Abstract
Loss or gain of whole chromosome, the form of chromosome instability commonly associated with cancers is thought to arise from aberrant chromosome segregation during cell division. Chromosome segregation in mitosis is orchestrated by the interaction of kinetochores with spindle microtubules. Our studies show that NEK2A is a kinetochore-associated protein kinase essential for faithful chromosome segregation. However, it was unclear how NEK2A ensures accurate chromosome segregation in mitosis. Here we show that NEK2A-mediated Hec1 (highly expressed in cancer) phosphorylation is essential for faithful kinetochore microtubule attachments in mitosis. Using phospho-specific antibody, our studies show that NEK2A phosphorylates Hec1 at Ser165 during mitosis. Although such phosphorylation is not required for assembly of Hec1 to the kinetochore, expression of non-phosphorylatable mutant Hec1S165 perturbed chromosome congression and resulted in a dramatic increase in microtubule attachment errors, including syntelic and monotelic attachments. Our in vitro reconstitution experiment demonstrated that Hec1 binds to microtubule in low affinity and phosphorylation by NEK2A, which prevents aberrant kinetochore-microtubule connections in vivo, increases the affinity of the Ndc80 complex for microtubules in vitro. Thus, our studies illustrate a novel regulatory mechanism in which NEK2A kinase operates a faithful chromosome attachment to spindle microtubule, which prevents chromosome instability during cell division.
Keywords: chromosome instability, mitosis, kinetochore-microtubule attachment, Hec1, NEK2A, phosphorylation
Introduction
Chromosomal instability (CIN) has been recognized as a hallmark of human cancer and is caused by continuous chromosome missegregation during cell division. Proper chromosome segregation requires a faithful physical link between spindle microtubules and centromeric DNA via a protein supercomplex called kinetochore (Cleveland et al., 2003). In addition to providing a physical link between chromosomes and spindle microtubules, the kinetochore has an active function in orchestrating chromosome movements through microtubule motors and correcting spindle microtubule attachment to kinetochore via checkpoint sensors located at or near it (Yao et al., 2000; Ke et al., 2003; Fang and Fang 2007). Recent studies show that CIN results from high incidence of aberrant kinetochore microtubule attachments such as syntelic attachment (Storchova et al., 2006), which promotes tumorigenesis in p53-null cells (Fujiwara et al., 2005). An outstanding question is how kinetochore microtubule attachment errors are generated and corrected during cell division.
The mitotic checkpoint is one pathway that prevents segregation errors by controlling the time of anaphase until all chromosomes achieve proper attachments to the spindle microtubule (Musacchio and Salmon 2007). Another process to prevent errors is to stabilize and destabilize the kinetochore-microtubule connection (Cleveland et al., 2003). Mounting evidence demonstrates that the Ndc80 complex, comprised of Hec1 (highly expressed in cancer), Nuf2, Spc24 and Spc25, constitutes an important kinetochore-microtubule connection in vivo and in vitro (Cheeseman et al., 2006; DeLuca et al., 2006). Hec1 was initially identified as an Rb-binding protein and subsequently demonstrated as an evolutionarily conserved kinetochore protein (Durfee et al., 1993). Our recent study revealed that Ndc80 complex provides a link between mitotic motor CENP-E and kinetochore (Liu et al., 2007).
Mitosis is orchestrated by kinase-signaling cascades that govern the spatiotemporal control of accurate chromosome segregation. The key switch for the onset of mitosis is the archetypal cyclin-dependent kinase, Cdc2. Besides Cdc2, there are three protein serine/threonine kinase families, the Polo kinases, Aurora kinases and the never in mitosis A-related kinases (NEK) (Nigg, 2001; Fry 2002; Ke et al, 2003), which participates in mitotic progression. Aneuploidy and chromosome instability are two of the most common abnormalities in cancer cells, which arise from aberrant chromosome segregation during mitosis. Emerging evidence suggests that aberrant regulation of mitotic kinases including NEK2 is implicated in solid tumor formation (Hayward and Fry 2006). However, it has remained elusive as whether and how perturbation of NEK2A signaling results in aberrant kinetochore plasticity and chromosome instability.
We have recently showed that NEK2A kinase activity is essential for faithful chromosome segregation (Chen et al., 2002; Lou et al, 2004; Fu et al, 2007). To elucidate the mechanistic insight underlying NEK2A regulation in mitosis, we examined the function of NEK2-mediated Hec1 phosphorylation in microtubule-kinetochore attachment. Our study revealed that NEK2A-mediated phosphorylation of Hec1 is essential for a stable kinetochore-microtubule connection, which prevents CIN during cell division.
Results
Ser165 of Hec1 is phosphorylated by NEK2A in mitosis
Our recent studies revealed that NEK2A is a kinetochore-associated kinase active in spindle checkpoint signaling (Lou et al, 2004; Fu et al, 2007). To delineate the regulatory function of NEK2A in kinetochore protein–protein interactions, we conducted a quick search for NEK2A-binding proteins using a ‘high-content’ far-western assay and had identified an NEK2A-Sgo1 interaction (Fu et al, 2007). This assay validated that NEK2A binds to Hec1 (Supplementary Figure 1).
If Hec1S165 is a cognate substrate of NEK2A (Chen et al, 2002), suppression of NEK2A would abolish the phosphorylation of Hec1S165 in vivo. As shown in Figure 1a, while siRNA treatment has suppressed NEK2A protein accumulation (lower panel) it did not alter Hec1 levels (upper panel). Probing the same blot with a phospho-Hec1S165-specific antibody (Chen et al, 2002) demonstrated that depletion of NEK2A abolished Ser165 phosphorylation in mitosis (Figure 1b, lane 2). Western blot analysis of phospho-Ser10 on histone 3 confirmed the mitotic status of samples (Figure 1b, lower panel). Interestingly, suppression of NEK2A retained a faster mobility shift of Hec1 band (Figure 1a; compare lanes 1 with 2), suggesting that Hec1 is indeed phosphorylated by NEK2A in mitosis.
Figure 1.
Ser165 of Hec1 is a substrate of NEK2A in mitosis. (a) Aliquots of HeLa cells were transfected with NEK2A siRNA and scramble control as described in the ‘Materials and methods‘. Twenty-four hours after the transfection, cells were synchronized with 10 ng ml−1 nocodazole for additional 18h before harvested for western blotting analyses of Hec1 (upper panel) and efficiency of NEK2A depletion (lower panel). *Signify the mobility shift. (b) Same blot used in (a) was stripped and re-probed with an anti-phospho-Hec1 (Ser165) antibody and an anti-phospho-histone3 (Ser10) antibody. (c) Lysates from synchronized G1 and mitotic HeLa cells ectopically expressing green fluorescent protein (GFP)-Hec1 and GFP-Hec1S165A mutant were prepared and incubated with an anti-GFP rabbit antibody coupled to protein A beads. After extensive wash, an aliquot of beads containing GFP-Hec1 from mitotic cells was treated with λ-phosphatase (PPase). Immunoblotting with an anti-Hec1 antibody indicated that both wild-type and mutant Hec1 were expressed at comparable levels. Cell cycle status is mentioned at the bottom. (d) Same blot used in (c) was stripped and re-probed with an anti-phospho-Hec1 (Ser165) antibody. Note that only GFP-Hec1 isolated from mitotic cells reacted with the phospho-Ser165 antibody.
To validate if Hec1S165 is NEK2A substrate in mitosis, we transfected green fluorescent protein (GFP)-tagged wild-type Hec1 and non-phosphorylatable S165A mutant into synchronized HeLa cells. The transfected cells were harvested at G1 and M phase. Interestingly, wild-type GFP-Hec1 from mitotic cells displays a slower mobility compared to that from interphase cells (Figure 1c). The mobility shift was confirmed to be phosphorylation-related as λ-phosphatase (PPase) treatment caused Hec1 to shift to the faster migrating form, indicating that Hec1 is phosphorylated in mitosis. In contrast, GFP-Hec1S165A from mitotic cells displays no mobility shift, suggesting that Ser165 is a major phosphorylation site in mitosis. The phosphorylation of GFP-Hec1 in mitosis was also confirmed with phospho-Ser165 antibody (Figure 1d). Thus, we conclude that Hec1S165 is phosphorylated by NEK2A in mitosis.
Phosphorylation of Hec1 is essential for faithful chromosome segregation
To examine whether NEK2A-mediated phosphorylation is essential for Hec1 localization to the kinetochore, plasmids expressing GFP-tagged wild-type, non-phosphorylatable (Hec1S165A) and phospho-mimicking Hec1 (Hec1S165E) were transfected into HeLa cells, and fusion proteins were scored for their kinetochore localization relative to CENP-H under fluorescence microscopy. As shown in Figure 2A, the kinetochore distribution of GFP-Hec1 proteins (wild type, Hec1S165A and Hec1S165E) was readily observed as the merged images show the co-distribution of GFP-Hec1 proteins with CENP-H (Figures 2A, d; 2B, d′ and 2C, d″), suggesting that exogenous Hec1 proteins are able to assemble onto the kinetochore regardless of the phosphorylation status of Ser165. These data suggest that Hec1S165 phosphorylation is not required for kinetochore targeting of Hec1, consistent with the architect of Ndc80 complex resolved by structural biology (Ciferri et al, 2007).
Figure 2.
Phosphorylation of Hec1 is essential for faithful chromosome congression. (A–C) HeLa cells were transiently transfected to express green fluorescent protein (GFP)-Hec1 wild type and mutants as described in the ‘Materials and methods‘. These transfected cells were fixed 36h after the transfection and processed for immunostaining with anti-CENP-H antibody (CENP-H, red), 4–6-diamidino-2-phenylindole (DAPI; DNA, blue) and GFP (GFP-Hec1, green). Bars, 10 μm. (A) Exogenous expression of GFP-Hec1 localized to kinetochore and chromosomes achieved metaphase alignment. (a–d) Optical images collected from a GFP-Hec1-expressing metaphase HeLa cell. (B) Exogenous expression of GFP-Hec1S165A localized to kinetochore but chromosomes failed to congress to the equator. Lagging chromosomes were correlated with the high-level expression of GFP-Hec1S165A (a′–d′; arrows). (C) Exogenous expression of GFP-Hec1S165E localized to kinetochore chromosomes achieved metaphase alignment. (D) Schematic illustration showing how kinetochore positions were measured by the distance from the nearest pole along the pole–pole axis, and normalized for pole–pole distance. (E) Profiles of kinetochore position of HeLa cells expressing wild-type Hec1, Hec1S165A and Hec1S165E. Positions were binned in increments of 0.1.
While the distribution pattern of all three GFP-Hec1 proteins (wild type, S165A and S165E) was similar (Figures 2A, b; B, b′ and C, b″), the GFP-Hec1S165A-expressing cells contain more lagging chromosomes that were readily apparent (Figure 2B, b′). As the kinetochore position relative to the pole has been previously proposed as an accurate reporter for judging chromosome misalignment (Lampson and Kapoor, 2005), we measured this distance in >100 kinetochore pairs in which both kinetochores were in the same focal plane, in both wild-type, S165A and S165E Hec1-expressing cells. Kinetochore positions were surveyed by the distance from the nearest pole along the pole–pole axis, and normalized for pole–pole distance (Figure 2D). Our quantitative analysis indicated a relatively uniform distribution of kinetochores along the length of the spindle in GFP-Hec1S165A-overexpressing cells (Figure 2E). Importantly, expression of GFP-Hec1S165A resulted in a significant increase in cells bearing misaligned chromosomes (33.9±6.6%; P<0.05). Only a minority of GFP-Hec1 and GFP-Hec1S165E-expressing cells displayed misaligned chromosomes (6±2.1% for GFP-Hec1 and 8±2.5% for GFP-Hec1S165E). Thus, we conclude that phosphorylation of Ser165 by NEK2A is essential for faithful chromosome congression.
To determine whether the centromeres of these lagging chromosomes are stretched, we also measured inter-kinetochore distances in the subset of GFP-Hec1S165A-overexpressing cells with metaphase alignment (Yao et al., 2000). The aligned metaphase chromosomes in Hec1S165A-overexpressing cells exhibited decreased centromere stretch compared with wild-type Hec1-expressing cells (Supplementary Table 1), suggesting that these cells segregate their chromosomes without achieving the proper extent of centromere stretch.
NEK2A phosphorylation is essential for proper chromosome-microtubule attachment
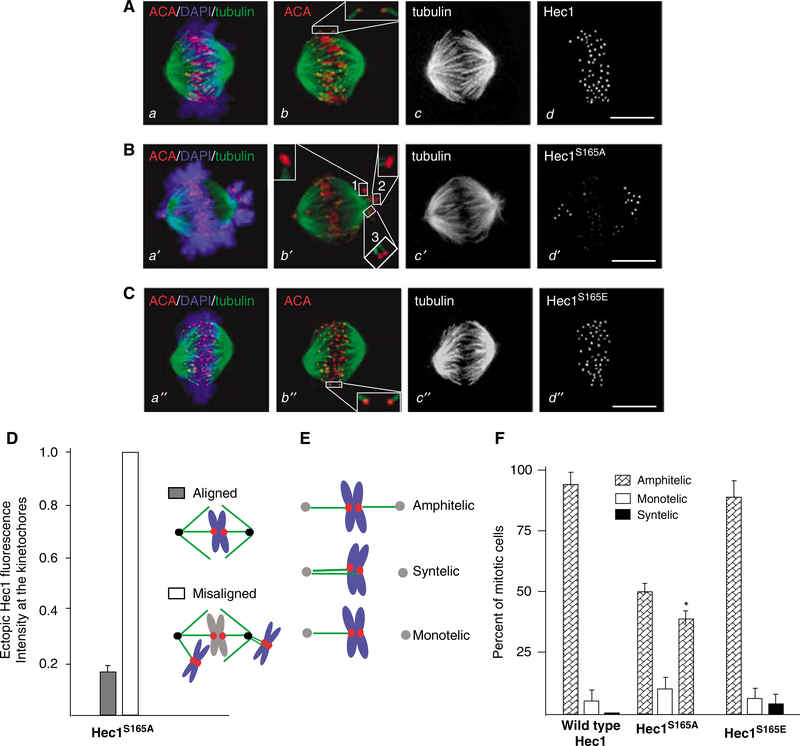
Our observations of delayed chromosome congression, lagging chromosomes and decreased centromere stretch in GFP-Hec1S165A-overexpressing cells suggest that non-phosphorylatable Hec1 results in abnormal interactions between the kinetochores and spindle microtubules. To validate this hypothesis, we probed for the presence of cold-stable kinetochore microtubules in GFP-Hec1S165A-overexpressing cells. As shown in Figure 3A, the spindle remained intact after cold treatment, with microtubule fibers clearly attached to each kinetochore stained with ACA (Figures 3A, a and C, a″, red), in cells overexpressing wild-type Hec1 and Hec1S165E. In Hec1S165A-overexpressing cells, cold-stable kinetochore-microtubule fibers were present on both aligned chromosomes and chromosomes near the pole. Quantitative analysis of GFP fluorescence intensity of GFP-Hec1S165A-expressing cells indicates that the GFP intensity of the kinetochore near the pole is about 6.1-fold higher than that of an aligned kinetochore (Figure 3D), consistent with the notion that overexpression of Hec1S165A produces dominant effect in chromosome segregation and/or alignment.
Figure 3.
Inhibition of Hec1 phosphorylation results in aberrant kinetochore-microtubule attachments. (A–C) Immunofluorescence images of HeLa cells expressing wild-type Hec1 (A, a–d), Hec1S165E (B, a′–d′) or Hec1S165E (C, a″–d″). Cells were treated as described in the ‘Materials and methods‘. Each chromosome (blue) has two sister kinetochores (red) that stain with CREST serum (ACA). In HeLa cells expressing wild-type Hec1 (A, a–d) or Hec1S165E (C, a″–d″), almost all microtubules (green) emanating from the oppose poles attach to each of sister kinetochores. However, in Hec1S165A-expressing cells (B, a′–d′), several examples with both kinetochores of a sister pair attached to microtubules emanating from same pole were observed (B, b′). 1, 2 and 3 show higher magnification micrographs for three pairs of kinetochores aberrantly attached to spindle microtubules. (D) Quantification of Hec1S165A fluorescence intensity of kinetochores of lagging chromosome compared to that of chromosomes aligned at the equator. (E) Schematic illustration of kinetochore-microtubule attachment orientation. (F) Quantification of kinetochore-microtubule attachment orientation in HeLa cells expressing wild-type Hec1, Hec1S165A and Hec1S165E, respectively. Error bars represent s.e.; n=3 preparations (~100 mitotic cells). *P<0.001.
We next examined the microtubule attachment and the orientation of sister kinetochores on chromosomes in Hec1S165A-overexpressing cells. To this end, we performed confocal scanning microscopy on HeLa cells that were stained with anti-tubulin antibody and anti-CREST serum. To show individual kinetochores more clearly, we present optical sections in insets while use maximal intensity projections of the entire Z-stack for volume view. We scored kinetochore attachment in which both kinetochores were in the same focal plane, in all three GFP-Hec1 (wild type, Hec1S165A, Hec1S165E)-expressing cells. In an Hec1S165A-expressing cell shown in Figure 3B, it is readily apparent from the confocal projection that Hec1S165A overexpression resulted in lagging chromosome near the poles, while all chromosomes were aligned around the equator in the cells expressing wild-type Hec1 and Hec1S165E mutant. Enlargement of kinetochore-microtubule attachment indicates that some cold-stable microtubules display syntelic orientation and monotelic orientation (drew in Figure 3E). In contrast, most spindle microtubules exhibit amphitelic configuration in control cells expressing wild-type Hec1 and Hec1S165E. We surveyed approximately 100 mitotic cells for kinetochore attachment orientation, in which both kinetochores were in the same focal plane, from Hec1S165A-overexpressing cells and the controls. Our survey indicates syntelic orientation is a typical feature of Hec1S165A-overexpressing cells. As shown in Figure 3F, a summary from three different experiments show that expression of Hec1S165A resulted in significant increases in cells bearing syntelic attachment (38.7±5.4%), compared to the control (8.7±3.5% for phospho-mimicking Hec1S165E-overexpressing cells). Therefore, we conclude that phosphorylation of Hec1S165 is essential for proper attachment of the kinetochore to spindle microtubules.
NEK2A phosphorylation regulates Ndc80 binding for microtubules
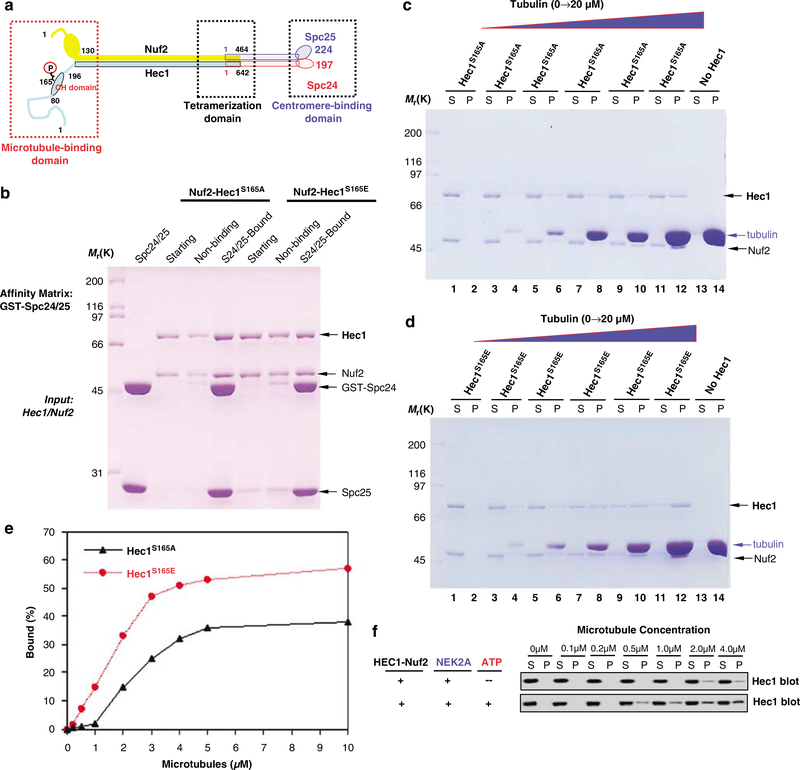
Our previous study in budding yeast demonstrated that Ser165 phosphorylation is essential for maintaining chromosome stability during cell division (Chen et al., 2002). Specifically, NEK2A-mediated phosphorylation allowed for a stable kinetochore-microtubule attachment (Figure 3). We sought to examine the assembly of Ndc80 complex using recombinant Hec1S165E and Hec1S165A proteins. However, phosphorylation of Ser165 did not alter the association of Hec1 with Nuf2 nor with Spc24/25 (Figure 4b), consistent with the structural analysis of human Ndc80 complex (Ciferri et al., 2005).
Figure 4.
NEK2A phosphorylation regulates Ndc80 binding to microtubules. (a) Schematic illustration of Ndc80 complex structure with functional domains highlighted. (b) GST-Spc24/25 on glutathione agarose beads was used to absorb phosphor-mimicking and non-phosphorylatable Hec1. Both Spc24/25 bound and flow-through materials were separated by SDS–polyacrylamide gel electrophoresis (PAGE) and visualized by Coomassie blue stain. (c) Purified Hec1S165A–Nuf2 complex (10 μg) was incubated with preformed microtubule followed by co-sedimentation. Equal proportion of materials was separated on SDS-PAGE and visualized by Coomassie blue stain. (d) Purified Hec1S165E–Nuf2 complex (10 μg) was incubated with preformed microtubule followed by co-sedimentation. Equal proportion of materials was analyzed as in (c). (e) Graph showing the microtubule-binding activities of the Hec1S165A–Nuf2 and Hec1S165E–Nuf2 complexes. The average of two experiments is plotted based on western blotting of Hec1. (f) Microtubule co-sedimentation analysis of wild-type Hec1 complex with Nuf2 (detected by probing for Hec1) in the presence of NEK2A alone (no phosphorylation) or NEK2A plus ATP (phosphorylation).
Another potential mechanism for the aberrant attachment seen in Figure 3 is that inhibition of NEK2A phosphorylation decreases the affinity of Ndc80 microtubule binding, we therefore carried out a co-sedimentation assay using phospho-mimicking mutants of Hec1 proteins incubated with preformed microtubules. The Coomassie blue-stained gel in Figure 4c shows that the Nuf2-Hec1S165A complex is co-sedimented with taxolstabilized microtubules in a dose-dependent manner (Figure 4c). Neither protein was pelleted in the absence of microtubules. A dramatic increase in microtubule binding was observed for Hec1S165E mutant (Figures 4d and e). To eliminate the potential artifact using mutant proteins, we assessed microtubule binding after in vitro phosphorylation of wild-type Hec1 by NEK2A. A dramatic increase in microtubule binding was observed after incubation with both NEK2A and ATP, but not with NEK2A in the absence of ATP (Figure 4f). ATP addition on its own, without NEK2A, had no effect (not shown). In addition, the microtubule-binding activity of Hec1S165A and Hec1S165E was unaffected by treatment of NEK2A and ATP (not shown). Thus, phosphorylation of Hec1S165 by NEK2A directly modulates the microtubule-binding affinity of the Ndc80 complex. These results demonstrate that promotion in microtubule-binding affinity of Ndc80 after phosphorylation of Hec1 may contribute to NEK2A-dependent stabilization of kinetochore-microtubule attachments in vivo.
Discussion
We have characterized the functional relevance of NEK2A phosphorylation of Hec1 in kinetochore-microtubule attachment. On the basis of a combination of an in vivo high-resolution phenotypic analysis (Figure 3) with in vitro biochemical reconstitution (Figure 4), we propose that the NEK2A-mediated phosphorylation of Hec1Ser165 modulates the core microtubule-binding activity of the kinetochore. These findings offer a mechanistic link between the NEK2A-mediated phosphorylation of Hec1 and proper attachment of spindle microtubules to kinetochores.
Several studies suggest that the Hec1 complex is involved in formation of the end-binding sites for microtubule plus ends within the kinetochore outer plate, which can maintain attachment under the strong pulling forces generated at kinetochores of bioriented chromosomes (DeLuca et al., 2005). Recent studies demonstrate that Ndc80 complex cooperates with Mis12 and KNL-1 protein complexes to constitute a microtubule-kinetochore binding core (Cheeseman et al., 2006). Our in vitro reconstitution study demonstrated the role of NEK2A kinase in Ndc80-microtubule binding. It would be of great interest to evaluate how NEK2A phosphorylation of Ser165 regulates the globular domain of Hec1 at atomic resolution and whether this phosphorylation facilitates the higher order organization of metaphase kinetochores essential for end capture of spindle microtubules during chromosomal congression.
Loss or gain of whole chromosomes, the form of CIN most commonly associated with human cancers, arising from the failure of accurate segregation of chromosomes in mitosis. The mitotic checkpoint is one pathway that prevents segregation errors by blocking the onset of anaphase until all chromosomes make proper attachments to the spindle. Another process that prevents errors is stabilization and destabilization of connections between kinetochore and spindle microtubules, in which spatiotemporal regulation of kinetochore architecture and microtubule attachment are integrated into mitotic orchestration. The question remains as to how these two pathways are coordinated to ensure accurate chromosome segregation. We observed that many chromosomes in Hec1S165A-expressing cells show syntelic orientation, with both kinetochores attached to the cold-stable spindle microtubules originating from a single pole. Syntelic orientation could result from the collapse of a bipolar spindle, but our observations suggest that Hec1S165A-expressing cells entering mitosis gain this syntelic orientation by de novo capture of microtubules by both kinetochores in a sister pair. Syntelic orientation is a common error early in meiotic spindles (Nicklas 1971), but its prevalence in early mitosis is less clear. A systematic analysis of the frequency at which syntelic orientation of chromosomes occurs by spontaneous mistakes during mitosis has not been documented. Ault and Rieder (1994) propose that syntelic orientation of chromosomes is likely to be a common error in mitosis, and that a specific mechanism must exist for correcting it, as has been demonstrated in meiosis. However, it has been difficult to study syntelic orientation in mitosis because it is normally transient, and presumably rapidly corrected. In Eg5 inhibitor monastrol-treated cells, as many as 70% of the chromosomes can be syntelically oriented at steady state. Two mechanisms for correction of syntelic error have been proposed from meiotic studies, where the first event is the microtubule capture from the opposite pole (Nicklas and Kubai 1985) or microtubule release at the attached pole (Ault and Nicklas 1989). In this regard, it would be of great interest to systematically study phospho-regulation of Hec1 in live cells to examine whether and how syntelic orientation is corrected in mitosis.
Recent studies showed that NEK2A phosphorylates Hec1 and this phosphorylation is required for faithful chromosome separation in yeast (Chen et al., 2002). Our recent study has confirmed the critical role of NEK2A in chromosome segregation in mammalian cells (Lou et al., 2004; Fu et al., 2007). However, NEK2A is not required for assembly of Hec1 to kinetochore (Figures 2A–C), suggesting that other regulatory mechanisms exist to govern the assembly of Ndc80 complex. Two recent elegant studies illustrated that mitotic kinase Aurora B also regulates the globular domain of Hec1 (Cheeseman et al., 2006; DeLuca et al., 2006), which reduces the affinity of the Ndc80 complex for microtubules in vitro and perhaps corrects improper kinetochore-microtubule connections in vivo. Therefore, it would be of great interest to elucidate the respective contribution of Aurora B and NEK2A in orchestrating kinetochore-microtubule attachment in real-time chromosome movements.
Taken together, our finding of phospho-regulation of Hec1 by NEK2A demonstrates a critical role of Hec1 in kinetochore-microtubule attachment. The fact that inhibition of Hec1 phosphorylation abrogates microtubule attachment to the kinetochore and induces chromosome missegregation demonstrates the importance of Hec1 phosphorylation in faithful chromosome segregation and the maintenance of genomic stability in mitosis.
Materials and methods
Recombinant protein production
NEK2A, Hec1 and Ndc80 complexes were expressed in bacteria as fusion proteins and purified as described (Lou et al., 2004; Ciferri et al, 2005). GST-Nuf2-Hec1 protein complex was cleaved with Precision Proteinase (Amersham, Piscataway, NJ, USA) and dialyzed against microtubule-binding buffer BRB80 (80 mM 1,4-piperazinediethanesulfonic acid (PIPES), pH 6.8, 1 mM ethylene glycol tetraacetic acid, 1 mM MgCl2) as descried (Cheeseman et al., 2006).
Microtubule co-sedimentation assay
For microtubule-binding reactions, 5 μg purified Hec1S165A and Hec1S165E proteins in BRB80 media were incubated with different concentrations of preformed microtubules (0–20 μM; Cytoskeleton Inc., CO, USA) at room temperature for 20 min. In some cases, wild-type Hec1 protein was phosphorylated by NEK2A in the presence of ATP before incubation with preformed microtubules as described (Cheeseman et al., 2006). The reaction was then pelleted for 10 min at 80000 r.p.m. in a TLA100 rotor at 25 °C. Equivalent volumes of supernatant (S) and pellet (P) fractions were separated on SDS–polyacrylamide gel electrophoresis (PAGE) and stained with Coomassie brilliant blue.
DNA construction, pull-down assay, transfection, RNA interference, cells synchronization and analysis of kinetochore position are presented in the Supplemental Information online.
Supplementary Material
Acknowledgements
We would like to thank Drs W-H Lee and A Musacchio for gift reagents. This work was supported by grants from Chinese 973 project (2002CB713700, 2007CB914503), Chinese Academy of Sciences (KSCX1-YW-R65 and KSCX2-YW-H10), Chinese 863 project (2001AA215331 and), Chinese Ministry of Education (20020358051; 20050358061 and 111 project B07007 to XD), Chinese Natural Science Foundation (90508002, 30121001 to XY; 30500183 to XD; 30570850 to JZ; 30600222 to JY), National Institutes of Health (DK56292, CA132389, CA89019, and CA92080) and a GCC Breast Cancer Research Grant to XY. The facilities were supported in part by NIH/NCRR/RCM1 Grant G-12-RR03034. SER and XY are GCC eminent scholars.
Abbreviations
- Hec1
highly expressed in cancer
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Ault JG, Nicklas RB. (1989). Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma 98: 33–39. [DOI] [PubMed] [Google Scholar]
- Ault JG, Rieder CL. (1994). Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol 6: 41–49. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. (2006). The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127: 983–997. [DOI] [PubMed] [Google Scholar]
- Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH. (2002). Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem 277: 49408–49416. [DOI] [PubMed] [Google Scholar]
- Ciferri C, De Luca J, Monzani S, Ferrari KJ, Ristic D, Wyman C et al. (2005). Architecture of the human Ndc80-hec1 complex, a critical constituent of the outer kinetochore. J Biol Chem 280: 29088–29095. [DOI] [PubMed] [Google Scholar]
- Ciferri C, Musacchio A, Petrovic A. (2007). The Ndc80 complex: hub of kinetochore activity. FEBS Lett 581: 2862–2869. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Mao Y, Sullivan KF. (2003). Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell 114: 87–98. [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Dong Y, Hergert P, Strauss J, Hickey JM, Salmon ED et al. (2005). Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell 16: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. (2006). Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127: 969–982. [DOI] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen PL, Yeh SH, Yang Y, Kilburn AE et al. (1993). The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev 7: 555–569. [DOI] [PubMed] [Google Scholar]
- Fang L, Fang G. (2007). Centromere cohesion: regulating the guardian. Cell Res 17: 664–665. [DOI] [PubMed] [Google Scholar]
- Fry AM. (2002). The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene 21: 6184–6194. [DOI] [PubMed] [Google Scholar]
- Fu G, Ding X, Yuan K, Aikhionbare F, Yao J, Cai X et al. (2007). Phosphorylation of human Sgo1 by NEK2A is essential for chromosome congression in mitosis. Cell Res 17: 608–618. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. (2005). Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437: 1043–1047. [DOI] [PubMed] [Google Scholar]
- Hayward DG, Fry AM. (2006). Nek2 kinase in chromosome instability and cancer. Cancer Lett 237: 155–166. [DOI] [PubMed] [Google Scholar]
- Ke Y, Dou Z, Zhang J, Yao X. (2003). Function and regulation of Aurora/Ipl1p kinase family in cell division. Cell Res 13: 69–81. [DOI] [PubMed] [Google Scholar]
- Lampson MA, Kapoor TM. (2005). The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat Cell Biol 7: 93–98. [DOI] [PubMed] [Google Scholar]
- Liu D, Ding X, Du J, Cai X, Huang Y, Ward T et al. (2007). Nuf2 interacts with CENP-E and is essential for a stable spindle microtubule-kinetochore attachment. J Biol Chem 282: 21415–21424. [DOI] [PubMed] [Google Scholar]
- Lou Y, Yao J, Zereshki A, Dou Z, Ahmed K, Wang H et al. (2004). NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J Biol Chem 279: 20049–20057. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. (2007). The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8: 379–393. [DOI] [PubMed] [Google Scholar]
- Nicklas RB. (1971). Mitosis. Adv Cell Biol 2: 225–297. [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Kubai DF. (1985). Microtubules, chromosome movement, and reorientation after chromosomes are detached from the spindle by micromanipulation. Chromosoma 92: 313–324. [DOI] [PubMed] [Google Scholar]
- Nigg EA. (2001). Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2: 21–32. [DOI] [PubMed] [Google Scholar]
- Storchova Z, Breneman A, Cande J, Dunn J, Burbank K, O’Toole E et al. (2006). Genome-wide genetic analysis of polyploidy in yeast. Nature 443: 541–547. [DOI] [PubMed] [Google Scholar]
- Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW. (2000). CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat Cell Biol 2: 484–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.