Abstract
Clinicians must have access to the best available evidence to inform point-of-care decisions about the use of remdesivir in patients with COVID-19. This article provides updated advice from the American College of Physicians for clinicians based on an updated rapid review.
Key Question 1
What are the effectiveness and harms of remdesivir in hospitalized patients with coronavirus disease 2019 (COVID-19)?
Key Question 2
Do effectiveness and harms in hospitalized patients with COVID-19 vary by symptom duration, disease severity, and treatment duration?
Background Update
On 22 October 2020, the U.S. Food and Drug Administration (1) approved the use of remdesivir for treatment of COVID-19 in patients aged 12 years or older and weighing at least 40 kg who require hospitalization. Remdesivir is the first drug to receive federal approval as treatment for COVID-19.
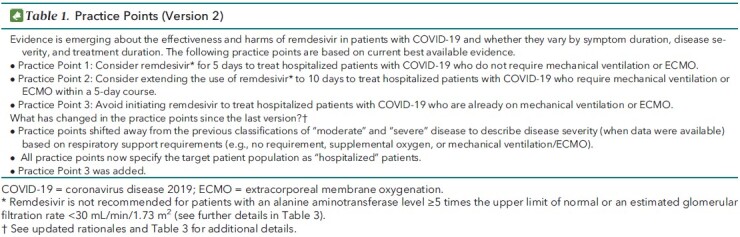
The Scientific Medical Policy Committee (SMPC) of the American College of Physicians (ACP) is maintaining rapid, living practice points on the use of remdesivir as a treatment for COVID-19 (Table 1). This is version 2 of the ACP practice points, which serves to update version 1 that was published on 5 October 2020 (2, 3). This version is based on an updated systematic evidence review done by the U.S. Department of Veterans Affairs (VA) Evidence Synthesis Program in Minneapolis, Minnesota, which has been updated through 7 December 2020 (Appendix) (4). The target audience for these practice points includes clinicians, the public, and public health professionals. The target patient population includes all hospitalized, nonpregnant, adult patients with COVID-19. This version was approved by the ACP's Executive Committee of the Board of Regents on behalf of the Board of Regents on 21 December 2020 and submitted to Annals of Internal Medicine on 18 December 2020. Updates are currently planned for every 2 months through December 2021.
Table 1. Practice Points (Version 2).
Overview of New Evidence
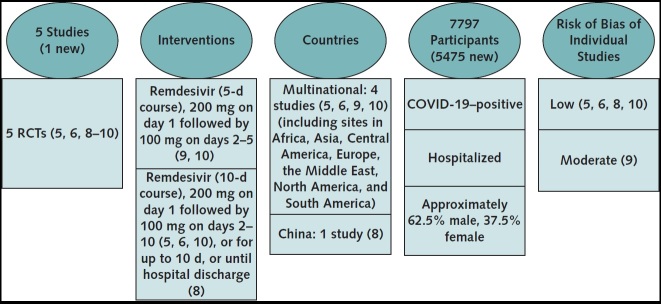
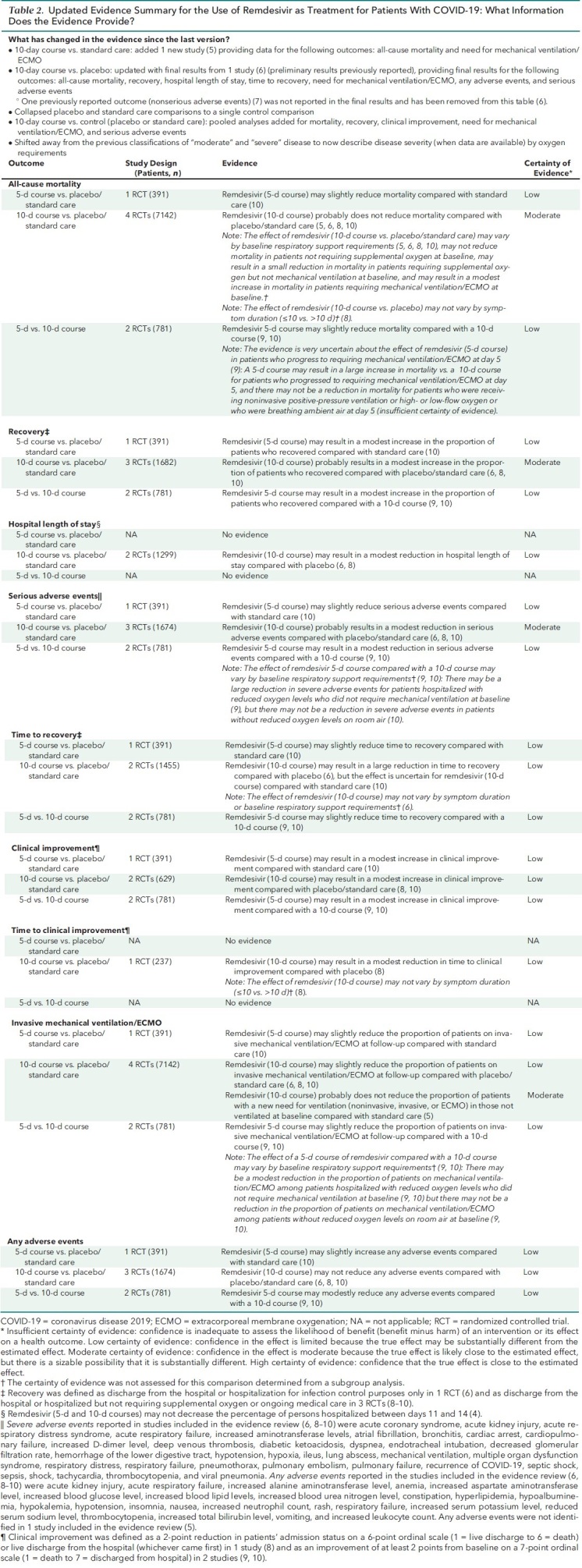
The evidence update identified 1 new study (4, 5). The new study evaluated a 10-day course of remdesivir versus standard care and provides new data for all-cause mortality (critical outcome) and new need for ventilation (noninvasive ventilation, invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation [ECMO]) among patients not requiring ventilation at the time of drug initiation (important outcome). The update also reports the final results from 1 study (6) that was included in version 1 as preliminary findings (7) comparing a 10-day course of remdesivir versus placebo for the following outcomes: all-cause mortality, recovery, hospital length of stay, time to recovery, proportion of patients on mechanical ventilation or ECMO, any adverse events, and serious adverse events. Table 2 and the accompanying systematic evidence review summarize changes in the findings (4).
Table 2. Updated Evidence Summary for the Use of Remdesivir as Treatment for Patients With COVID-19: What Information Does the Evidence Provide?
The evidence update did not identify any new evidence comparing a 5-day course of remdesivir versus placebo or standard care or a 5-day course versus a 10-day course.
Updated Practice Points and Rationales (Version 2)
The Figure and Table 2 summarize the updated evidence. Considering the recent U.S. Food and Drug Administration approval for use only in hospitalized patients, we have modified the practice points to specify the target patient population as hospitalized. Table 3 presents clinical considerations. Thresholds applied to determine the magnitude of effect for critical and important outcomes, prespecified by the evidence review team, are provided in Table 4. Table 5 identifies additional evidence gaps. Appendix Table presents the data estimates supporting the practice points.
Figure. Updated evidence description.
The evidence search and assessment were done by the U.S. Department of Veterans Affairs Evidence Synthesis Program in Minneapolis, Minnesota (4). Updated search for evidence, done through 7 December 2020, aimed to identify RCTs evaluating remdesivir for treatment of COVID-19. COVID-19 = coronavirus disease 2019; RCT = randomized controlled trial.
Table 3. Clinical Considerations.
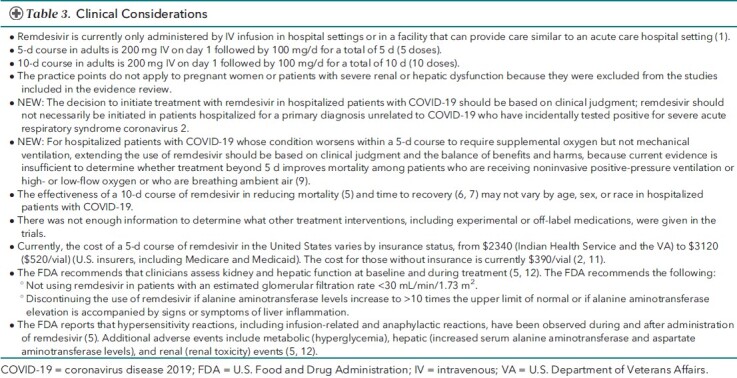
Table 4. Thresholds for Determining Magnitude of Effect*.
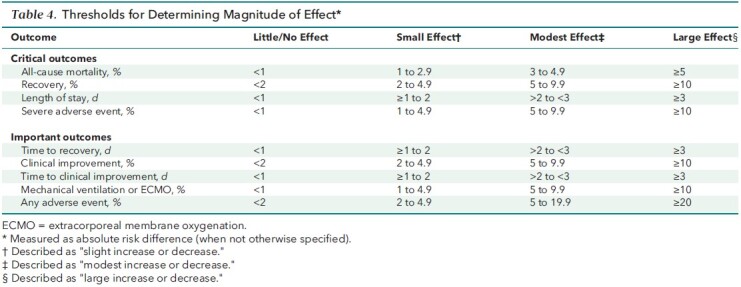
Table 5. Evidence Gaps.

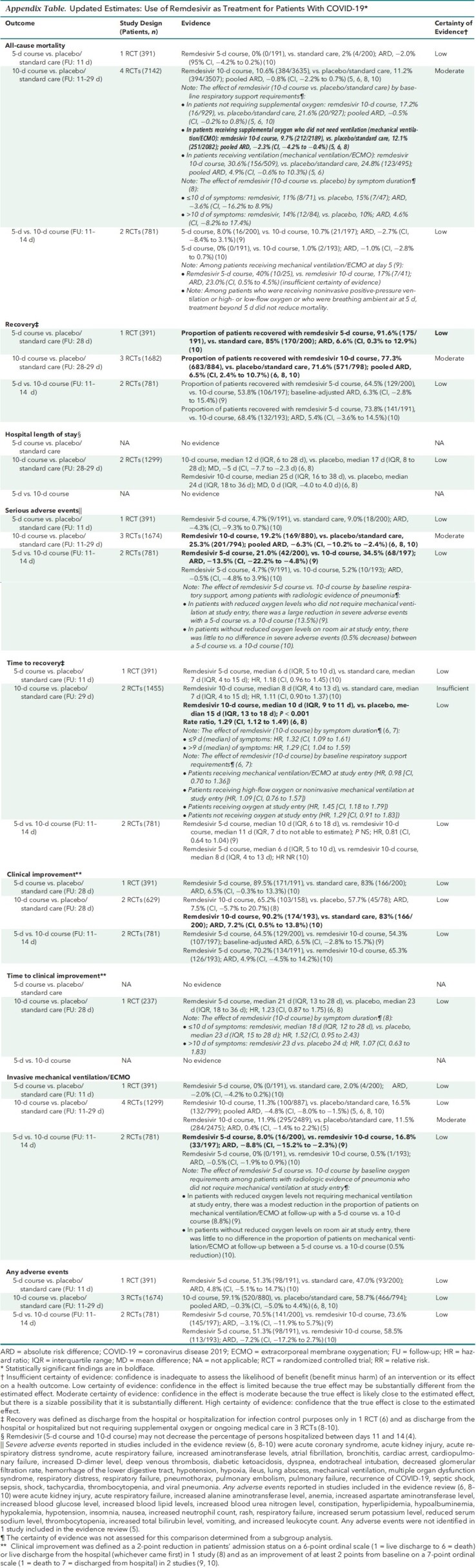
Appendix Table. Updated Estimates: Use of Remdesivir as Treatment for Patients With COVID-19.
Practice Point 1: Consider Remdesivir for 5 Days to Treat Hospitalized Patients With COVID-19 Who Do Not Require Mechanical Ventilation or ECMO
Updated Rationale
As in the previous version of the practice points, current evidence suggests an overall net benefit of remdesivir in patients with COVID-19 who do not require invasive mechanical ventilation or ECMO and suggests that 5 days of treatment may be as effective as 10 days, with no increase in potential harms (2). Despite low certainty, the SMPC judged it reasonable to provide clinical advice for the use of a 5-day course of remdesivir in hospitalized patients, particularly considering the limited availability of effective treatments for hospitalized patients with COVID-19. The language of the practice point was changed from “use remdesivir” to “consider remdesivir” to highlight the importance of clinical judgment when making decisions with individual patients about whether to begin remdesivir treatment. Given that disease severity definitions (mild, moderate, severe, and critical) vary widely among the included studies and among different organizations (for example, World Health Organization [WHO], National Institutes of Health, and U.S. Food and Drug Administration) (2), ACP has shifted away from the previous classifications of “moderate” and “severe” in this version. Rather than using these classifications, we use respiratory support requirements as a proxy for disease severity because we expect this to be more clinically useful given the inconsistent definitions. As a result, we have combined the previously separated practice points for patients with moderate COVID-19 and severe COVID-19 who do not require mechanical ventilation or ECMO into a single statement.
The evidence update did not result in any changes to our previous overall assessment of the balance of benefits and harms. There continues to be a net benefit with both a 5-day and 10-day course compared with placebo or standard care. No new studies were identified reporting on a 5-day course; however, the results for recovery and clinical improvement were updated with longer-term results and show no changes to previous conclusions. Although the inclusion of new data resulted in a finding of little to no effect of a 10-day course on mortality (previously reported as a small reduction) and the new need for ventilation (previously not reported), there remains a reported benefit for recovery (modest increase), clinical improvement (modest increase), and length of stay (modest reduction), along with fewer serious adverse events (modest difference) among those treated. Further, patient compliance data from the final report of 1 study comparing a 10-day course versus placebo continue to show that a 10-day course (10 doses) was used in fewer than half of the patients receiving remdesivir (41.2%), and an even lower percentage of patients (38.1%) received fewer than 10 doses because they recovered and were discharged from the hospital (2, 3, 6). Finally, there are no new studies directly comparing a 5-day versus a 10-day course.
An important area of uncertainty relates to the use of remdesivir in patients who do not require supplemental oxygen at hospitalization, although we expect that most patients with a diagnosis of COVID-19 are admitted with respiratory signs and symptoms. A newly reported pooled subgroup analysis comparing a 10-day course versus placebo or standard care showed that there may be a small reduction in mortality among patients requiring supplemental oxygen (but not mechanical ventilation) at the time of drug initiation, but that there may be little to no difference in mortality in patients not requiring supplemental oxygen at the time of drug initiation (5, 6, 8). In consideration of limited treatment options for COVID-19, the SMPC considered the evidence as insufficient to advise against considering the use of remdesivir in patients who do not require supplemental oxygen at the time of drug initiation. Further research is needed on treatment effects by oxygenation status at baseline.
Hence, our past conclusions are unchanged; in patients not requiring invasive mechanical ventilation or ECMO at the time of drug initiation, a 5-day course of remdesivir may be superior to a 10-day course for the following outcomes, with no evidence of increased harm with the shorter duration: mortality (slight reduction), recovery (modest increase), time to recovery (slight reduction), clinical improvement (modest increase), and the proportion of patients on invasive mechanical ventilation or ECMO at follow-up (slight reduction).
Practice Point 2: Consider Extending the Use of Remdesivir to 10 Days to Treat Hospitalized Patients With COVID-19 Who Require Mechanical Ventilation or ECMO Within a 5-Day Course
Updated Rationale
The previous version of the practice points concluded that evidence suggests a reduction in mortality with extension of remdesivir treatment to 10 days that outweighs potential harms among patients with COVID-19 who progress to requiring mechanical ventilation or ECMO by day 5 (2). This conclusion was based on evidence suggesting a net benefit for a 10-day course of remdesivir in these patients compared with placebo or standard care and on a post hoc analysis considering variation in disease severity (respiratory support requirements) when comparing a 5-day course with a 10-day course of remdesivir (9). The post hoc analysis found that treatment beyond 5 days did not improve mortality among patients who were receiving noninvasive positive-pressure ventilation or high- or low-flow oxygen or who were breathing ambient air; however, among patients with COVID-19 who progressed to requiring mechanical ventilation or ECMO at day 5, continued treatment through 10 days resulted in lower mortality (9).
The updated evidence report now rates the post hoc analysis (previously not rated) as insufficient, but the direction of effect still suggests potential benefit (based on this post hoc analysis and the overall findings for a 10-day course versus placebo or standard care). The SMPC also considered that currently, with limited availability of other effective treatments to manage hospitalized patients with COVID-19, extending treatment to 10 days is a consideration, particularly for patients who have not demonstrated any adverse effect profile while receiving the 5-day course.
Practice Point 3: Avoid Initiating Remdesivir to Treat Hospitalized Patients With COVID-19 Who Are Already on Mechanical Ventilation or ECMO
New Rationale
Our current understanding of COVID-19 progression is that patients who are admitted on mechanical ventilation or ECMO have likely progressed beyond the viral stage of the illness to the inflammatory stage and are less likely to improve from antivirals; hence, it is important to avoid any additional toxicity from remdesivir, unless there is evidence for potential benefit. This understanding is consistent with findings from a newly reported pooled subgroup analysis of 3 studies comparing a 10-day course of remdesivir versus placebo or standard care, which showed that remdesivir may result in a modest increase in mortality in patients receiving mechanical ventilation or ECMO at the time of drug initiation (5, 6, 8). This is also consistent with previously reported post hoc findings from 1 study that showed no improvement in time to recovery with a 10-day course among patients receiving invasive mechanical ventilation or ECMO at baseline (6). Studies evaluating the effectiveness of a 5-day course have not investigated the effect of baseline COVID-19 severity.
Although the evidence base is limited, the SMPC considers these findings a signal that the potential harms of remdesivir may outweigh the potential benefits in patients who are receiving invasive mechanical ventilation or ECMO at baseline and cautions against initiating remdesivir treatment in these patients.
Appendix: Overview of Practice Points Development Process and Methods
Practice Points Development Process
The SMPC, in collaboration with staff from ACP's Department of Clinical Policy, developed these practice points on the basis of a rapid and living systematic evidence review done by the VA Evidence Synthesis Program in Minneapolis, Minnesota (2). The SMPC comprises 11 internal medicine physicians representing various clinical areas of expertise and 1 public (nonclinician) member and includes members with expertise in epidemiology, evidence synthesis, healthy policy, and guideline development. In addition to contributing clinical, scientific, and methodological expertise, Clinical Policy staff provided administrative support and liaised among the SMPC, the evidence review funding entity and evidence team, and the journal. Clinical Policy staff and the SMPC reviewed and prioritized potential topic suggestions from ACP members, SMPC members, and ACP governance. A committee subgroup, including the SMPC chair, worked with staff to draft the key questions and led the development of the practice points. Clinical Policy staff worked with the subgroup and an independent evidence review team to refine the key questions and determine appropriate evidence synthesis methods for each key question. Via conference calls and e-mail, Clinical Policy staff worked with the committee subgroup to draft the practice points on the basis of the results of the rapid and living systematic evidence review. The full SMPC reviewed and approved the final practice points. Before journal submission, ACP's Executive Committee of the Board of Regents also reviewed and approved the practice points on behalf of the ACP Board of Regents. The evidence review team will continually update the evidence review. ACP will update the practice points based on the evidence review using the same process as the first version described above. Updates are currently planned for every 2 months through December 2021. The SMPC will continuously assess the priority of the topic and the overall state of evidence, including the anticipated rate of new evidence, and may choose to modify the update intervals accordingly (any modifications will be described in an Update Alert).
Methodological Differences From the WHO Guideline
On 20 November 2020, the WHO published an update of its “Therapeutics and COVID-19: Living Guideline” (13). In this guideline, the WHO “suggests against administering remdesivir in addition to standard care, in hospitalized patients with COVID-19, regardless of disease severity” (conditional recommendation). A review of current, publicly available documents (13–15) showed that there are 3 important methodological differences between the WHO guideline and the ACP practice points that may contribute to differing conclusions between ACP and WHO.
• The WHO guideline is based on a network meta-analysis comparing multiple drug treatments. The ACP practice points were developed on the basis of a VA Evidence Synthesis Program living systematic evidence review with the sole focus of evaluating the benefits and harms of remdesivir in hospitalized patients (4).
• The WHO guideline considered the effect of remdesivir regardless of its duration of use, whereas the ACP practice points focused specifically on the effectiveness and comparative effectiveness of differing durations of remdesivir use—5 days and 10 days compared with placebo or standard care or the other duration.
• The WHO guideline did not make a recommendation based on disease severity. WHO requested subgroup analyses from its network meta-analysis team and judged the credibility to be insufficient when assessing the variation in effectiveness of remdesivir by disease severity (WHO severity classifications). ACP provides clinical advice based on disease severity (baseline oxygen requirements). ACP considered subgroup analyses reported within the individual studies and those done de novo by the authors of the supporting rapid, living systematic review (4).
Footnotes
This article was published at Annals.org on 9 February 2021
* This paper, written by Amir Qaseem, MD, PhD, MHA; Jennifer Yost, PhD, RN; Itziar Etxeandia-Ikobaltzeta, PharmD, PhD; George M. Abraham, MD, MPH; Janet A. Jokela, MD, MPH; Mary Ann Forciea, MD; Matthew C. Miller, MD; and Linda L. Humphrey, MD, MPH, was developed for the Scientific Medical Policy Committee of the American College of Physicians. Individuals who served on the Scientific Medical Policy Committee from initiation of the project until its approval were Linda L. Humphrey, MD, MPH (Chair)†; Robert M. Centor, MD (Vice Chair)†; Elie A. Akl, MD, MPH, PhD‡; Rebecca Andrews, MS, MD†; Thomas A. Bledsoe, MD‡; Mary Ann Forciea, MD†; Ray Haeme†§; Janet A. Jokela, MD, MPH†; Devan L. Kansagara, MD, MCR‡; Maura Marcucci, MD, MSc‡; Matthew C. Miller, MD†; and Adam Jacob Obley, MD‡. Approved by the ACP Executive Committee of the Board of Regents on behalf of the Board of Regents on 21 December 2020.
† Author.
‡ Nonauthor contributor.
§ Nonphysician public representative.
Update Alerts: The authors have specified in the Background Update section and Appendix the interval and stop date for updates to this Practice Points article. As Annals receives updates, they will appear in the Comments section of the article on Annals.org. Reader inquiries about updates that are not available at approximately the specified intervals should be submitted as comments to the article.
References
- 1. U.S. Food and Drug Administration. FDA approves first treatment for COVID-19. Accessed at www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 on 3 November 2020.
- 2. Wilt TJ, Kaka AS, MacDonald R, et al. Rapid Response: COVID-19: Remdesivir for Hospitalized Adults. Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs; 2020. VA ESP Project 09-009.
- 3. Wilt TJ , Kaka AS , MacDonald R , et al. Remdesivir for adults with COVID-19. A living systematic review for an American College of Physicians Practice Points. Ann Intern Med. 5 October 2020. [Epub ahead of print].. doi: 10.7326/M20-5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaka AS, MacDonald R, Greer N, et al. Major update: remdesivir for adults with COVID-19. A living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann Intern Med. 9 February 2021. [Epub ahead of print]. doi:10.7326/M20-8148 [DOI] [PMC free article] [PubMed]
- 5. WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results. N Engl J Med. 2 December 2020. [Epub ahead of print]. doi:10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed]
- 6. Beigel JH , Tomashek KM , Dodd LE , et al; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. [PMID: ] doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beigel JH , Tomashek KM , Dodd LE . Remdesivir for the treatment of Covid-19—preliminary report. Reply [Letter]. N Engl J Med. 2020;383:994. [PMID: ] doi: 10.1056/NEJMc2022236 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y , Zhang D , Du G , et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [PMID: ] doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldman JD , Lye DCB , Hui DS , et al; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827-1837. [PMID: ] doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spinner CD , Gottlieb RL , Criner GJ , et al; GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048-1057. [PMID: ] doi: 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Day D. An open letter from Daniel O'Day, Chairman & CEO, Gilead Sciences. Stories @Gilead. 29 June 2020. Accessed at https://stories.gilead.com/articles/an-open-letter-from-daniel-oday-june-29 on 7 December 2020.
- 12. Lexicomp. Remdesivir: drug information. Accessed at www.uptodate.com/contents/remdesivir-united-states-investigational-agent-refer-to-prescribing-and-access-restrictions-drug-information on 18 June 2020.
- 13. World Health Organization. Therapeutics and COVID-19: living guideline. Accessed at www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline on 7 December 2020. [PubMed]
- 14. Siemieniuk R , Rochwerg B , Agoritsas T , et al. A living WHO guideline on drugs for Covid-[WEB_ONLY]19. BMJ. 2020;370:m3379. [PMID: ] doi: 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Summary: what is this living guideline? Accessed at https://app.magicapp.org/#/guideline/nBkO1E on 7 December 2020.