Abstract
Case series
Patients: Male, 29-year-old • Male, 37-year-old
Final Diagnosis: Noncardiogenic pulmonary edema
Symptoms: Dyspnea
Medication: —
Clinical Procedure: —
Specialty: Critical Care Medicine • Pulmonology
Objective:
Challenging differential diagnosis
Background:
Naloxone remains the mainstay for the treatment of opioids overdose both in the clinical and public settings. Naloxone has been showing relative safety, leading to trivial adverdse effects which are mostly due to acute withdrawal effects, but when used in patients with known long-term addiction, it usually requires additional dosing or rapid infusion to achieve detoxification effects in a timely manner or to sustain the effects after they fade away. In some patients this has resulted in fatal adverse effects, including non-cardiogenic pulmonary edema (NCPE), which may require intensive care for those patients. Whether the higher dose is the cause has been debatable and not enough studies have looked into this subject.
Case Reports:
Here, we report a series of 2 cases where 2 young patients were given naloxone following opioid overdose. Both our patients required frequent dosing due to insufficient response or owing to the washout of the naloxone effect shortly after, given its short half-life. Although the administered doses were different, both patients developed the adverse effect of NCPE and required ventilator support.
Conclusions:
Evidence suggests that such a catastrophic adverse effect following the administration of such a critical medication, which is known to be relatively safe and is being publicized for saving lives, might limit its use and would require more attention and further studies to standardize a safe dose, limiting these life-threatening events and decreasing the need for unnecessary invasive respiratory support as well as admissions to intensive care units, which might create an additional burden on the health care system.
Keywords: Drug-Related Side Effects and Adverse Reactions, Heart Failure, Naloxone, Pulmonary Edema
Background
Naloxone remains the main opiate antagonist used to reverse the effects of opioid overdose, both in clinical settings and illicit drug use [1]. It was developed in the 1960s and is a relatively safe medication with minimal adverse effects, which include acute withdrawal symptoms (eg, vomiting); however, it is reported that rapid infusion or using higher doses of naloxone induces a catecholamines surge that has fatal adverse effects in rare situations, such as acute pulmonary edema, ventricular arrythmias, and cardiac arrest [2,3]. We report a series of 2 cases of young patients who tolerated a low dose of naloxone well but developed acute pulmonary edema after administration of higher-dose naloxone [4].
Case Reports
Case Presentation #1
A 29-year-old man with a history of heroin abuse was found unresponsive at home secondary to a reported heroin overdose. He responded well to 2 milligrams (mg) of naloxone in the field, and was brought to the hospital alert, awake, and oriented to person, place, and time. The patient was monitored for several hours in the Emergency Department (ED) and then was discharged home the same day after an uneventful hospital course. A few hours after discharge, he was brought back to the hospital unresponsive after another overdose of heroin. He received repeated doses of naloxone to a total of 8 mg until he became responsive. Two hours later, the patient developed severe shortness of breath, hypoxemia, and altered mental status, necessitating endotracheal intubation. The patient’s vital signs were within normal limits (blood pressure of 132/89 mmHg, heart rate of 98 beats per minute, respiratory rate of 16/min, and body temperature 37°C). A chest examination was significant for bilateral basal crepitations only. Arterial blood gas (ABG) showed pH: 7.452 [7.350–7450], pCO2: 34.6 mmHg [35–50] mmHg, and pO2: 405 mmHg [85–106] mmHg. Troponin was 0.00 NG/ML (reference range <0.08 NG/ML) and lactate was 1.0 mmol/L (reference range 0.5–2.0 mmol/L). A chest radiograph showed bilateral infiltrates consistent with pulmonary edema (Figure 1). The patient was treated with IV 40 mg Lasix and was successfully extubated 7 h later. A repeat chest radiograph showed resolution of pulmonary infiltrates. The patient was saturating well at 94–96% on 3-L nasal cannula and transferred to the medical floors, where he was weaned off oxygen and observed for the following 24 h, where he was hemodynamically and clinically stable, then discharged home.
Figure 1.
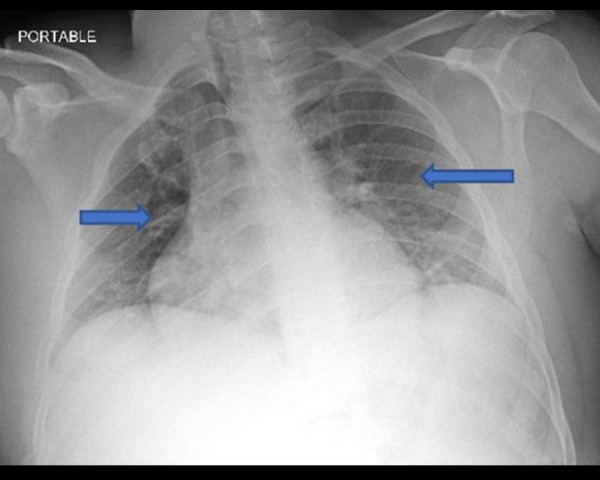
Blue arrows showed bilateral infiltrates consistent with pulmonary edema.
Case Presentation #2
A 37-year-old man with a medical history of intravenous heroin use and cocaine use presented to the ED due to a suspected heroin overdose. In the field the patient was given 2 mg of naloxone, but remained obtunded in the ED and was given 0.4 mg naloxone. A urine drug screen on admission was positive for cocaine and heroin but negative for other illicit substances. Alcohol level was 0.00, troponin level was <0.02 NG/ML (reference range <0.08 NG/ML), B-Type natriuretic peptide (BNP) was 18 pg/mL (reference range 0–100 pg/mL) on admission. Vitals on admission were blood pressure of 120/57 mmHg, heart rate of 84 beats per minute, respiratory rate 19/ min, and temperature 36.8°C, and oxygen saturation 96% on room air. His mental status improved after being given naloxone in the ED, but he became hypoxic and in respiratory distress eventually requiring bilevel positive airway pressure (BiPAP). Cardiac electrocardiography showed sinus tachycardia and an emergent chest X-ray showed interval development of diffuse reticulonodular opacities (Figure 2). Repeat vitals were blood pressure 104/55 mmHg, heart rate 130 beats per minute, respiratory rate 30/min, temperature of 37.3°C, and oxygen saturation 84%. Arterial blood gas was measured, showing pH 7.359, pCO2 43.5 mm Hg (reference: 35–50 mm Hg), pO2 83.6 mm Hg (reference: 85.0–106.0 mm Hg), oxygen saturation 96.2%, and bicarbonate level 23.9 mmol/L (reference: 22–26 mmol/L). He was transferred to the Intensive Care Unit (ICU) on bilevel positive airway pressure (BiPAP) due being at a low threshold for endotracheal intubation. While in the ICU, he was given IV 40 mg Lasix daily, with significant improvement of symptoms. After a short uneventful ICU course, he was weaned off the BiPAP and transitioned to 4-L nasal cannula and transferred to the medical floors and remained vitally stable with an unremarkable physical exam.
Figure 2.
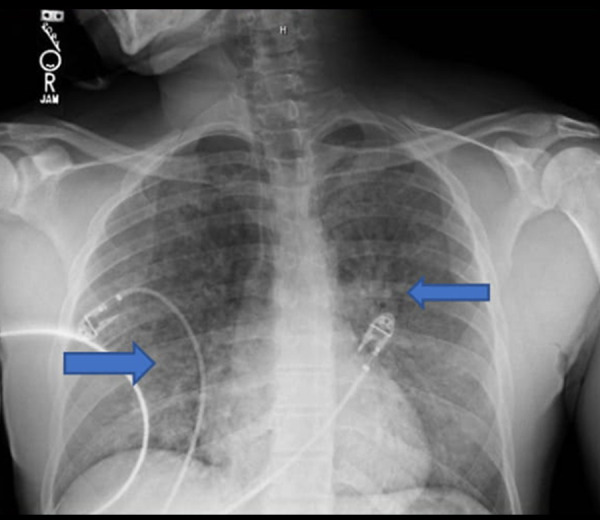
Blue arrows showed diffuse reticulonodular opacities.
Discussion
Naloxone hydrochloride (Narcan) is a competitive opioid antagonist that was initially approved by the FDA in 1971 for the emergency treatment of opioid overdose as witnessed by respiratory and central nervous system depression [5]. Opioids used by addicts usually have a long half-life, while naloxone’s half-life is relatively short, so the respiratory symptoms recur shortly after its effect has subsided [3]. Due to this short half-life, patients may require higher doses or faster rates of infusion for the reversal of the opioid overdose, which causes a surge in the catecholamines release, leading to manifestation of adverse effects, including non-cardiogenic pulmonary edema and/or cardiac arrythmias [2].
The fluid balance in the lung and risk for development of pulmonary edema are critically determined by a multitude of factors, including pulmonary capillary and arterial pressures. Catecholamines were shown to contribute to the formation of pulmonary edema in patients with neurogenic as well as high-altitude pulmonary edema (HAPE) by a non-human experiment conducted by Rassler et al 2003 [6]. The responsible mechanisms for the increase in epinephrine in plasma following the administration of naloxone include its direct antagonizing effects on µ-opioid receptors in the adrenal medulla, and neutrally mediated changes of central sympathetic out-flow has been proposed as an explanation for the naloxone-induced non-cardiogenic acute pulmonary edema [7]. Whether this is a dose-dependent adverse effect remains unclear, as it has been reported with low doses of naloxone as well as shown by Partridge et al in 1986 [8]. However, the fact that our patient developed acute pulmonary edema after administration of 8 mg, but not 2 mg, of naloxone suggests a dose-dependent relationship. This finding reinforces the current recommendation of the American Heart Association to gradually titrate up naloxone to the least effective dose to prevent these serious adverse effects [9]. Our 2 patients had no known significant past medical history to otherwise explain the acute pulmonary edema. We excluded cardiac etiologies due to negative troponin level, BNP, and normal echocardiography studies. Furthermore, because of the chronological occurrence of pulmonary edema after naloxone administration and because no other medications known to cause such adverse effect were administered at the time, we believe that naloxone was the causative agent.
Conclusions
Naloxone remains the drug of choice in managing opioid overdose but can rarely cause non-cardiogenic acute pulmonary edema. This adverse effect is apparently dose-dependent. Therefore, healthcare providers should aim to use the least effective dose.
Footnotes
Conflict of Interests
None.
References:
- 1.Jiwa N, Sheth H, Silverman R. Naloxone-induced non-cardiogenic pulmonary edema: A case report. Drug Saf Case Rep. 2018;5:20. doi: 10.1007/s40800-018-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rzasa Lynn R, Galinkin J. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9:63–88. doi: 10.1177/2042098617744161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dorp E LA, Yassen A, Dahan A. Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf. 2007;6:125–32. doi: 10.1517/14740338.6.2.125. [DOI] [PubMed] [Google Scholar]
- 4.Al Azzawi M, Alshami A, Hossain M. Naloxone-induced acute non cardiogenic pulmonary edema is it dose dependent. Am J Resp Crit Care Med. 2020;201:A4931. [Google Scholar]
- 5.Adapt Pharma Inc NARCAN (naloxone hydrochloride) nasal spray. FDA Access Data. 2015:1–18. [Google Scholar]
- 6.Rassler B, Reissig C, Briest W, et al. Pulmonary edema and pleural effusion in norepinephrine-stimulated rats – hemodynamic or inflammatory effect? Mol Cell Biochem. 2003;250:55–63. doi: 10.1023/a:1024942132705. [DOI] [PubMed] [Google Scholar]
- 7.Taff RH. Pulmonary edema following naloxone administration in a patient without heart disease. Anesthesiology. 1983;59:576–77. doi: 10.1097/00000542-198312000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Partridge BL, Ward CF. Pulmonary edema following low-dose naloxone administration. Anesthesiology. 1986;65:709–10. doi: 10.1097/00000542-198612000-00037. [DOI] [PubMed] [Google Scholar]
- 9.Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special circumstances of resuscitation. Circulation. 2015;132:S501–18. doi: 10.1161/CIR.0000000000000264. [DOI] [PubMed] [Google Scholar]


