Abstract
Background
Left ventricular non-compaction (LVNC) cardiomyopathy is a persistence of abnormal foetal myocardium and is a rare cause of cardiomyopathy in the peripartum period. Unlike other causes of peripartum cardiomyopathy which typically improve, LVNC has significant long-term personal and family implications and needs lifelong follow-up.
Case summary
We describe a unique case of a 30-year-old woman who developed cardiomyopathy in the peripartum period which was revealed on cardiovascular magnetic resonance imaging to be due to occult LVNC. Our patient also had Ebstein’s anomaly, which is a known LVNC association.
Discussion
Cardiomyopathy in the peripartum period can be a decompensation of previously asymptomatic subclinical cardiomyopathy. It is important to assess for LVNC in patients presenting with this. Cardiovascular magnetic resonance imaging is the gold-standard imaging modality and allows accurate diagnosis of LVNC, associated structural complications and rare associations such as Ebstein’s anomaly. Left ventricular non-compaction is irreversible and has implications for patients and their family members.
Keywords: Peripartum cardiomyopathy, Echocardiography, Cardiovascular magnetic resonance, Left ventricular non-compaction, Ebstein’s anomaly, Case report
Learning points
Cardiomyopathy in the peripartum period can be a decompensation of previously asymptomatic subclinical cardiomyopathy.
Accurately assessing for left ventricular non-compaction (LVNC) when investigating patients with cardiomyopathy in the peripartum period is important.
Cardiovascular magnetic resonance imaging is the gold-standard imaging assessment for peripartum cardiomyopathy and in particular LVNC, which can be missed on echocardiography.
Cardiovascular magnetic resonance imaging allows detailed assessment of LVNC and its complications such as thrombus formation and subtle subclinical features such as elevated T1 times, which indicate subtle myocardial fibrosis even before left ventricular systolic dysfunction (LVSD) or scarring on late enhancement imaging is seen.
Left ventricular non-compaction has long-term implications for patients. There is a risk of ventricular arrhythmias, sudden death, embolic events, and progressive LVSD is typical. It also has implications for family members due to its inheritable nature necessitating family screening.
Ebstein’s anomaly in LVNC is a known rare association. This combination is important to recognize as Ebstein’s anomaly itself can be progressive and associated with other congenital heart defects.
Introduction
Left ventricular non-compaction (LVNC) is a structural abnormality of left ventricular myocardium characterized by persistent ‘spongy’ Foetal myocardium. It is of uncertain aetiology and mechanism but generally considered genetic in nature. Left ventricular non-compaction is a rare but important cause of cardiomyopathy in the peripartum period, given its association with embolic phenomenon, heart failure, ventricular arrhythmias, sudden death, and genetic inheritance requiring family screening.1–3 Peripartum cardiomyopathy (PPCM) is idiopathic and essentially a diagnosis of exclusion so it is important to ensure that causes such as LVNC are actively sought and excluded.
Timeline
| Time | Events |
|---|---|
| Three years prior | Unconfirmed neurological event. |
| Day 1 | Following the normal vaginal delivery of her 3rd child, our patient presented with exertional dyspnoea and examination revealed a resting sinus tachycardia with a soft systolic murmur. |
| Day 14 | Echocardiography showed a dilated and severely impaired left ventricle (LV) with an ejection fraction of 20%, moderate functional mitral regurgitation, and mild tricuspid regurgitation. |
| Day 15 | Commenced on conventional LVSD medications |
| Six months | Cardiovascular magnetic resonance imaging (CMR) showed mildly dilated and mildly impaired LV with hypertrabeculation at the apical, mid-anterolateral and mid-inferior segments, and papillary muscles. The right ventricle was of normal size and systolic function. There was mildly increased tricuspid valve-mitral valve offset (11 mm/m2) consistent with the known phenomenon of associated Ebstein’s anomaly. |
| Twelve months | Repeat CMR demonstrated persistent myocardial and papillary muscle hypertrabeculation and mild global LVSD. Native (pre-contrast) LV T1 times were mildly elevatedsuggestive of the presence of subtle diffuse myocardial fibrosis. |
| Current | Our patient is currently asymptomatic on supportive conventional LVSD/heart failure treatment and long-term anticoagulation. Annual CMR follow-up is planned. |
Case presentation
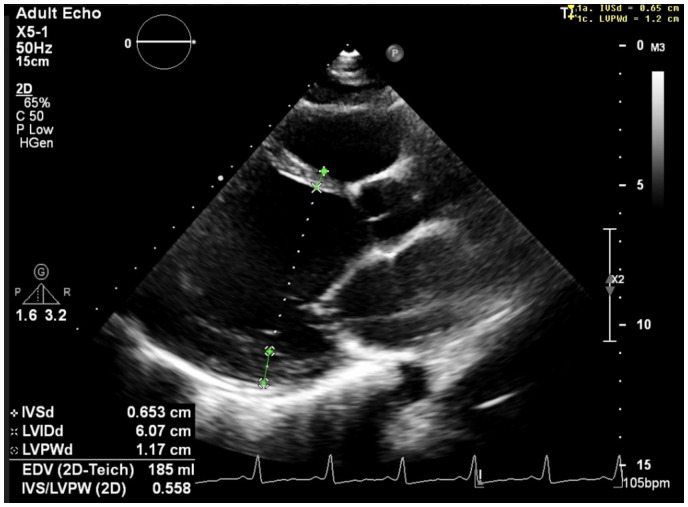
We present the case of a 30-year-old woman with a history of an unexplained neurological event 2 weeks after the normal vaginal delivery of her 2nd child. She lost power in her right arm whilst hovering. She was assessed at a local hospital, by which time her weakness had resolved and her symptoms were attributed to post-delivery pain medication. She did not have any imaging or an echo at this time. She had no other relevant past medical history. Following the normal vaginal delivery of her 3rd child, 3 years after this event, she described exertional dyspnoea (NYHA Class II). On examination, she had a resting sinus tachycardia and a soft systolic murmur with no further relevant examination findings. An electrocardiogram performed showed left ventricular hypertrophy (Figure 1). Echocardiography performed 2 weeks post-partum demonstrated a dilated and severely impaired left ventricle (LV) with ejection fraction of 20% (Figure 2), moderate functional mitral regurgitation, and mild tricuspid regurgitation. The study was performed by a senior echocardiographer who mentioned ‘prominent left ventricular trabeculations’ on the study report (Figure 2) but did not quantify this (ratio of non-trabeculated to trabeculated myocardium) or further assess with intravenous echocardiographic contrast.
Figure 1.
Electrocardiogram showing left ventricular hypertrophy.
Figure 2.
Three-chamber echocardiographic view showing dilated left ventricle [LVID (end-diastolic dimension) 6.1 cm, normal reference 5.3< cm]. LVID, left ventricular internal dimension.
Echocardiographic findings were felt consistent with PPCM due to the temporal relationship with delivery and she was commenced on conventional left ventricular systolic dysfunction (LVSD) medications (angiotensin-converting enzyme-inhibitor, beta-blocker), Bromocriptine (standard regime of 2.5 mg bd for 2 weeks followed by 2.5 mg od for 6 weeks, to augment LV recovery in PPCM by cleaving implicated 16 kDa prolactin), and anticoagulated (given previous unexplained neurological event concerning for a transient ischaemic attack). Relevant cardiomyopathy screening blood tests (thyroid function, ferritin, full blood count, C-reactive protein, autoimmune screen) were unremarkable. Bromocriptine was given according to the 2018 ESC Clinical Practice Guidelines.4 As breastfeeding is contraindicated with this drug, our patient did not breastfeed.
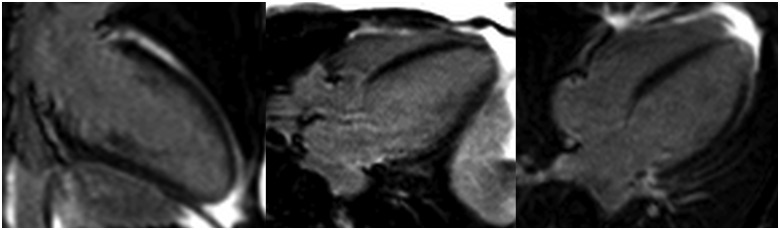
She underwent cardiovascular magnetic resonance imaging (CMR) at 1.5T field strength 6 months later, which confirmed a mildly dilated left venrticular end diastolic volume (LVEDV) 177 mL [reference <175mL], LVEDVI 97 mL/m2 [reference <96], left ventricular ejection fraction (LVEF) 52% [reference >57], and mildly impaired LV with hypertrabeculation at the apical, mid-anterolateral and mid-inferior segments, and papillary muscles.5 The right ventricle (RV) was of normal size and systolic function. There was mildly increased tricuspid valve-mitral valve offset (11 mm/m2) consistent with the known phenomenon of associated Ebstein’s anomaly (Figure 3). Due to the LV dilatation and volume overload, the basal diameter of the ventricle was enlarged on the four-chamber view, reducing the end-diastolic offset between the tricuspid and mitral valves to 12.7 mm (6.9 mm/m2), making it challenging to appreciate in echocardiography (Figure 5). There was no significant valve dysfunction, no infarction, no significant scarring or fibrosis, and no left ventricular thrombus (Figure 4). Findings were keeping with LVNC, meeting the most commonly used Petersen CMR criteria (end-diastolic non-compacted to compacted ratio of >2.3 in long-axis imaging5) (Figures 3 and 4). Due to the LV dilatation and volume overload, the basal diameter of the ventricle was enlarged on the four-chamber view, reducing the end-diastolic offset between the tricuspid and mitral valves to 12.7 mm, making it challenging to appreciate the increased offset (Figure 5).
Figure 3.
Four-chamber echocardiographic demonstrating prominent left ventricular trabeculations. Left: Dilated left ventricle with arrowed lines illustrating deep myocardial recesses (pathological trabeculations). Right: Corresponding image with colour Doppler overlay illustrating blood flow into deep recesses. LV, left ventricle; RV, right ventricle.
Figure 5.
Four-chamber image from baseline echocardiogram demonstrating that due to LV dilatation the basal diameter of the ventricle was enlarged on four-chamber imaging reducing the end-diastolic offset between the tricuspid and mitral valves to 12.7 mm (6.9 mm/m2), making it extremely challenging to appreciate the increased offset and hence Ebstein’s anomaly.
Figure 4.
Cardiovascular magnetic resonance imaging: four-chamber cine still image demonstrating dilated left ventricle with (A) non-compacted myocardium [end-diastolic non-compacted to compacted ratio >2.3 (5), asterisk], (B) non-compacted and underdeveloped anterolateral papillary muscle (white arrow), and (C) increased tricuspid valve to mitral valve offset of 11 mm/m2 (normal range <8 mm/m2) due to apical displacement of septal tricuspid valve leaflet.
Repeat CMR 12 months later confirmed persistent myocardial and papillary muscle hypertrabeculation and mild global LVSD (LVEDV 170 mL, LVEDVI 98 mL/mL2, LVEF 49%). Native (pre-contrast) LV T1 times were mildly elevated (Figure 6) confirming the presence of subtle diffuse myocardial fibrosis. The conclusion was that the PPCM was due to decompensation of occult and previously undiagnosed and asymptomatic LVNC. Although systolic function improved compared with the episode of PPCM, it remained mildly impaired long term.
Figure 6.
Cardiovascular magnetic resonance imaging: Native T1 mapping showing mildly elevated global T1 values (1031 ms at 1.5T) globally in keeping with global myocardial fibrosis (this image shows single region of interest).
She is currently asymptomatic on supportive conventional LVSD/heart failure treatment and long-term anticoagulation. She was last seen in November 2020 for her annual CMR and clinical follow-up. She and her family have been referred to an inheritable cardiac conditions clinic for genetic and family screening for LVNC.
Discussion
Peripartum cardiomyopathy is left ventricular systolic dysfunction in late pregnancy or the peripartum period and is a diagnosis of exclusion. Key differential diagnoses include decompensation of pre-existing known or undiagnosed cardiomyopathies such as dilated cardiomyopathy, previous myocarditis, drug-induced cardiomyopathy, and inheritable cardiomyopathies such as LVNC. Other causes include valvular disease, particularly mitral and aortic stenosis, and conditions such as shunts and pulmonary arterial hypertension. These may be unmasked in pregnancy due to increased circulating plasma volume and cardiac output during pregnancy.6,7
Recent data suggest that up to 80% of subjects with PPCM without LVNC recover to normal left ventricular systolic function (LVEF ≥55%), with most of this recovery occurring within the first 6-month post-conventional heart failure medication treatment.6
Patients with cardiomyopathy in the peripartum period due to decompensated LVNC have a poorer prognosis due to the progressive, irreversible nature of LVNC.8,9 Patients require lifelong follow-up to monitor systolic function given the typical sequela of progressive LVSD and heart failure, particularly with regard to future pregnancies. This condition predisposes to ventricular arrhythmias, which can require implantation of an implantable cardioverter-defibrillator and sudden cardiac death. The non-compacted myocardium also predisposes to thrombus formation and embolic phenomenon potentially requiring anticoagulation. The previous unexplained neurological event, likely a transient ischaemic attack, in our patient was likely due to this. Due to the inheritable nature of LVNC, family screening is crucial.8–10
There was no particular physiological rationale to our patient presenting only after her 3rd baby, following two previous normal vaginal deliveries. Her only symptom was of exertional dyspnoea and was investigated due to persistent tachycardia. It is possible she had cardiomyopathy in all of her pregnancies, which worsened over time to become symptomatic. She has been advised against future pregnancy. An implantable cardiac defibrillator was discussed but our patient declined to have one.
Left ventricular non-compaction is a rare phenomenon. Hence, there are only a very small number of case reports of LVNC in pregnancy (<7 in PubMed) and a recent systematic review11 identified only 30 cases throughout the literature base.
Left ventricular non-compaction needs to be considered in all patients with LVSD in the peripartum period. There is a spectrum of increased ventricular trabeculation: it can be seen in the normal population, in patients with congenital heart disease (ranging from patent ductus arteriosus, atrial septal defect, and VSD to more severe associations such as Ebstein’s anomaly) and in LVNC, which makes the diagnosis a difficult one. Left ventricular non-compaction should be considered in those patients with symptoms such as impaired systolic function, arrhythmia, and embolic events, neuromuscular disorders and those with a family history.
Echocardiography is body habitus dependent and detailed views of the LV myocardium are essential in assessing for LVNC. The ratio of non-trabeculated to trabeculated myocardium must be quantified and the use of intravenous ultrasound contrast should be utilized where standard echocardiographic imaging is suboptimal. On echocardiography, the Jenni criteria (end-systolic ratio >2.0 on short-axis imaging) is most commonly used.12 Despite our patient being assessed by a senior echocardiographer and prominent trabeculations mentioned, CMR was required to confirm the diagnose LVNC and hence is the gold-standard diagnostic test for LVNC. Cardiovascular magnetic resonance imaging is safe in the 2nd or 3rd trimesters of pregnancy and in the peripartum period, has no radiation and the use of Gadolinium contrast is not contraindicated in the pregnancy or breastfeeding. There should be a low threshold for performing CMR where echo cannot accurately assess for LVNC.5
There is a known association of LVNC with Ebstein’s anomaly [increased offset between tricuspid and mitral valves (>8 mm/m2) leading to atrialization of the RV].12 This will also require lifelong follow-up due to its progressive nature that can result in RV failure, severe tricuspid regurgitation, and atrial arrhythmias.13 Wherever one congenital heart defect (Ebstein’s anomaly here) is present, it is crucial to assess for the presence of other congenital heart defects and CMR allows optimal assessment for this and exclusion of extracardiac abnormalities often associated with congenital heart defects.
Conclusion
Left ventricular non-compaction as a cause of PPCM must be actively sought out and excluded as it can be easily missed on a routine echocardiogram. There are long-term implications of LVNC causing cardiomyopathy in the peripartum period as it is generally progressive, there is a predisposition to arrythmias and embolic events, and there is a need for family screening due to the genetic component. This is in contrast to most other peripartum cardiomyopathies that improve with time.
Patient perspective
I did not realize anything was wrong with my heart because I felt so well after the birth of my baby. I am really grateful to the doctors for picking my condition up, especially since it might affect my children. It is good that I only have to have magnetic resonance imaging scans as follow-up as I did not want to have lots of computed tomography scans due to the radiation.
Lead author biography

Emily Evans is a radiology trainee with a subspeciality interest in paediatric congenital cardiac imaging. She currently works at Birmingham Children's Hospital but has also trained with Dr Jamal Khan, Imaging Cardiologist, at University Hospital Coventry and Warwickshire.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P. et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J 2007;29:270–276. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnet D, Council on Epidemiology and Prevention et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 3. Hussein A, Karimianpour A, Collier P, Krasuski RA.. Isolated noncompaction of the left ventricle in adults. J Am Coll Cardiol 2015;66:578–585. [DOI] [PubMed] [Google Scholar]
- 4. Regitz-Zagrosek V, Roos-Hesselink J, Blomstrom-Lundqvist C, Cifkova R, Dr Bonis M, Lung B. et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147–3197. [DOI] [PubMed] [Google Scholar]
- 5. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH. et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46:101–105. [DOI] [PubMed] [Google Scholar]
- 6. Honigberg MC, Givertz MM.. Peripartum cardiomyopathy. BMJ 2019;364:k5287. [DOI] [PubMed] [Google Scholar]
- 7. Bhattacharyya A, Basra SS, Sen P, Kar B.. Peripartum cardiomyopathy: a review. Texas Heart Inst J 2012;39: [PMC free article] [PubMed] [Google Scholar]
- 8. Weir-McCall JR, Yeap PM, Papagiorcopulo C, Fitzgerald K, Gandy S, Lambert M. et al. Left ventricular noncompaction: anatomical phenotype or distinct cardiomyopathy? J Am Coll Cardiol 2016;68:2157–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oechslin EH, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R.. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000;36:493–500. [DOI] [PubMed] [Google Scholar]
- 10. Stöllberger C, Finsterer J, Blazek G.. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol 2002;90:899–902. [DOI] [PubMed] [Google Scholar]
- 11. Bardhi E, Faralli I, Deroma M, Galoppi P, Ventriglia F, Giancotti A.. Non-compaction cardiomyopathy in pregnancy: a case report of spongy myocardium in both mother and foetus and systematic review of literature. J Matern Fetal Neonatal Med 2019;1:1–8. [DOI] [PubMed] [Google Scholar]
- 12. Jenni R, Oechslin E, Schneider J, Attenhofer JC, Kaufmann PA.. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001;86:666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vermeer AM, Van Engelen K, Postma AV, Baars MJ, Christiaans I, De Haij S. et al. Ebstein anomaly associated with left ventricular noncompaction: an autosomal dominant condition that can be caused by mutations in MYH7. Am J Med Genet C Semin Med Genet 2013;163C:178–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.