observed a loss of the melatonin circadian rhythm with the acrophase at 06:00 hours in patients with delirium. Additionally, fluctuations in melatonin secretion were moderate in patients with delirium. However, circadian variation and a diurnal curve of melatonin secretion were observed in patients in the control group. These findings suggest that the physiological pattern of melatonin secretion is greatly disturbed among patients in the ICU.
In addition to abolished rhythmicity, the peak and mean melatonin values were significantly lower in the delirium group than in the control group. Similar to our observations, some studies have reported that patients with brain injury show reduced melatonin levels and more pronounced rhythm disturbances.28
Cortisol is a glucocorticoid hormone involved in the hypothalamic–pituitary–adrenal axis and it plays an important role in coordinating adaptive responses to stressful events. Cortisol secretion also shows circadian variation. In the present study, altered cortisol rhythms and higher cortisol levels were found in the delirium group compared with the control group. Additionally, there were significant differences between daytime and nighttime levels of cortisol secretion in the control group, while these diurnal changes were not observed in the delirium group. Other studies have also reported an association between higher cortisol levels and lower cognitive ability.29,30 Cortisol levels are increased in patients with severe sepsis and brain dysfunction31 and such an increase is associated with postoperative delirium.13,32
Although several studies have investigated the potential risk factors for delirium among patients in the ICU, their findings have been heterogeneous and no definitive conclusions have been achieved.33,34 Moreover, changes in sleep-related biomarkers may precede the symptoms of delirium.23,32,35 In our study, REM sleep, melatonin levels, and cortisol levels were independently associated with an increased risk of delirium among patients in the ICU, which validated our hypothesis. An REM stage of ≤1.05%, a mean melatonin value ≤422.09 pg/mL, and a mean cortisol value ≥212.14 ng/mL showed good sensitivity and specificity for the diagnosis of delirium in the ICU.
Melatonin and melatonin receptor agonists are widely used agents in the therapy of sleep disturbances. Consistent with our findings, there is also some evidence for the efficacy of melatonin for delirium in the ICU. Some studies have reported that oral melatonin treatment improves sleep quality in healthy participants who are exposed to a simulated ICU environment and patients with critical illness.36,37 Taken together, these previous studies and our results support the notion that sleep quality is associated with melatonin and cortisol secretion. Recent studies have shown that melatonin treatment decreases postoperative delirium and delirium in older adults who are admitted to acute care facilities.38,39 However, two studies reported that treatment with 3 mg of melatonin did not prevent delirium in non-ICU patients.40,41 Therapy with melatonin and ramelteon (a melatonin receptor agonist) minimizes the effect of risk factors on development of delirium. This is a natural form of therapy, which prevents the circadian rhythm being disturbed by the ICU environment.42 To a certain extent, these discrepant findings confirm that there are potential differences in the pathogenesis of simple delirium and ICU-related delirium.
This study has several limitations. First, the study was in a single center and comprised a small sample. Larger, multicenter studies are required to verify our preliminary findings. Second, PSG recordings and biological data were collected only for 24 hours. Therefore, we did not perform a before and after comparison, which might be important for determining the incidence of delirium in the ICU.
Conclusion
In the present study, patients with delirium showed markedly less REM sleep compared with patients without delirium. Furthermore, serum melatonin levels were lower, while cortisol levels were higher in patients with delirium than in those without delirium. Decreased REM sleep, lower melatonin levels, and higher cortisol levels were independently associated with an increased risk of delirium. Therefore, improvement in sleep and readjustment of circadian rhythmicity may be therapeutic targets for prevention and treatment of delirium in the ICU. Further research is required to understand the underlying physiological relationships and develop effective prevention and therapeutic strategies.
Acknowledgments
We would like to thank the patients and other staff at the Department of Pulmonary and Critical Care Medicine of Binzhou Medical University Hospital.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was supported by the National Natural Science Foundation of China (No. 81670078), the Science and Technology Development Plan of Shandong Province (No. 2014WS0187), and the Science Development Plan of Binzhou Medical University (No. BY2012KJ21).
ORCID iD: Xiaozhi Wang https://orcid.org/0000-0003-0497-7882
References
- 1.Ely EW, Shintani A, Truman B, et al . Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 291: 1753–1762. [DOI] [PubMed] [Google Scholar]
- 2.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010; 38: 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014; 383: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients - importance of sleep deprivation. Crit Care 2009; 13: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinhouse GL. Delirium and sleep disturbances in the intensive care unit: can we do better? Curr Opin Anaesthesiol 2014; 27: 403–408. [DOI] [PubMed] [Google Scholar]
- 6.Knauert MP, Haspel JA, Pisani MA. Sleep Loss and Circadian Rhythm Disruption in the Intensive Care Unit. Clin Chest Med 2015; 36: 419–429. [DOI] [PubMed] [Google Scholar]
- 7.Boesen HC, Andersen JH, Bendtsen AO, et al. Sleep and delirium in unsedated patients in the intensive care unit. Acta Anaesthesiol Scand 2016; 60: 59–68. [DOI] [PubMed] [Google Scholar]
- 8.Medrzycka-Dabrowska W, Lewandowska K, Kwiecien-Jagus K, et al. Sleep Deprivation in Intensive Care Unit – Systematic Review. Open Med (Wars) 2018; 13: 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyko Y, Jennum P, Nikolic M, et al. Sleep in intensive care unit: The role of environment. J Crit Care 2017; 37: 99–105. [DOI] [PubMed] [Google Scholar]
- 10.Trompeo AC, Vidi Y, Locane MD, et al. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol 2011; 77: 604–612. [PubMed] [Google Scholar]
- 11.Elliott R, McKinley S, Cistulli P, et al. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013; 17: R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun T, Han F, Sun Y, et al. [Research of 24-hour dynamic sleep monitoring and melatonin changes in patients with delirium in intensive care unit]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26: 726–729. [DOI] [PubMed] [Google Scholar]
- 13.Kazmierski J, Banys A, Latek J, et al. Cortisol levels and neuropsychiatric diagnosis as markers of postoperative delirium: a prospective cohort study. Crit Care 2013; 17: R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol 1999; 34: 721–732. [DOI] [PubMed] [Google Scholar]
- 15.Ho KM, Lee KY, Williams T, et al. Comparison of Acute Physiology and Chronic Health Evaluation (APACHE) II score with organ failure scores to predict hospital mortality. Anaesthesia 2007; 62: 466–473. [DOI] [PubMed] [Google Scholar]
- 16.Ely EW, Barr J. Pain/agitation/delirium. Semin Respir Crit Care Med 2013; 34: 151–152. [DOI] [PubMed] [Google Scholar]
- 17.Iber C. and American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Westchester, IL: American Academy of Sleep Medicine, 2007, pp.7–9. [Google Scholar]
- 18.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redline S, Kirchner HL, Quan SF, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med 2004; 164: 406–418. [DOI] [PubMed] [Google Scholar]
- 20.Ficca G, Salzarulo P. What in sleep is for memory. Sleep Med 2004; 5: 225–230. [DOI] [PubMed] [Google Scholar]
- 21.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci 2010; 11: 114–126. [DOI] [PubMed] [Google Scholar]
- 22.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev 2011; 35: 1154–1165. [DOI] [PubMed] [Google Scholar]
- 23.Evans JL, Nadler JW, Preud'homme XA, et al. Pilot prospective study of post-surgery sleep and EEG predictors of post-operative delirium. Clin Neurophysiol 2017; 128: 1421–1425. [DOI] [PubMed] [Google Scholar]
- 24.Brzezinski A. Melatonin in humans. N Engl J Med 1997; 336: 186–195. [DOI] [PubMed] [Google Scholar]
- 25.Olofsson K, Alling C, Lundberg D, et al. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand 2004; 48: 679–684. [DOI] [PubMed] [Google Scholar]
- 26.Marseglia L, Aversa S, Barberi I, et al. High endogenous melatonin levels in critically ill children: a pilot study. J Pediatr 2013; 162: 357–360. [DOI] [PubMed] [Google Scholar]
- 27.Boyko Y, Holst R, Jennum P, et al. Melatonin Secretion Pattern in Critically Ill Patients: A Pilot Descriptive Study. Crit Care Res Pract 2017; 2017: 7010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int 2007; 24: 45–61. [DOI] [PubMed] [Google Scholar]
- 29.Geoffroy MC, Hertzman C, Li L, et al. Morning salivary cortisol and cognitive function in mid-life: evidence from a population-based birth cohort. Psychol Med 2012; 42: 1763–1773. [DOI] [PubMed] [Google Scholar]
- 30.Gaysina D, Gardner MP, Richards M, et al. Cortisol and cognitive function in midlife: the role of childhood cognition and educational attainment. Psychoneuroendocrinology 2014; 47: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen DN, Huyghens L, Zhang H, et al. Cortisol Is an Associated-Risk Factor of Brain Dysfunction in Patients with Severe Sepsis and Septic Shock. Biomed Res Int 2014; 2014: 712742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Munster BC, Bisschop PH, Zwinderman AH, et al. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn 2010; 74: 18–23. [DOI] [PubMed] [Google Scholar]
- 33.Zaal IJ, Devlin JW, Peelen LM, et al. A systematic review of risk factors for delirium in the ICU. Crit Care Med 2015; 43: 40–47. [DOI] [PubMed] [Google Scholar]
- 34.Yamada C, Iwawaki Y, Harada K, et al. Frequency and risk factors for subsyndromal delirium in an intensive care unit. Intensive Crit Care Nurs 2018; 47: 15–22. [DOI] [PubMed] [Google Scholar]
- 35.Simons KS, Van Den Boogaard M, Hendriksen E, et al. Temporal biomarker profiles and their association with ICU acquired delirium: a cohort study. Crit Care 2018; 22: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care 2008; 12: R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HW, Zheng BL, Jiang L, et al. Effect of oral melatonin and wearing earplugs and eye masks on nocturnal sleep in healthy subjects in a simulated intensive care unit environment: which might be a more promising strategy for ICU sleep deprivation? Crit Care 2015; 19: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth 2010; 4: 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Aama T, Brymer C, Gutmanis I, et al. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry 2011; 26: 687–694. [DOI] [PubMed] [Google Scholar]
- 40.De Jonghe A, Van Munster BC, Goslings JC, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ 2014; 186: E547–E556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaiswal SJ, McCarthy TJ, Wineinger NE, et al. Melatonin and Sleep in Preventing Hospitalized Delirium: A Randomized Clinical Trial. Am J Med 2018; 131: 1110–1117.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewandowska K, Malkiewicz MA, Sieminski M, et al. The role of melatonin and melatonin receptor agonist in the prevention of sleep disturbances and delirium in intensive care unit - a clinical review. Sleep Med 2020; 69: 127–134. [DOI] [PubMed] [Google Scholar]