Abstract
Background:
The first arteriovenous fistulas were created at the wrist more than 60 years ago. Basic surgical construction techniques remain unchanged with mobilization and repositioning of the vessels followed by a sutured anastomosis. We used the Ellipsys device to construct percutaneous radiocephalic–arteriovenous fistulas at the wrist and report the results.
Methods:
Data were reviewed retrospectively for all patients who had a percutaneous radiocephalic–arteriovenous fistula created during a 6-month period. Each individual underwent ultrasound vessel mapping in addition to physical examination. When a radiocephalic–arteriovenous fistula was feasible and a communicating vein ⩾ 2 mm in diameter was noted in the distal forearm along with a radial artery ⩾ 2 mm, a percutaneous radiocephalic–arteriovenous fistula was considered and reviewed with the patient.
Results:
Four individuals met the criteria to consider a percutaneous radiocephalic–arteriovenous fistula and all elected to have the procedure performed. Ages were 54–85 years. Three were diabetic and one was female. All percutaneous radiocephalic–arteriovenous fistulas were technically successful. Two individuals had not yet started dialysis therapy. Successful and repetitive cannulation for the two individuals with catheters was initiated at 4 and 8 weeks post procedure. The two pre-dialysis patients had physiologic arteriovenous fistula maturation (6 mm vein diameter and >500 mL/min flow) at 4 and 12 weeks. There were no procedural or late complications and none required intervention. Follow-up was 8–23 months (mean 16 months).
Conclusion:
The success of these percutaneous radiocephalic–arteriovenous fistulas suggests that use of the Ellipsys device will be applicable at the wrist in selected patients where appropriate vessel sizes and configurations are found.
Keywords: Arteriovenous fistula, percutaneous, radiocephalic, Cimino, Ellipsys, endo–arteriovenous fistula
Introduction
The first surgical arteriovenous fistulas (AVF) for hemodialysis were reported in 1966 by James Cimino and colleagues and remain the preferred method of vascular access for hemodialysis.1–3 These surgical radiocephalic–AVFs (sRC-AVF) were created at the wrist between the cephalic vein and the radial artery by Ken Appell and were cannulated the following day. However, while AVF options and locations have expanded greatly, the basic construction techniques remain unchanged with mobilization and repositioning of the vessels followed by a sutured anastomosis. These techniques are all associated with the potential for vessel rotation or kinking with transposition, injury, or devascularization of the vein; high blood flow turbulence; elevated wall sheer stress; and technical misadventure; frequently resulting in outflow venous hyperplasia, stenosis and access thrombosis. Surgical techniques and outcomes have been described for sRC-AVFs with many reports finding cumulative and functional patency rates of roughly 50%.4–7
Recently the Federal Drug Administration has approved two new devices for creation of percutaneous arteriovenous fistulas (pAVF) in the United States. Both had been available in Europe previously and both devices establish pAVF inflow in the mid-arm using the proximal radial or ulnar arteries for inflow and the deep communicating vein (DCV, that is, perforating vein) for outflow.8–10 Percutaneous AVFs have been created in patients where a sRC-AVF is not feasible. Longer term follow-up has become available for the Ellipsys device (Avenu Medical, San Juan Capistrano, CA, USA) and have shown functional patency > 90% at 2 years.11,12
The Ellipsys system as approved uses a single catheter with superficial venous access cannulation creating a direct fused anastomosis with thermal energy and pressure between the DCV and the proximal radial artery with both venous and arterial vessels remaining in their native position. We used the Ellipsys device to construct successful pRC-AVFs using a more distal communicating vein from the cephalic vein at the wrist to the radial vein adjacent to the radial artery, recreating an original Cimino AVF and report the results (Figures 1–3).
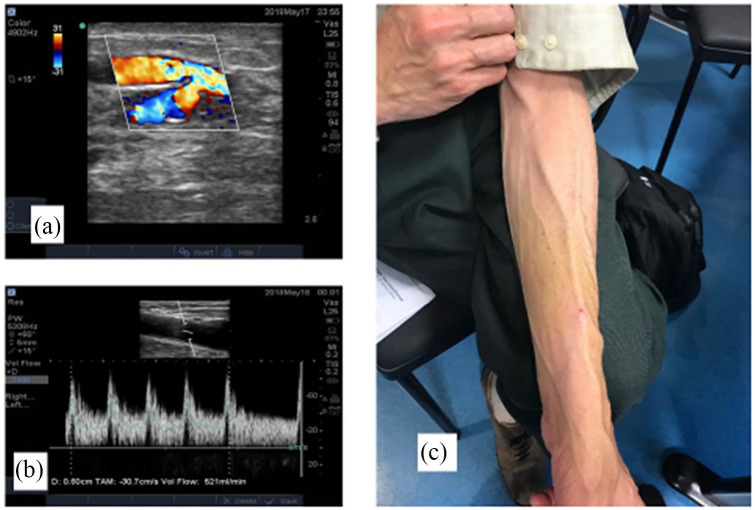
Figure 1.
(a) Duplex ultrasound image shows a percutaneous radiocephalic–arteriovenous fistula (pRC-AVF) created with the communicating vein at the wrist using the Ellipsys device, (b) brachial artery flow volume 521 mL/min post operatively, and (c) matured percutaneous pRC-AVF, initial cannulation was successful at 4 weeks.
Figure 2.
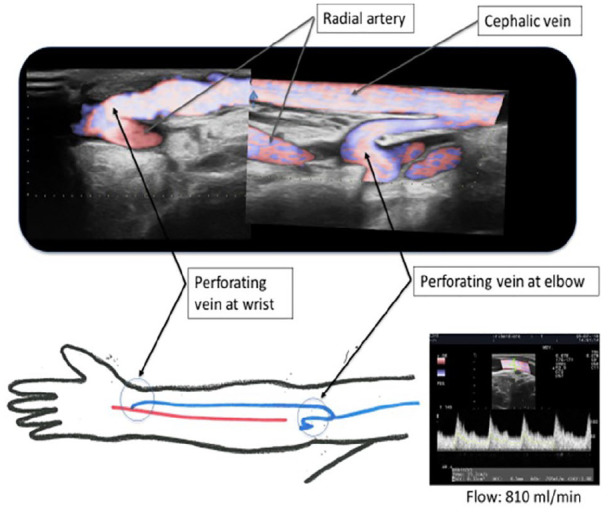
A composite duplex ultrasound (US) image shows an Ellipsys percutaneous radiocephalic–AVF and a schematic diagram of the radial artery AVF inflow at the wrist with mid-arm outflow through the deep communicating vein (perforating vein) in the cubital fossa. US flow volume of 810 mL/min is documented 6 months following AVF creation.
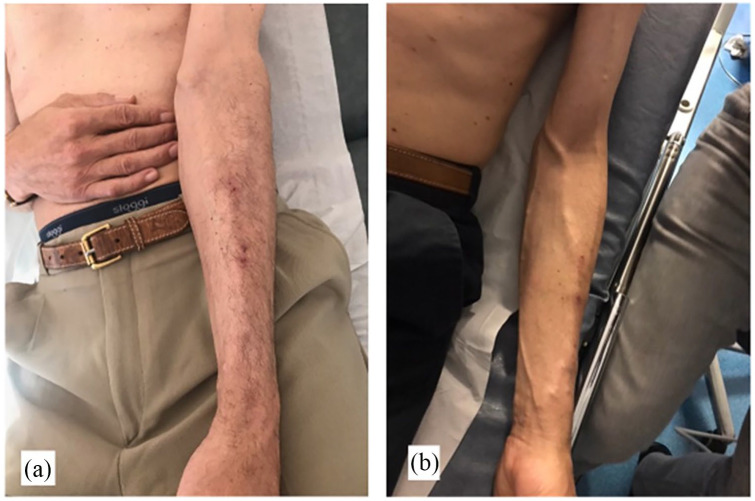
Figure 3.
Photos show clinical images of a mature Ellipsys percutaneous radiocephalic–AVF: (a) Flow at 4 weeks was 436 mL/min, initial cannulation was successful at 4 weeks, and (b) same patient at 6-month follow-up with 810 mL/min flow.
Methods
This study is a retrospective analysis of data collected for consecutive vascular access patients, focusing on those individuals who had a pRC-AVF created. All patients referred for a permanent hemodialysis vascular access during a 6-month period underwent duplex ultrasound vessel mapping in addition to physical examination. When a RC-AVF was feasible and a communicating vein ⩾ 2 mm in diameter was noted in the distal forearm along with a radial artery ⩾ 2 mm, a pRC-AVF was considered and discussed with the patient. Informed consent was obtained for each individual regarding device use variance from the manufacturer’s instructions for use. The procedures were performed as outpatients at a tertiary medical center in Paris, France.
The pAVF technique with the Ellipsys device has been previously described with video images available online.13 Briefly, the pRC-AVF Ellipsys procedures required a distal communicating vein between the deep and superficial venous systems ⩾ 2 mm diameter and a radial artery at the wrist with an internal luminal diameter ⩾ 2 mm. The pAVF device is introduced over a single venous guidewire, advanced through the communicating vein and then through the vein wall into the radial artery. The Ellipsys device captures the arterial and venous walls, creating a fused anastomosis using pressure with thermal resistance heating. Balloon dilatation (5 × 20 mm) of the communicating vein extending into the anastomosis completes the procedure. The same balloon size was used when creating a pRC-AVFs for the individuals in this study as the patients selected had vessels similar in size and appearance to those at the more proximal pAVF site. All Ellipsys pAVF procedures are completed entirely with duplex ultrasound imaging and without contrast or radiation exposure.
Technical success was confirmed by doppler ultrasound examination demonstrating pRC-AVF flow through the anastomosis, communicating vein, and outflow cephalic vein. Maturation was defined as blood flow of 500 mL/min in the mid-brachial artery with pRC-AVF outflow diameter > 6 mm. Functional success was repeated and reliable dialysis access at the prescribed flow rate to include two dialysis sessions within 4 days and with catheter removal or without need for catheter placement. Primary patency was time from pRC-AVF creation to first intervention and cumulative patency was time from pRC-AVF creation to access abandonment. The study was approved by the Institutional Review Board (Comité d’Evaluation des Protocoles et d’Aide à la Recherche, Protocol Evaluation and Research Assistance Committee—CEPAR) and was in accordance with the Declaration of Helsinki. Participants provided informed written consent for publication of patient information and images.
Results
Four individuals met the criteria to consider a pRC-AVF and all elected to have the procedure performed. Ages were 54–85 and three were male. Three patients were diabetic and one had previous access surgery. Two had a dialysis catheter at time of the percutaneous procedure and two individuals had not yet started dialysis therapy. The pRC-AVF sites were established by the position of an adequate communicating venous branch and varied in location by 5–10 cm from the wrist.
All pRC-AVFs were technically successful. Successful and repetitive two needle cannulation for the two individuals with catheters was initiated at 4 and 8 weeks post procedure. The two pre-dialysis patients had physiologic AVF maturation (6 mm vein diameter and >500 mL/min flow) at 4 and 12 weeks. One of these eventually started dialysis using the pRC-AVF without incident and the other patient’s renal status remained stable at the end of the study period. All three individuals requiring dialysis had functional pRC-AVF success.
There were no procedural complications, none have required intervention, and there have been no late complications. No patient required an outflow coil embolization either during the pRC-AVF procedure or later during the follow-up period. Both primary and cumulative (secondary) patency rates were 100% during the study period. Procedure times for the pRC-AVFs were 38, 28, 23, and 18 min. Follow-up was 8–23 months (mean 16 months). Access flow volumes were 168, 343, 212, and 521 mL/min post-operatively and 550, 615, 810, and 905 mL/min at 6 months, respectively, as measured in the brachial artery.
Discussion
The first recommendation for permanent hemodialysis vascular access remains a RC-AVF at the wrist; however, several reports have found up to 50% failure rates with the classic Cimino sRC-AVF.2–7 Criticisms of these distal AVFs include not only the high rate of access failure but also the frequent need for interventions and prolonged times for maturation and successful repetitive cannulation. Our practice for many years has been to limit sRC-AVF construction to those patients with vessels that meet acceptable criteria (vein 2.5 mm and artery 2.0 mm in diameters and otherwise normal in appearance) by physical and duplex ultrasound examinations, where success and prompt maturation can be expected.14–16 We found over 90% cumulative patency at 2 years in sRC-AVFs but this practice restricts the number of sRC-AVFs to such individuals, approximately 10% of our new vascular access patients.14 Importantly, primary patency in this select group of sRC-AVF patients was only 48% at 2 years.14 In anatomically appropriate patients where a communicating vein at the wrist is ⩾2 mm in diameter and in continuity with both the cephalic and radial veins, an Ellipsys pRC-AVF may offer quicker maturation, fewer interventions, and shorter procedure times in addition to a non-surgical option for patients averse to even a minor surgical operation.
This study presents the novel creation of a RC-AVFs at the wrist in select patients established with a minimally invasive percutaneous technique, avoiding not only a surgical incision but also the potential problems associated with surgical vessel relocation and manipulation. These patients all had an adequate distal communicating vein leading to the radial vein adjacent to the radial artery as identified by duplex ultrasound examination. The prevalence of this anatomy and the true population that may benefit is currently unknown but clearly, these patients are not common and may be more widely appreciated with recognition of this access option. Each individual had prompt access maturation without complications and all remained functional without intervention.
This pAVF site option for the Ellipsys device varied from the instructions for use (off-label use) regarding the AVF site in both the European and US regulatory bodies; however, it conformed with specifications for the identified vessels (radial artery and a communicating vein), vessel size and proximity requirements, and technical use of the device. Initial studies of the Ellipsys pAVF found superior patency and fewer complications when compared to surgical AVFs.9–10 The original Ellipsys pivotal trial has now accrued 2-year follow-up data demonstrating functional cumulative patency of 92.7%, and the initial report by Mallios et al. has been extended to 2-year follow-up with cumulative patency > 90%.11,12 Our observation of these four pRC-AVFs is that they result in a percutaneous access that is very similar to other pAVF sites previously reported and establish a moderate (adequate) flow AVF with few, if any, complications. These first patients required no interventional procedures but as more individuals are treated, some will undoubtedly need balloon angioplasty or other therapy. We note that with increasing experience, the number of necessary interventions for pAVF maturation and salvage has decreased.12,13 This report includes only four patients; however, it demonstrates the feasibility of creating successful pRC-AVFs. Individuals were not specifically evaluated for a pRC-AVF but this procedure was considered when a fistula at the wrist was an option. We were careful to create RC-AVFs only in patients where we expected prompt success; therefore, pRC-AVFs candidates were limited in number to the portion of those individuals with suitable anatomy. Further investigation is needed to clarify how this application of new vascular access technology will be appropriately incorporated into clinical practice. Other evaluations may include the possibility that somewhat smaller forearm veins may eventually become successful AVFs with this technique and the potential for some centers to find higher patency rates for RC-AVFs using this procedure in selected patients.
Conclusion
The success of these percutaneous radiocephalic–AVFs at the wrist suggests that use of the Ellipsys device will be applicable at the wrist in selected patients where appropriate vessel sizes and configurations are found.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.M. and W.C.J. are consultants and hold stock options in Avenu Medical, Inc.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alexandros Mallios  https://orcid.org/0000-0003-0641-6050
https://orcid.org/0000-0003-0641-6050
William C Jennings  https://orcid.org/0000-0003-3599-0609
https://orcid.org/0000-0003-3599-0609
References
- 1. Brescia MJ, Cimino JE, Appell K, et al. Chronic hemo-dialysis using venipuncture and a surgically created arterio-venous fistula. N Engl J Med 1966; 275: 1089–1092. [DOI] [PubMed] [Google Scholar]
- 2. National Kidney Foundation. K/DOQI clinical practice guidelines for vascular access, 2000. Am J Kidney Dis 2001; 37(Suppl. 1): S137–S181. [DOI] [PubMed] [Google Scholar]
- 3. Fistula First. Catheter Last—FFCL, http://esrdncc.org/ffcl (accessed 19 January 2020).
- 4. Biuckians A, Scott EC, Meier GH, et al. The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 2008; 47(2): 415–421. [DOI] [PubMed] [Google Scholar]
- 5. Kim YO, Song HC, Yoon SA, et al. Preexisting intimal hyperplasia of radial artery is associated with early failure of radiocephalic arteriovenous fistulas. Am J Kidney Dis 2003; 41(2): 422–428. [DOI] [PubMed] [Google Scholar]
- 6. Pflederer TA, Kwok S, Ketel BL, et al. A comparison of transposed brachiobasilic fistulae with nontransposed fistulae and grafts in the fistula first era. Semin Dial 2008; 21(4): 357–363. [DOI] [PubMed] [Google Scholar]
- 7. Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 2002; 62(4): 1109–1124. [DOI] [PubMed] [Google Scholar]
- 8. Hull JE, Jennings WC, Cooper RI, et al. The pivotal multicenter trial of ultrasound-guided percutaneous arteriovenous fistula creation for hemodialysis access. J Vasc Interv Radiol 2018; 29(2): 149–158. [DOI] [PubMed] [Google Scholar]
- 9. Mallios A, Jennings WC, Boura B, et al. Early results of percutaneous arteriovenous fistula creation with the Ellipsys vascular access system. J Vasc Surg 2018; 68(4): 1150–1156. [DOI] [PubMed] [Google Scholar]
- 10. Rajan DK, Ebner A, Desai SB, et al. Percutaneous creation of an arteriovenous fistula for hemodialysis access. J Vasc Interv Radiol 2015; 26: 484–490. [DOI] [PubMed] [Google Scholar]
- 11. Beathard GA, Jennings WC, Litchfield T. Two-year cumulative patency of endovascular arteriovenous fistula. J Vasc Access 2020; 21: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mallios A, Bourquelot P, Franco G, et al. Midterm results of percutaneous arteriovenous fistula creation with the Ellipsys Vascular Access System, technical recommendations, and an algorithm for maintenance. J Vasc Surg 2020; 72(6): 2097–2106. [DOI] [PubMed] [Google Scholar]
- 13.https://www.youtube.com/watch?v=CG46KQoK5LU (accessed 19 January 2020).
- 14. Jennings WC, Kindred MG, Broughan TA. Creating radiocephalic arteriovenous fistulas: technical and functional success. J Am Coll Surg 2009; 208(3): 419–425. [DOI] [PubMed] [Google Scholar]
- 15. Jennings WC, Parker DE. Creating arteriovenous fistulas using surgeon-performed ultrasound. J Vasc Access 2016; 17(4): 333–339. [DOI] [PubMed] [Google Scholar]
- 16. Jennings WC, Mallios A, Blebea J. Upper extremity permanent hemodialysis access placement. In: Darling RC, Ozaki CK. (eds) Master techniques in surgery: vascular surgery: hybrid, venous, dialysis access, thoracic outlet, and lower extremity procedures (Ch.16). Philadelphia, PA: Wolters Kluwer, 2015, pp. 151–163. [Google Scholar]