Abstract
Background:
Peginterferon beta-1a was developed for treatment of relapsing–remitting multiple sclerosis (RRMS) to provide an interferon with increased exposure to facilitate adherence by reducing frequency of application. This non-interventional observational study investigated the adherence to peginterferon beta-1a in real-world clinical practice settings.
Methods:
This prospective study was conducted from 1/2015 to 1/2018 at 77 German MS sites. Adult patients with RRMS (previously treated or treatment-naïve) receiving peginterferon beta-1a (125 µg SC every 2 weeks) were eligible for participation. Data were documented every 3 months over 2 years (nine visits). The primary endpoint was the percentage of patients with overall adherence defined as ⩽10% of injections not administered throughout the 24-month observation period. Secondary endpoints included persistence, patient satisfaction, efficacy (relapse activity, disability progression), and tolerability. Patients were invited to participate in an individualised patient support programme.
Results:
Out of 250 enrolled patients, 190 (aged 18–74 years, 75.3% female) were included in the efficacy analysis. Of those, 74 patients completed the study; 33.2% were treatment-naïve. The proportion of patients with an overall adherence of >90% was 75.7% (95% CI 67.9–81.6). The annualised relapse rate was 0.17. Compared with previous therapies, the scores for treatment satisfaction and convenience were markedly higher with peginterferon beta-1a. Overall, 87.4% participated in the patient support programme, and 47.8% of patients reported adverse events.
Conclusions:
Adherence to the bi-weekly treatment with peginterferon beta-1a was very high. Although adherence could have been positively influenced by the well-accepted patient support programme, the extent could not be unequivocally evaluated. Clinical disease activity remained low. Peginterferon beta-1a was well tolerated, and there were no new relevant safety findings.
Keywords: adherence, multiple sclerosis, patient satisfaction, peginterferon beta-1a, real-world, relapse
Introduction
Disease-modifying therapies (DMT) of mild-to-moderately active forms of relapsing multiple sclerosis (RMS) with interferon (IFN) beta have been established for more than 20 years with a well-recognised efficacy and safety profile.1 However, adverse effects, such as injection-related reactions and anxiety due to self-injection, may impair adherence and subsequently reduce treatment responses.2–5 Furthermore, route and frequency of administration have been shown to affect adherence.6–8 For instance, studies demonstrated better adherence to oral administrations compared with injections and to less frequent dosing regimens.7 In order to address the need for a safe and effective injectable treatment with reduced dosing frequency, pegylated IFN (peginterferon) beta-1a was developed9 to optimise the pharmacokinetic and pharmacodynamic properties of the unpegylated molecule, resulting in improved therapeutic potency.10 Compared with non-pegylated IFNs, peginterferon beta-1a is characterised by a longer half-life, increased bioavailability and exposure, and decreased renal clearance.9,11 Results from the randomised placebo-controlled phase III ADVANCE trial led to the approval of peginterferon beta-1a by both the European Medicines Agency and the Food and Drug Administration in 2014.12 Meanwhile, sustained efficacy of peginterferon beta-1a over 5 years on relapse rate, disability progression, and radiological parameters has been shown.13 However, data on the use of peginterferon beta-1a in real-world practice are limited. The current non-interventional study was initiated to investigate treatment adherence to peginterferon beta-1a in an uncontrolled setting under real-life conditions.
Methods/patients
Study design and patients
This prospective, open-label, non-interventional multi-centre post-marketing surveillance study was conducted at 77 sites across Germany from January 2015 to January 2018. The study design was reviewed and approved by the independent ethics committee of the medical faculty of the Heinrich-Heine-University Düsseldorf, Germany (approval no. 4836), and is consistent with the ethical standards included in the Declaration of Helsinki of 1964 and its later amendments. Male and female patients aged 18 years or older diagnosed with RMS, intended to receive or already receiving (but not for more than 5 weeks prior to inclusion) treatment with peginterferon beta-1a (Plegridy®, Biogen GmbH) were eligible for inclusion. Patients could be either treatment-naïve or previously treated with any DMT. Additionally, patients were invited to participate in the individualised treatment-accompanying patient support programme pleg2care. All patients were required to provide their written informed consent prior to enrolment. Bias was minimised by enrolling patients in consecutive order rather than by physicians’ choice. The observation period comprised 24 months, including one baseline and eight follow-up visits, every 3 months.
Study procedures
Prescription of peginterferon beta-1a was at the discretion of the treating neurologist and not associated with the enrolment status in this study. The V1 (baseline) procedures included obtaining informed consent, eligibility check, review of demographics, medical history (including MS history and concomitant diseases) and previous MS treatment (including treatment satisfaction). All patients received injection training at the baseline visit. The procedures at follow-up visits included assessment of adherence, response to treatment [relapses, change in Expanded Disability Status Scale (EDSS) score], and adverse events (AE). Treatment satisfaction was assessed at month 6, 12, 18, and 24 with the Treatment Satisfaction Questionnaire for Medication (TSQM) questionnaire, a validated, psychometrically sound measure for the assessment of patients’ satisfaction with medication.14 The TSQM has been validated in patients with RMS and has been used to measure satisfaction with DMTs. At month 3, patients were asked to complete the Multiple Sclerosis Treatment Concerns Questionnaire (MSTCQ),15 comparing the current therapeutic regimen with peginterferon beta-1a with their most recent DMT, and a modified version of the Adherence Determinants Questionnaire,16 which ranges from 0 to 100, whereby higher values indicate more utility, more severity, more susceptibility, and more support than barriers. All procedures performed within the scope of this study were in accordance with routine clinical practice.
Patient support programme
The accompanying treatment support programme was designed to address the needs of the individual patient and involved the neurologist/MS nurse, MS service centre, mobile treatment support (mobile app with memory features for medication and appointment alerts) and a website with exclusive access by patients with peginterferon beta-1a subscriptions (ensured via the identification number for pharmaceutical products in Germany). The treatment support programme provided injection training, information (e.g. side effects management), individual support, and aimed at accomplishing a sustainable improvement in adherence and response to treatment.
Outcomes
The primary endpoint was the percentage of adherent patients, with an overall adherence defined as ⩽10% of missed injections during the 24-month observation period (based on patient diaries). Secondary endpoints were: (a) the adherence between two successive physician–patient contacts in which all peginterferon beta-1a injections were administered (defined as 3-month adherence); (b) persistence defined as the time between start of observation and any discontinuation of treatment; (c) patient satisfaction with peginterferon beta-1a treatment (TSQM-9,14 modified adherence determinants questionnaire,16 patient support programme questionnaire); (d) efficacy (relapse activity, disability progression); (e) and tolerability.
Statistical analysis
Descriptive statistical analysis of all collected data was performed using SAS® version 9.2 (SAS Institute Inc., Cary, NC, USA). Subgroup analyses included stratification by previous treatment status (treatment-naïve versus previously treated). Persistence on study treatment was analysed by Kaplan–Meier analysis. A censoring event was the end of observation on treatment (last contact date). Due to the observational nature of the study, no confirmatory hypothesis testing was performed, and all statistical tests (e.g. 95% CIs, ANOVA) were considered exploratory. Generally, no imputation methods (such as last observation carried forward) were applied for missing data. No formal sample size estimations for group comparisons were done. In total, 400 patients were planned to be enrolled. The safety analysis set included patients who had received at least one injection of peginterferon beta-1a. For all other reported results, the efficacy analysis set was used. Patients were excluded from the efficacy analyses under the following conditions: missing date for the baseline visit, missing visit dates for a subsequent post-baseline visit, treatment with peginterferon beta-1a for more than 5 weeks prior to enrolment.
Results
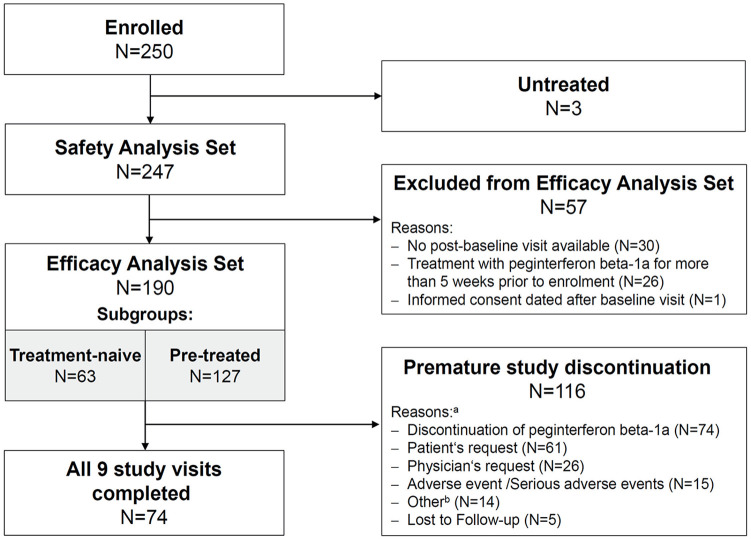
In total, 250 patients from 77 study sites were enrolled (Figure 1). Of those, 247 received at least one injection of peginterferon beta-1a and were included in the safety analysis set. From the safety analysis set 57 patients were excluded due to missing post-baseline data (N = 30), prior treatment with peginterferon beta-1a (N = 26) or informed consent dated after baseline visit (N = 1). Thus the efficacy analysis set comprised the remaining 190 patients. Of these, 74 patients completed all nine study visits, whereas 116 patients prematurely terminated the study (Figure 1).
Figure 1.
Patient disposition.
aMultiple reasons possible.
bDocumentation included skin reaction, therapy switch, pregnancy, elective intervention, new lesions in magnetic resonance imaging, therapy switch to second line, relapse, flu-like symptoms, intense (and persisting) side effects, lack of effectiveness, patient’s request to switch back to prior treatment.
Baseline demographics are summarised in Table 1. Mean age was 41.2 ± 11.9 years; patients were predominantly female (75.3%). Sixty-three patients (33.2%) were treatment-naïve. The remaining 127 patients had one (46.8%), two (12.1%), or more than two (7.9%) previous MS therapies, respectively (Table 1). These were predominantly injectable DMTs (81.9%). The most common reasons for discontinuing the previous therapy were patient’s request (45.3%), adverse drug reactions (ADRs) (28.4%), and needle fatigue (24.7%). The predefined term ‘patient’s request’ comprised the sub-categories lack of motivation, lack of confidence in therapy efficacy, lack of understanding of needfulness of therapy, cognitive problems, and other. Most frequent ADRs occurring during previous treatment were injection site reactions, flu-like symptoms and/or gastro-intestinal disorders.
Table 1.
Baseline demographics.
| Parameter | N = 190 |
|---|---|
| Female, n (%) | 143 (75.3%) |
| Mean (±SD) age, years | 41.2 ± 11.9 |
| Mean (±SD) disease durationa, years | 8.6 ± 9.5 |
| Mean (±SD) number of relapses within the previous 12 months | 0.5 ± 0.7 |
| Mean EDSS (±SD) | 1.8 ± 1.3 |
| Previous therapyb | 127 (66.8%) |
| Injectables | 89/127 (67.9%) |
| Interferon beta-1a IM | 51/127 (40.2%) |
| Interferon beta-1a SC | 27/127 (21.3%) |
| Interferon beta-1b SC | 13/127 (10.2%) |
| Glatiramer acetate | 10/127 (7.9%) |
| Natalizumab | 3/127 (2.4%) |
| Oral | 21/127 (19.8%) |
| Dimethyl fumarate | 15/127 (11.8%) |
| Teriflunomide | 7/127 (5.5%) |
| Azathioprine | 1/127 (0.8%) |
Time between diagnosis and baseline.
Multiple answers possible.
Percentages for individual prior therapies denote the most recent treatment before study start.
EDSS, Expanded Disability Status Scale; SD, standard deviation.
Adherence
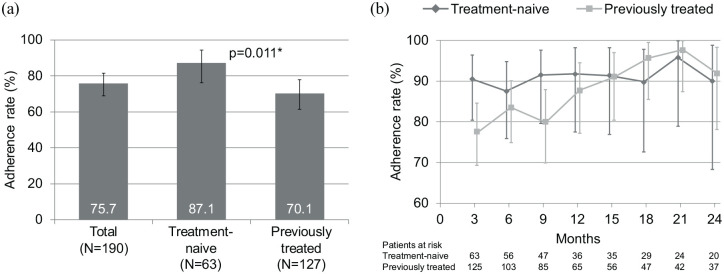
The proportion of patients with an overall adherence of >90% was 75.7% (95% CI 67.9; 81.6). The subgroup analysis showed that the proportion of patients with an overall adherence of >90% was significantly higher among treatment-naïve patients compared with those who received previous therapies [87.1% (95% CI 76.1; 94.3) versus 70.1% (95% CI 61.3; 77.9); p = 0.011] (Figure 2a). The 3-months adherence rate remained stable throughout the study in treatment-naïve patients, but appeared to increase in the previously treated group (Figure 2b).
Figure 2.
Adherence rates overall and by subgroup (a) and 3-month adherence rates by subgroup (b). Adherence rate is defined as the proportions of patients achieving a total adherence of >90.0%.
Vertical error bars indicate the 95% confidence intervals.
*Fisher’s Exact Test.
Treatment persistence with peginterferon beta-1a
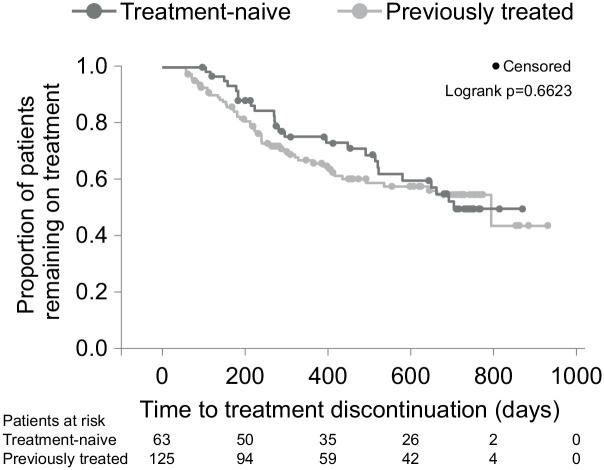
Overall persistence, expressed as median time on treatment according to the Kaplan–Meier estimate, was 793 days [95% CI 579; – (upper limit of CI not estimable)] with no significant difference between treatment-naïve and previously treated patients [703 days (95% CI 520; –) versus 793 days (492; –), log-rank p = 0.6623] (Figure 3). The estimated time to treatment discontinuation of 25% of patients (75th percentile persistence) was 271 days (95% CI 214; 386) for the overall population, and 396 (95% CI 214; 522) versus 239 days (95% CI 184; 328) for the treatment-naïve and previously treated subgroups, respectively. Overall, at months 12 and 24, the proportions of patients remaining on treatment as estimated from the Kaplan–Meier analysis were 68.9 % (95% CI 61.3; 75.4) and 52.5% (95% CI 43.6; 60.2), respectively. The most common reasons for treatment discontinuation were patient’s request (22.6%), lack of efficacy (13.2%) as determined by MS relapse (3.7%), EDSS deterioration (2.1%) or magnetic resonance imaging activity (6.3%), and ADR (10.5%).
Figure 3.
Kaplan–Meier plot for persistence on study (time to treatment discontinuation) by subgroups. A censoring event was the end of observation on treatment (last contact date). The numbers provided for patients at risk indicate those patients who are still in the study and thus at risk of discontinuing treatment. The planned observation period covering nine visits was 24 months (730 days); however, due to real-life circumstances, some patients had longer intervals between visits, thus leading to a longer observation period to complete all nine visits.
Treatment satisfaction
At month 6, 133 patients (85 previously treated) had completed the TSQM-9 questionnaire. The mean values after 6 months on study (V3) were 74.1 ± 19.8 points for the global satisfaction domain, 69.4 ± 21.4 points for the effectiveness domain, and 84.6 ± 15.3 points for the convenience domain. These mean values remained stable during the course of the study. Statistically significant differences between subgroups were not observed. Compared with previous therapies, the scores for treatment satisfaction and convenience were markedly higher with peginterferon beta-1a (Figure 4). Out of 90/133 patients with available responses regarding general satisfaction, the majority of patients were very satisfied (42.2%) or extremely satisfied (22.2%) with peginterferon beta-1a at the last documented visit.
Figure 4.
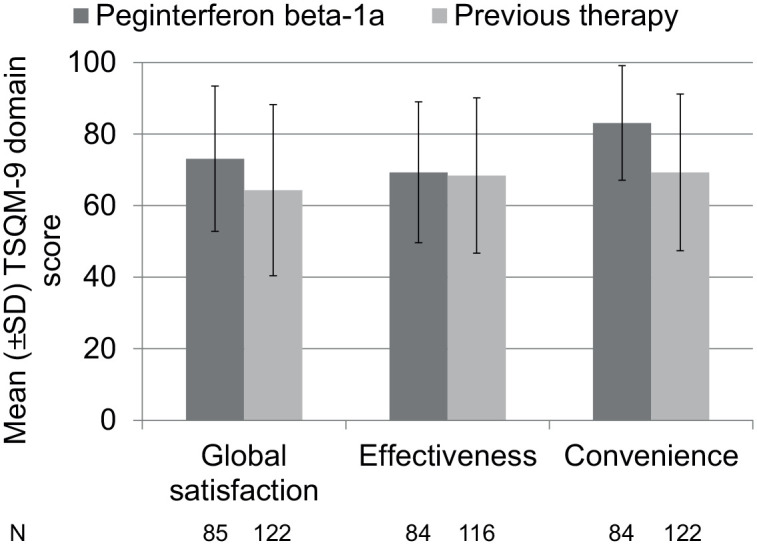
TSQM-9 scores after 6 months on peginterferon beta-1a (subgroup of patients with previous therapy) compared with previous therapy.
Adherence determinants
Adherence determinant questionnaire responses at month 3 indicated that on average patients were well aware of the necessity to adhere to their treatment schedule (mean perceived utility 77.0 ± 15.9), assessed their disease as moderate in severity (mean perceived severity of MS 30.8 ± 17.6), felt moderately vulnerable due to their disease (mean perceived susceptibility 44.1 ± 19.2), and perceived more support than barriers regarding adherence to their treatment schedule (mean ratio of support versus barriers 85.1 ± 15.7).
Patient support programme pleg2care
In total, 166 patients (87.4%) participated in the patient support programme pleg2care (N = 148 at baseline, N = 18 additional patients joined during the study). Patients who declined participation stated most frequently that they already felt sufficiently informed and trained by their treating physician. Pre-treatment status had no statistically significant impact on participation in the programme (p = 0.853). Among those patients participating in pleg2care, only 40 patients provided an assessment at end of study. Of those, 75.0% stated that their expectations were met or completely met and that they felt well or very well supported. Some 97.5% could address all their questions within the scope of pleg2care and felt well advised, and 45.0% reported that the patient support programme was helpful regarding the 14-day injection regimen. In a post hoc secondary analysis comparing the adherence rate between patients who participated in the support programme versus those who didn’t, no significant difference was found between the adherence rates. However, the group of patients not participating in pleg2care was very small (N = 24) so that the analysis was clearly underpowered in that regard.
Comparison with previous therapy
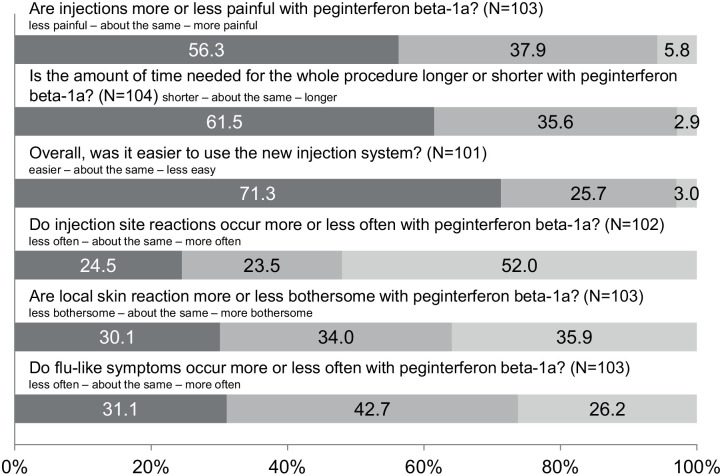
According to the questionnaire completed by 104 previously treated patients at month 3, more than half of the patients stated that the peginterferon beta-1a injections were less painful (56.3%), the time for the procedure was shorter (61.6%), and the use of the peginterferon beta-1a injection system was easier (71.3%) when compared with the previous MS therapy (Figure 5). On the contrary, 52% of the patients felt that they experienced injection site reactions more frequently than with their previous DMT. Regarding the impact of injection site reactions or the frequency of flu-like symptoms, the answers were balanced when patients compared peginterferon beta-1a with their previous DMT.
Figure 5.
Burden of therapy: comparison of peginterferon beta-1a treatment with the most recent disease-modifying therapy.
Effectiveness
Overall, the annualised relapse rate (ARR) was 0.17 (95% CI 0.14; 0.39) relapses per patient year. Among treatment-naïve patients the ARR was higher compared with previously treated patients [0.23 (95% CI 0.08; 0.23) versus 0.14 (95% CI 0.12; 0.25)], but the difference was not statistically significant (p = 0.122). No relevant changes from baseline were observed in the EDSS score during the study period (median 1.5).
Safety
Among the 247 patients included in the safety analysis set, any AEs were reported in 47.8% of patients, any serious AEs (SAEs) in 6.9% and any possibly drug-related SAEs in 3.2%. Treatment-naïve patients consistently showed higher incidences across all analysed AEs than those with previous MS therapy (Table 2). Most commonly occurring AEs were flu-like symptoms (15.4%) and MS relapse (14.2%).
Table 2.
Most common adverse events (incidence ⩾4.0% in the total population) by subgroup and in the total population.
| AE | Treatment-native (N = 82) | Previously treated (N = 165) | Total (N = 247) |
|---|---|---|---|
| Any AE | 46 (56.1%) | 72 (43.6%) | 118 (47.8%) |
| Flu-like symptoms | 20 (24.4%) | 18 (10.9%) | 38 (15.4%) |
| MS relapse | 17 (20.7%) | 18 (10.9%) | 35 (14.2%) |
| Drug ineffective | 6 (7.3%) | 9 (5.5%) | 15 (6.1%) |
| Injection site erythema | 7 (8.5%) | 7 (4.2%) | 14 (5.7%) |
| Fatigue | 5 (6.1%) | 8 (4.8%) | 13 (5.3%) |
| Headache | 6 (7.3%) | 6 (3.6%) | 12 (4.9%) |
| Skin reaction | 5 (6.1%) | 5 (3.0%) | 10 (4.0%) |
| Any SAE | 11 (13.4%) | 6 (3.6%) | 17 (6.9%) |
| MS relapse | 7 (8.5%) | 3 (1.8%) | 10 (4.1%) |
AE, adverse event; MS, multiple sclerosis; SAE, serious adverse event.
Discussion
Under systematically assessed real-world conditions, patients treated with peginterferon beta-1a remained highly adherent, regardless of their prior treatment status. The most common reasons for switching from a previous DMT were patient’s request, ADRs or needle fatigue. The increasing adherence rates of the previously treated group over the course of the study suggest that these reasons did no longer apply after switching to peginterferon beta-1a. It appears that treatment confidence lost during the previous injectable treatment is gradually regained with peginterferon beta-1a. Notably, treatment satisfaction was reported to be higher compared with previous treatment. This is consistent with observations from the ALLOW study, where treatment satisfaction (also assessed via TSQM) increased significantly after switching to peginterferon beta-1a.14 In line with previous observations,17 significantly more treatment-naïve patients achieved an overall adherence >90% compared with previously treated patients. Frequency of administration is known to affect adherence7,8,18 which is reflected in increasing adherence rates from daily injectable glatiramer acetate (49–78%) to every-second-day SC IFN beta-1b (49–91%), thrice weekly SC IFN beta-1a (67–88%) to once weekly IM IFN beta-1a (77–94%).19 Accordingly, even higher adherence rates would be anticipated with a bi-weekly administration interval. Results from the present study corroborate this expectation. The 3-month adherence, which describes the adherence between two successive physician–patient contacts in which all peginterferon beta-1a injections were administered, ranged throughout the study between 88% and 91% for treatment-naïve patients and 78% and 98% for previously treated patients. However, it must be borne in mind that a direct comparison to historical and published adherence rates is not feasible due to different measures and definitions applied for adherence.20 For instance, here six injections of peginterferon beta-1a in 3 months are compared with 15 injections of SC IFN beta-1b in 1 month.20 Another factor known to affect adherence is treatment satisfaction. TSQM-9 results showed increased treatment satisfaction with peginterferon beta-1a in comparison to the previous treatment. This observation is consistent with results from the randomised open-label phase IIIb study ALLOW that investigated treatment satisfaction in patients switching from non-PEGylated IFN beta therapy to peginterferon beta-1a.14 Also the accompanying patient support programme may have positively influenced adherence, for patient education has been identified as a valuable factor to promote adherence.21,22 By the end of the study, several patients no longer required the patient support programme because they felt that they had become sufficiently well informed.
Typically in clinical study settings treatment persistence is expressed as the median time on treatment as estimated by the Kaplan–Meyer analysis. Since the median treatment persistence appeared to be longer than the planned observation period of 24 months, we additionally report the estimated 75th percentile treatment persistence. The estimated period of 396 and 239 days for the treatment-naïve and previously treated patients, respectively, exceeds the 75th percentile treatment persistence for interferon beta-1b, IM interferon beta-1a and glatiramer acetate reported in a retrospective study (approximately 142–185 days for treatment-naïve; 92–170 days for previously treated patients).23 The improved treatment persistence of peginterferon beta-1a may be attributed to the less frequent, that is, bi-weekly dosing interval, which is a key factor to promote adherence.7 Our results for peginterferon beta-1a concur with the observations of Agashivala et al.23 with respect to treatment-naïve patients remaining longer on treatment than those who received prior MS medications. However, treatment-naïve patients on peginterferon beta-1a remain 2.1–2.8-fold longer on treatment than patients on the other interferons or glatiramer acetate, whereas for previously treated patients persistence on peginterferon beta-1a is 1.4–2.6-fold longer compared with other injectables. Thus, treatment burden appears to be a relevant factor for persistence, implying that patients may benefit from receiving peginterferon beta-1a as first-line treatment. However, head-to-head comparisons would be required to confirm this hypothesis.
In terms of effectiveness, data from this real-world study were consistent with clinical trials. The ARR was similarly low compared with that observed at year 2 of the ADVANCE trial (0.17 versus 0.178).24 The effectiveness in terms of sustained EDSS stability was consistent with the ADVANCE trial25 as well as with the 5-year long-term extension trial.13 As was to be expected in a real-world setting, patients had less active disease, as indicated by the rather low mean baseline EDSS (1.8), compared with those enrolled in the ADVANCE study (2.5).
Peginterferon beta-1a was well tolerated under real-world conditions; considerably fewer patients reported flu-like symptoms (15.4%) and injection site reactions (5.7%) than during the 2 years of the ADVANCE study (51% and 64%, respectively).24 However, slightly more than half of the patients reported that injection site reactions with peginterferon beta-1a occurred more frequently than during their most recent previous MS therapy. Given that the majority of patients received prior injectables with more frequent dosing intervals, this result was unexpected and did not reflect results obtained from a head-to-head comparison between non-PEGylated IFN and peginterferon beta-1a.26 It may well be speculated that patients were more sensitised towards the occurrence of injection site reactions if enrolled in the support programme. Data from the open-label study ALLOW indicated that erythema is the most commonly observed injection site reaction associated with peginterferon beta-1a, but its impact on daily activities is minimal.21 The rate of discontinuation due to ADR was higher compared with the rate over 2 years in the ADVANCE study (10.5% this study versus 6% ADVANCE study).24 Although treatment adherence was already high, it may be interesting to explore the potential for further improvement if the time of occurrence of ADR were better predictable. To this end a clinical study investigating delayed ADR is needed.
This study is limited by its non-interventional design which allows the identification of associations, but excludes the conclusion of causal relationships. Furthermore, due to the small number of patients, data could only be analysed descriptively and did not allow any predictive analyses with regards to regression models. In view of the vast treatment landscape of MS agents, the recruitment of patients willing to commit to participation in a non-interventional study over 2 years was challenging. Nevertheless, although the pre-planned number of 400 patients was not reached, clinical data from randomised clinical trials could be replicated. However, our results should be interpreted with caution due to the declining numbers of patients over the course of the study. This is not uncommon in real life and reflects the switching behaviour of patients as other treatment options for MS, such as oral dimethyl fumarate, had become available at the time the study was conducted. Due to the non-interventional design of the study, it is not uncommon that patients do not complete all questionnaires. Another limitation of this study is the possible confounding factor of the pleg2care programme in influencing adherence. A post hoc analysis was performed with the aim to explore this limitation. Unfortunately, due to the small sample size of patients not participating in pleg2care, the extent of the support programme influencing adherence and satisfaction could not be unequivocally evaluated. The strength of this study is represented by the use of validated scores to measure treatment satisfaction27 and adherence determinants.16
The use of Kaplan–Meier survival curves to analyse treatment persistence provides considerable advantages over conventional analyses. In an observational study setting it takes into account patients who had not yet stopped peginterferon beta-1a therapy by their last follow-up, therefore providing a more accurate estimate of persistence. Hence, the study provides a comprehensive view of adherence to peginterferon beta-1a treatment in the real-world setting of RRMS in Germany.
In conclusion, peginterferon beta-1a is a novel interferon associated with high efficacy and low injection frequency in comparison with non-PEGylated interferons. Adherence to the bi-weekly treatment with peginterferon beta-1a was excellent during the study. To what extent this was mediated by the educational aspects of the accompanying patient support programme could not be conclusively determined due to the small number of non-participants. Clinical disease activity remained low with an ARR of 0.17 and maintenance of patients’ EDSS. Patient-reported outcomes confirmed patients’ awareness of disease and necessity for treatment, and patients were very satisfied with their treatment plan. Peginterferon beta-1a was well tolerated, and there were no new relevant safety findings.
Acknowledgments
We thank Dr. Petra Jöstingmeyer (med:unit GmbH, Cologne, Germany) who provided medical writing services. Biogen GmbH provided funding for medical writing support in the development of this article. The authors had full editorial control of the manuscript and provided their final approval.
Footnotes
Conflict of interest statement: TM: personal fees from Biogen, Novartis, Teva, Roche, Merck Serono; non-financial support from Biogen, Merck Serono. KRW, KT: employees of Biogen. IN: Speaker honoraria from Merck Serono, Genzyme; travel grants from Biogen, Merck Serono, Genzyme, Teva, Novartis. MJ: The author declares that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by Biogen GmbH, München, Germany.
ORCID iD: Til Menge  https://orcid.org/0000-0003-3459-8867
https://orcid.org/0000-0003-3459-8867
Contributor Information
Til Menge, Centre for Neurology and Neuropsychiatry, LVR-Klinikum Düsseldorf, Medical Faculty, Heinrich-Heine-University Düsseldorf, Bergische Landstr. 2, Düsseldorf, 40629, Germany.
Karin Rehberg-Weber, Biogen GmbH, München, Germany.
Kirsi Taipale, Biogen GmbH, München, Germany.
Ilias Nastos, Neurological Specialist Practice, Bochum, Germany.
Marek Jauß, Ecumenical Hainich Hospital gGmbH, Mühlhausen/Thüringen, Germany.
References
- 1. Limmroth V, Putzki N, Kachuck NJ. The interferon beta therapies for treatment of relapsing-remitting multiple sclerosis: are they equally efficacious? A comparative review of open-label studies evaluating the efficacy, safety, or dosing of different interferon beta formulations alone or in combination. Ther Adv Neurol Disord 2011; 4: 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43: 655–661. [DOI] [PubMed] [Google Scholar]
- 3. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352: 1498–1504. [PubMed] [Google Scholar]
- 4. Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996; 39: 285–294. [DOI] [PubMed] [Google Scholar]
- 5. Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig 2010; 30: 89–100. [DOI] [PubMed] [Google Scholar]
- 6. Lugaresi A. Addressing the need for increased adherence to multiple sclerosis therapy: can delivery technology enhance patient motivation? Expert Opin Drug Deliv 2009; 6: 995–1002. [DOI] [PubMed] [Google Scholar]
- 7. Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adherence 2010; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011; 28: 51–61. [DOI] [PubMed] [Google Scholar]
- 9. Baker DP, Pepinsky RB, Brickelmaier M, et al. PEGylated interferon beta-1a: meeting an unmet medical need in the treatment of relapsing multiple sclerosis. J Interferon Cytokine Res 2010; 30: 777–785. [DOI] [PubMed] [Google Scholar]
- 10. Bailon P, Won C-Y. PEG-modified biopharmaceuticals. Expert Opin Drug Deliv 2009; 6: 1–16. [DOI] [PubMed] [Google Scholar]
- 11. Hu X, Miller L, Richman S, et al. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol 2012; 52: 798–808. [DOI] [PubMed] [Google Scholar]
- 12. Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol 2014; 13: 657–665. [DOI] [PubMed] [Google Scholar]
- 13. Newsome SD, Scott TF, Arnold DL, et al. Long-term outcomes of peginterferon beta-1a in multiple sclerosis: results from the ADVANCE extension study, ATTAIN. Ther Adv Neurol Disord 2018; 11: 1756286418791143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hendin B, Naismith RT, Wray SE, et al. Treatment satisfaction significantly improves in patients with multiple sclerosis switching from interferon beta therapy to peginterferon beta-1a every 2 weeks. Patient Prefer Adherence 2018; 12: 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cramer JA, Cuffel BJ, Divan V, et al. Patient satisfaction with an injection device for multiple sclerosis treatment. Acta Neurol Scand 2006; 113: 156–162. [DOI] [PubMed] [Google Scholar]
- 16. DiMatteo MR, Hays RD, Gritz ER, et al. Patient adherence to cancer control regimens: scale development and initial validation. Psychol Assess 1993; 5: 102–112. [Google Scholar]
- 17. Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol 2009; 256: 568–576. [DOI] [PubMed] [Google Scholar]
- 18. Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 2011; 18: 69–77. [DOI] [PubMed] [Google Scholar]
- 19. Remington G, Rodriguez Y, Logan D, et al. Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care 2013; 15: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Menzin J, Caon C, Nichols C, et al. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm 2013; 19: S24–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendin B, Huang D, Wray S, et al. Subcutaneous peginterferon beta-1a injection-site reaction experience and mitigation: Delphi analysis of the ALLOW study. Neurodegener Dis Manag 2017; 7: 39–47. [DOI] [PubMed] [Google Scholar]
- 22. Stockl KM, Shin JS, Gong S, et al. Improving patient self-management of multiple sclerosis through a disease therapy management program. Am J Manag Care 2010; 16: 139–144. [PubMed] [Google Scholar]
- 23. Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol 2013; 13: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kieseier BC, Arnold DL, Balcer LJ, et al. Peginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler 2015; 21: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newsome SD, Kieseier BC, Liu S, et al. Peginterferon beta-1a reduces disability worsening in relapsing-remitting multiple sclerosis: 2-year results from ADVANCE. Ther Adv Neurol Disord 2017; 10: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu X, Shang S, Nestorov I, et al. COMPARE: pharmacokinetic profiles of subcutaneous peginterferon beta-1a and subcutaneous interferon beta-1a over 2 weeks in healthy subjects. Br J Clin Pharmacol 2016; 82: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes 2009; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]