Abstract
Objective
The association between COVID-19 and chemosensory loss has garnered substantial attention, however to date little is known about the real-life consequences of impairment in this unique patient population. The aim of this study is to evaluate the quality of life (QOL) and personal safety deficits experienced by patients with COVID-19 infection.
Study design
Prospective, longitudinal questionnaires.
Setting
National survey.
Methods
A longitudinal web-based nationwide survey of adults with COVID-19 and/or a sudden change in smell and taste was launched April 10, 2020. Previously published questions on chemosensory-related QOL and safety events were asked at the 6-month follow-up survey.
Results
As of February 10, 2021, 480 eligible respondents took the 6-month questionnaire, of whom 322 were COVID-19 positive. Impact on QOL was substantial with 96% of subjects reporting at least one of the defined deficits, and over 75% reporting at least 3 of these. “Reduced enjoyment of food” was the most common complaint (87%), while 43% of subjects self-reported depression. The prevalence of safety-related issues was common in this population, with over 57% reporting at least one, and 36% reporting 2 or more events. Of the events asked, the inability to smell smoke that others could perceive was the most common at 45%.
Conclusions
COVID-19 associated chemosensory losses have a real and substantial impact on both quality of life and safety, beyond mere inconvenience. The high prevalence of these issues despite a relatively short period of olfactory deficit should alert clinicians to the serious risks to an already vulnerable patient population.
Keywords: Smell; Taste; COVID-19; Coronavirus; Epidemiology; Quality of life, safety
1. Introduction
Since the emergence of the COVID-19 pandemic, widespread reports have confirmed chemosensory loss as a cardinal symptom of active infection. Over the past year a flurry of academic activity has characterized the phenomenon of COVID-associated smell and taste changes, and yielded important advances in understanding the pathophysiology, epidemiology, and natural history of these deficits [[1], [2], [3], [4], [5]]. Increasing literature is also demonstrating potential for long-term, even permanent health consequences of COVID-19 infection [6,7]. However to date little is known about the real-life consequences of smell and taste impairment in this unique population. A number of prior reports prior to the COVID-19 pandemic had demonstrated substantial impact of chemosensory dysfunction, irrespective of cause, on quality of life and personal safety [[8], [9], [10], [11], [12], [13]]. Thus it stands to reason that the same issues may be present in COVID-19 patients, thus adding to the already significant short and long-term burden of this disease. The aim of this study is to report quality of life (QOL) and personal safety data from a large, prospectively-collected nationwide cohort of subjects with chemosensory changes experienced during the COVID-19 pandemic. This data will seek to highlight to clinicians and the public that such deficits have substantial negative effects, beyond mere inconvenience.
2. Methods
A web-based nationwide survey was conducted of adults who had either been diagnosed with COVID-19 or experienced a sudden change in smell and taste since January 2020. Recruitment began April 10, 2020 through online social media platforms, and participants received follow-up surveys 14 days, 1 month, 3 months, and 6 months after enrollment. Following consent, patient demographics, symptoms, comorbidities, testing status, treatment, and recovery status were collected and managed using REDCap electronic data capture tool [14,15]. Questions regarding quality of life (QOL) and safety concerns were asked in the 6 month survey (Table 1 ). These questions were chosen from standard questions asked during clinical evaluations at the Virginia Commonwealth University Smell and Taste Center, and those used in prior work from our group, as reported in previously published work [8,12,13] in order to allow comparison with such historical data. All analyses were done using R statistical programming language (version 4.0.3; R Core Team, 2020). For this study, subjects were included in the study population if the following criteria were met: a) age over 18 years; b) subject reported a change in sense of smell occurring since January 2020; c) subject reported having been diagnosed with COVID-19 infection by a positive test result or diagnosis by a medical professional as reported at initial survey or any follow up survey. Continuous variables were summarized with means, standard deviations, and ranges, whereas categorical variables were summarized with frequencies and percentages. This study was approved by Virginia Commonwealth University Institutional Review Board (HM20019186).
Table 1.
Survey questions for quality of life and safety factors.
| Quality of life factors: While I was experiencing smell/taste loss, I experienced the following (check all that apply): |
|---|
| Reduced Enjoyment of Life |
| My enjoyment of food was reduced |
| My appetite was reduced |
| I lost weight |
| I missed the enjoyment of fragrances |
| I was depressed |
| I worried about body odors |
| None of these |
| Safety Factors: While I was experiencing smell/taste loss, I experienced the following (check all that apply): |
|---|
| I was unable to smell smoke that others noticed |
| I burned food on the stove or in the oven |
| I ingested spoiled foods |
| I was exposed to a gas leak and did not know it |
| I did not recognize the smell of soiled diapers |
| None of these |
3. Results
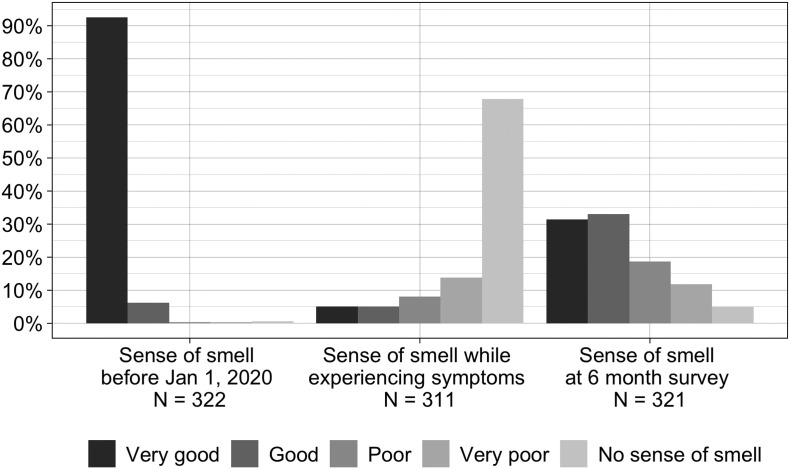
Through February 10, 2021, 2495 individuals completed the initial survey, 1378 had been sent and 480 completed the 6-month survey. Of these, 322 met study inclusion criteria and make up our study population. Table 2 details demographic information for this group. Over 98% of subjects rated their overall sense of smell as “good” or “very good” prior to January 2020, while. During COVID-19 symptoms only 10% reported their sense of smell as “good” or “very good,” and two thirds reported “no sense of smell.” At the time of 6 month survey only about one third indicated their current sense of smell was the same as compared to prior to January 2020, with 64% indicating sense of smell to be “good” or “very good” and 5% still reporting “no sense of smell.” (Fig. 1 ) Duration of smell alterations was less than a month in less than one quarter of respondents, and 6 months or longer in 67%. Over 45% indicated alterations in odor perception (parosmias), while over 25% reported smelling things that were not there (phantosmias). Patient reported taste status mirrored olfactory status, with over 99% rating their taste as good or very good prior to January 2020, with only 42% indicating their current sense of taste was the same as compared to that time.
Table 2.
Demographic characteristics, N = 322.
| N | (%) | |
|---|---|---|
| Sex | ||
| Male | 63 | (16.6%) |
| Female | 258 | (80.4%) |
| Race | ||
| White | 263 | (82.5%) |
| Hispanic or Latino | 26 | (8.2%) |
| Black or African American | 13 | (4.1%) |
| More than one | 11 | (3.5%) |
| Asian | 4 | (1.3%) |
| American Indian/ Alaska Native | 2 | (0.86%) |
| Mean (SD) | [Min, Max] | |
| Age (years; n = 322) | 41.57 (13.72) | [18.00, 78.00] |
| BMI (n = 297) | 27.61 (7.70) | [16.83, 86.17] |
Fig. 1.
Self-reported level of sense of smell in COVID-19 positive patients before January 2020 (baseline), during COVID-19 symptoms, and at time of 6 month survey.
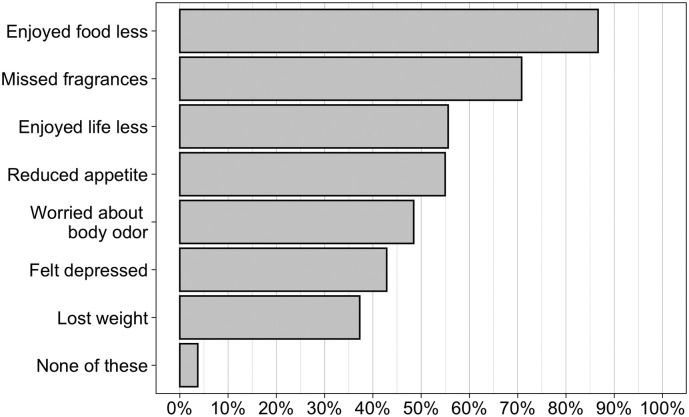
Impact on QOL was substantial (Fig. 2 ), with 96% of subjects reporting at least one of the defined quality of life deficits, and over 75% reporting 3 or more of these. It is noteworthy that “reduced enjoyment of food” was the most common complaint (87%), while 43% of patients self-reported depression.
Fig. 2.
Prevalence of Quality of Life issues in COVID-19 positive patients with loss of smell/taste, (N = 322).
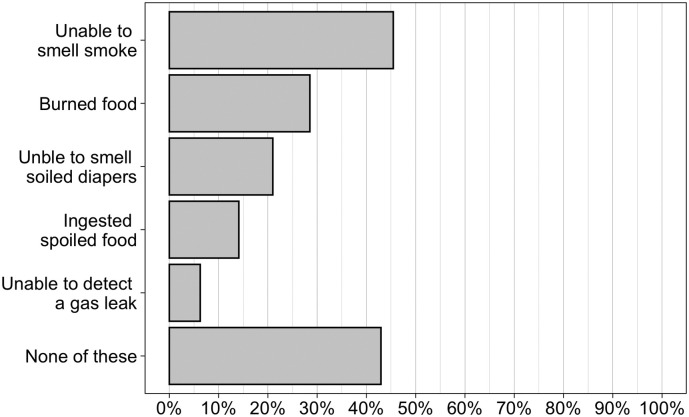
The prevalence of safety-related issues was common in this patient population, with over 57% reporting at least one, and 36% reporting 2 or more of these issues (Fig. 3 ). Of the events queried, the inability to smell smoke that others could perceive was the most common at 45%.
Fig. 3.
Prevalence of Safety issues in COVID-19 positive patients with loss of smell/taste. (N = 319).
4. Discussion
Since its onset over a year ago, the Coronavirus pandemic has exerted a harsh impact. Beyond the rising death toll, now over 2.4 million worldwide and 490,000 in the United States as of this writing, the economic effect has been staggering, between the direct costs of healthcare for COVID sufferers and the indirect costs on world economies [16,17]. Growing reports of patients experiencing long-lasting symptoms and persistent medical comorbidities inflicted by the virus – so-called “long-haulers” - have emerged indicating potential for health impacts well beyond the acute infection and suggesting a need to better assess long term health and quality of life consequences of the disease [6,7,18]. While previous reports have shown that in the case of smell and taste deficits associated with COVID, the majority improve or even resolve within weeks approximately one third or more have persistent deficits [[19], [20], [21], [22]]. Unfortunately for patients with persistent olfactory deficits no definitive treatments exist to effectively restore function. A number of treatments have been explored specifically for post-viral olfactory dysfunction, however current evidence only supports a potential benefit of olfactory training therapy [[23], [24], [25]]. Evaluation of specific therapies for COVID-19 associated olfactory loss is currently an area of exploration. For those with persistent losses, interventions are largely directed at advising compensatory strategies, including strict maintenance of smoke detectors and having others check perishable food items [26,27]. The data from the current study demonstrate a relatively high rate of QOL and safety issues in patients with COVID-19 related smell and taste loss. This is in line with Elkholi et al.'s recent online subjective survey study of QOL in COVID-19 patients, which showed 76% of patients reporting general decline in QOL, with “less awareness of personal hygiene” and “less interested in food and drink” as the most often cited most negative effects [27]. Those perceiving risks associated with their smell loss indicated “failure to perceive smoke/fire” as their main risk. Among other methodological differences with the current study, this work restricted subjects to those reporting complete anosmia, without self-reported parosmia and/or phantosmia, and without other non-smell manifestations of COVID-19 infection. While prior studies have demonstrated significant safety risks and quality of life impact of olfactory dysfunction irrespective of cause, the rather high prevalence of these issues in our cohort of COVID-19 affected subjects over a short period of time (maximum of roughly one year, given the approximate time of onset on the pandemic in the United States) is of concern [8,10,11]. Prior studies on the impact of chemosensory loss on QOL and safety, almost all retrospective in nature, have queried patients on the prevalence of these issues over the entire duration of their loss, which in many cases may be years or, in the case of congenital losses, lifelong [28].
As would be expected, the most common effects on QOL seen in our study relate to the hedonic appreciation of foods and fragrances. Yet, an alarmingly high percentage of patients report a decreased enjoyment of life in general (56%), depression (43%), loss of appetite (55%), and weight loss (37%). Previous studies showed somewhat lower rates of reduced enjoyment in life (25%) [8], with comparable rates of “mood changes” (68%) [9] and decreased appetite (56%) [9]. Both of these studies were comprised of a majority of patients with far longer durations of olfactory loss (over 96% greater than 1 year in the study by Miwa et al., over 47% greater than 2 years in the study by Temmel et al). This striking difference complicates comparison of these populations. One may speculate that patients with longer duration of olfactory deficits may have adopted compensatory strategies that may lessen their perceived QOL impact of their chemosensory loss, although Temmel et al. found no significant effect of duration of olfactory loss on the prevalence of “difficulties in daily life” among their study population [9,26]. In the case of depression, a substantial incidence has been reported with both COVID-19 and olfactory disorders [9,29]. Speth et al. recently used validated questionnaires for depression and anxiety to assess changes associated with COVID-19 at time of diagnosis [30]. They revealed a significant association between depression and anxiety scores and both age and chemosensory losses, but not with gender or smoking status, or other COVID-19 associated symptoms such as cough, fever, shortness of breath. The authors suggest that this may support emotional disturbances as a direct effect of COVID-19 on the central nervous system. Elkholi et al. reported a lower prevalence (15.8%) of depression in their study, however their questionnaire used an open-ended item asking the “main effect” on wellbeing, suggesting that overall prevalence in their group may be higher [27]. However both this and the current study did not assess baseline level of depression or other psychiatric disease prior to development of COVID-19 and associated olfactory deficits, nor have a control population of COVID-19 patients without olfactory deficits as a basis for comparison, thus making interpretation of these figures less straightforward.
The occurrence of personal safety risks or events, such as failure to detect smoke or gas leaks or ingestion of spoiled foods, has been studied in the non-COVID population. Generally incidence of such safety “events” increase with increasing degree of olfactory deficits [12,13,31]. The current data shows a higher percentage of patients having at least one safety event (57%) than previously reported by Pence et al. (approximately 40%), although the specific events queried differed slightly [13]. Most notably, our data showed 45% of subjects unable to smell smoke, higher than previous reported by Pence et al. (approximately 7%) with olfactory losses from varied etiologies. This may be accounted for by the higher risk of experiencing such events in younger and female subjects, given mean age (42 years) and disproportionate gender breakdown (80% female) suggest a higher risk of safety events in our population than in prior reports [12,13].
This study is not without limitations. Our study population shows a disproportionately high percentage of female and white subjects, which thus may not be a fair sampling of the nationwide population of COVID-19 sufferers. It is unclear if this is a result of the online means of solicitation for subject participants reaching these demographics, or reflects differing motivations to complete the surveys of access to the online survey. The retrospective nature of such a longitudinal survey is prone to both selection and recall bias. Patients with more severe, longer lasting or persistent symptoms may be more inclined to participate than those with lesser or no symptoms. This is clearly supported by the low rate of recovery to baseline smell function reported by our study population of about one third, far lower than the 70–90% recovery within 1 month figures reported by studies reporting on the natural history of these deficits [[19], [20], [21], [22]]. In addition, subjects retrospectively reporting on past symptom burden may under or over report based on memory. It has been well established that patient self-reports of olfactory dysfunction likely underestimate the true incidence of these deficits [32,33]. Perhaps most significantly, COVID-19 itself has very real and lasting effects on both physical and mental health. Our data do not allow control of other potential confounding factors, such as pre-existing non-COVID-related or other COVID-related medical comorbidities and socioeconomic factors, and thus precludes determination of the individual contribution of COVID-associated olfactory deficits to this burden. Irrespective, it is clear that those individuals with COVID-related chemosensory loss are at considerable risk for both impairment of QOL and adverse safety events.
5. Conclusions
These data demonstrate a substantial quality of life and personal safety burden attributable to olfactory deficits observed in subjects with COVID-19. Almost all reported quality of life deficits with almost half indicating depression. Likewise, over half experienced personal safety related issues. These numbers are striking in light of the relatively short duration of smell loss reported by the majority of subjects. Further work will better define factors predictive of this added morbidity of COVID-19. Until then, clinicians and the general public should be aware of and vigilant for the potentially serious consequences of chemosensory loss in this population.
CRediT authorship contribution statement
Conception: DHC, ERR, ZAK, RMC
Design: DHC, ERR, ZAK, RMC
Data Analysis: DHC, ERR, YS, SGB, RMC
Manuscript Drafting: DHC, ERR, SGB, ZAK, RMC
Final Approval: DHC, ERR, YS, SGB, ZAK, RMC
Declaration of competing interest
None.
Acknowledgments
Acknowledgement
Funded by a grant from the Medarva Research Foundation. The use of the REDCap Database was partially funded by the National Center for Research Resources (NCRR), Award Number UL1TR002649.
References
- 1.Coelho D.H., Kons Z.A., Costanzo R.M., Reiter E.R. Subjective changes in smell and taste during the COVID-19 pandemic: a national survey-preliminary results. Otolaryngol—Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2020 doi: 10.1177/0194599820929957. Published online May 19. 194599820929957. [DOI] [PubMed] [Google Scholar]
- 2.Karimi-Galougahi M., Raad N., Mikaniki N. Anosmia and the need for COVID-19 screening during the pandemic. Otolaryngol—Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2020;163(1):96–97. doi: 10.1177/0194599820925056. [DOI] [PubMed] [Google Scholar]
- 3.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. 2020;41(5) doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaye R, Chang CD, Kazahaya K, Brereton J, Denneny J. COVID-19 anosmia reporting tool: initial findings. Otolaryngol—Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. https://www.entnet.org/sites/default/files/uploads/kaye_covid-19_anosmia_reporting_tool_initial_findings.pdf. [DOI] [PubMed]
- 5.Carrillo-Larco R.M., Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: a systematic review. Wellcome Open Res. 2020;5:94. doi: 10.12688/wellcomeopenres.15917.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Disser N.P., De Micheli A.J., Schonk M.M., et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am. 2020;102(14):1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological Sequelae of COVID-19. Med Sci Monit Int Med J Exp Clin Res. 2020;e928996:26. doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miwa T., Furukawa M., Tsukatani T., Costanzo R.M., DiNardo L.J., Reiter E.R. Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg. 2001;127(5):497–503. doi: 10.1001/archotol.127.5.497. [DOI] [PubMed] [Google Scholar]
- 9.Temmel A.F.P., Quint C., Schickinger-Fischer B., Klimek L., Stoller E., Hummel T. Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg. 2002;128(6):635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 10.Altundag A., Tekeli H., Salihoglu M., et al. A study on olfactory dysfunction in Turkish population with using survey method and validated olfactory testing. Indian J Otolaryngol Head Neck Surg Off Publ Assoc Otolaryngol India. 2015;67(1):7–12. doi: 10.1007/s12070-014-0720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmedy F., Mazlan M., Danaee M., Abu Bakar M.Z. Post-traumatic brain injury olfactory dysfunction: factors influencing quality of life. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2020;277(5):1343–1351. doi: 10.1007/s00405-020-05823-0. [DOI] [PubMed] [Google Scholar]
- 12.Santos D.V., Reiter E.R., DiNardo L.J., Costanzo R.M. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130(3):317–319. doi: 10.1001/archotol.130.3.317. [DOI] [PubMed] [Google Scholar]
- 13.Pence T.S., Reiter E.R., DiNardo L.J., Costanzo R.M. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951–955. doi: 10.1001/jamaoto.2014.1675. [DOI] [PubMed] [Google Scholar]
- 14.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Home Johns Hopkins coronavirus resource center. https://coronavirus.jhu.edu/ Accessed February 17, 2021.
- 17.Ibn-Mohammed T., Mustapha K.B., Godsell J., et al. A critical analysis of the impacts of COVID-19 on the global economy and ecosystems and opportunities for circular economy strategies. Resour Conserv Recycl. 2021;164 doi: 10.1016/j.resconrec.2020.105169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryson W.J. Long-term health-related quality of life concerns related to the COVID-19 pandemic: a call to action. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2020 doi: 10.1007/s11136-020-02677-1. Published online October 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter E.R., Coelho D.H., Kons Z.A., Costanzo R.M. Subjective smell and taste changes during the COVID-19 pandemic: short term recovery. Am J Otolaryngol. 2020;41(6) doi: 10.1016/j.amjoto.2020.102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J Otolaryngol - Head Neck Surg J Oto-Rhino-Laryngol Chir Cervico-Faciale. 2020;49(1) doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RHW Cho, To ZWH, ZWC Yeung, et al. COVID-19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope. 2020;130(11):2680–2685. doi: 10.1002/lary.29056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otte M.S., Eckel H.N.C., Poluschkin L., Klussmann J.P., Luers J.C. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol (Stockh) 2020;140(12):1032–1035. doi: 10.1080/00016489.2020.1811999. [DOI] [PubMed] [Google Scholar]
- 23.Addison A.B., Wong B., Ahmed T., et al. Clinical olfactory working group consensus statement on the treatment of post infectious olfactory dysfunction. J Allergy Clin Immunol Published online January. 2021:13. doi: 10.1016/j.jaci.2020.12.641. [DOI] [PubMed] [Google Scholar]
- 24.Miwa T., Ikeda K., Ishibashi T., et al. Clinical practice guidelines for the management of olfactory dysfunction - secondary publication. Auris Nasus Larynx. 2019;46(5):653–662. doi: 10.1016/j.anl.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Kattar N., Do TM Unis G.D., Migneron M.R., Thomas A.J., ED McCoul. Olfactory training for postviral olfactory dysfunction: systematic review and meta-analysis. Otolaryngol—Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2021;164(2):244–254. doi: 10.1177/0194599820943550. [DOI] [PubMed] [Google Scholar]
- 26.Blomqvist E.H., Brämerson A., Stjärne P., Nordin S. Consequences of olfactory loss and adopted coping strategies. Rhinology. 2004;42(4):189–194. [PubMed] [Google Scholar]
- 27.SMA Elkholi, Abdelwahab M.K., Abdelhafeez M. Impact of the smell loss on the quality of life and adopted coping strategies in COVID-19 patients. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. January 19, 2021 doi: 10.1007/s00405-020-06575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croy I., Negoias S., Novakova L., Landis B.N., Hummel T. Learning about the functions of the olfactory system from people without a sense of smell. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speth M.M., Singer-Cornelius T., Oberle M., Gengler I., Brockmeier S.J., Sedaghat A.R. Mood, anxiety and olfactory dysfunction in COVID-19: evidence of central nervous system involvement? Laryngoscope. 2020;130(11):2520–2525. doi: 10.1002/lary.28964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonfils P., Faulcon P., Tavernier L., Bonfils N.A., Malinvaud D. Home accidents associated with anosmia. Presse Medicale Paris Fr 1983. 2008;37(5 Pt 1):742–745. doi: 10.1016/j.lpm.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Desiato V.M., Levy D.A., Byun Y.J., Nguyen S.A., Soler Z.M., Schlosser R.J. The prevalence of olfactory dysfunction in the general population: a systematic review and meta-analysis. Am J Rhinol Allergy. 2021;35(2):195–205. doi: 10.1177/1945892420946254. [DOI] [PubMed] [Google Scholar]
- 33.Nordin S., Monsch A.U., Murphy C. Unawareness of smell loss in normal aging and Alzheimer’s disease: discrepancy between self-reported and diagnosed smell sensitivity. J Gerontol B Psychol Sci Soc Sci. 1995;50(4):P187–P192. doi: 10.1093/geronb/50b.4.p187. [DOI] [PubMed] [Google Scholar]