Abstract
Introduction:
Liposomal irinotecan (nal-IRI) plus fluorouracil/leucovorin (5-FU/LV) has shown clinical benefit in patients with metastatic pancreatic adenocarcinoma (mPAC) who progressed on gemcitabine-based chemotherapy. However, its role in patients with mPAC previously treated with conventional irinotecan-containing chemotherapy has not been appropriately investigated.
Methods:
In this retrospective analysis, patients with mPAC who received nal-IRI plus 5-FU/LV after conventional irinotecan-containing regimen between January 2017 and March 2020, were identified from two referral cancer centers in South Korea. The ratio of time to progression (TTP) with nal-IRI plus 5-FU/LV to TTP with conventional irinotecan (TTPr) was analyzed with respect to the duration and cumulative dose of conventional irinotecan treatment.
Results:
In total, 35 patients treated with nal-IRI plus 5-FU/LV after the irinotecan-containing regimen were analyzed. The median age was 58 years and 16 (46%) patients were male. The median duration of conventional irinotecan therapy was 4.6 months at a median cumulative dose of 1230 mg. The objective response rate of nal-IRI plus 5-FU/LV was 2.9%, and stable disease was achieved in 11 (31.4%) patients. During the median follow-up of 9.2 [95% confidence interval (CI): 7.8–10.5] months, the median progression-free survival (PFS) and overall survival (OS) were 2.0 (95% CI: 1.4–2.6) months and 4.4 (95% CI: 3.6–5.7) months, respectively. The 6-month PFS and OS rates were 16.3% and 37.5%, respectively. The median TTPr was 0.41 (range, 0.07–2.07), showing a negative correlation with the cumulative dose of prior irinotecan therapy (R = −0.37, p = 0.041). A tentative negative correlation between TTPr and duration of prior irinotecan therapy was observed (R = −0.35, p = 0.062). The most common grade 3–4 toxicities were neutropenia (20%) and fatigue (8.6%).
Conclusion:
Nal-IRI plus 5-FU/LV showed modest effectiveness and manageable toxicities for patients with mPAC previously treated with conventional irinotecan-containing chemotherapy. The cumulative dose of prior conventional irinotecan therapy may be inversely correlated with the effectiveness of nal-IRI plus 5-FU/LV.
Keywords: Fluorouracil/leucovorin, irinotecan-containing chemotherapy, liposomal irinotecan, metastatic pancreatic adenocarcinoma
Introduction
Pancreatic adenocarcinoma (PAC) is a leading cause of cancer-related deaths worldwide as well as in South Korea.1,2 It is usually diagnosed at an advanced stage and has a high recurrence rate despite curative resection, with a 5-year survival rate of approximately 9%.
In the late 1990s, gemcitabine monotherapy showed significant improvement in overall survival (OS) compared with the fluorouracil (5-FU) treatment. Since then, it has been the standard first-line regimen for patients with advanced PAC.3 However, there had been limited progress in systemic treatment strategies for advanced PAC until 2010. As first-line treatment, new chemotherapy regimens such as FOLFIRINOX [5-FU, leucovorin (LV), irinotecan, and oxaliplatin] and gemcitabine plus albumin-bound paclitaxel (nab-paclitaxel) have significantly improved survival outcomes in patients with advanced PAC.4–8
Liposomal irinotecan (nal-IRI) is an intravenous liposomal formulation of irinotecan that consists of irinotecan sucrosofate salt encapsulated in a liposome particle. Preclinical studies have shown that the active metabolite of irinotecan, SN-38, in both nal-IRI and conventional irinotecan therapy cause similar tumor exposure, except that much lower doses of the former are needed.9 Driven by the promising efficacy of nal-IRI reported by a phase II study, NAPOLI-1, a phase III trial, investigated the effects of nal-IRI in patients with metastatic PAC (mPAC) who previously underwent gemcitabine-based treatment.10,11 This trial demonstrated that nal-IRI plus 5-FU/LV improved the OS, progression-free survival (PFS), and objective response rate (ORR) in patients with mPAC who progressed after prior gemcitabine-based therapy.11 Although the NAPOLI-1 trial included patients who previously received conventional irinotecan-containing chemotherapy, the small sample size (approximately 10% patients) was not enough to avoid skepticism about the efficacy of nal-IRI for these patients.
Therefore, we performed a multicenter retrospective analysis to evaluate the effectiveness and safety of nal-IRI with 5-FU/LV in patients with mPAC previously treated with conventional irinotecan-containing chemotherapy.
Materials and methods
Patients
This retrospective study aimed to evaluate the effectiveness and safety of nal-IRI plus 5-FU/LV in patients with mPAC who previously received conventional irinotecan-containing chemotherapy. Patients with histologically confirmed mPAC treated with nal-IRI plus 5-FU/LV were eligible for this study if they had previously received conventional irinotecan-containing chemotherapy as a neoadjuvant, adjuvant, or palliative therapy. The patients were enrolled from two referral cancer centers (Asan Medical Center and Ulsan University Hospital) in South Korea. Clinical data on patient characteristics, treatment history, and survival outcomes were retrospectively obtained by reviewing patient medical records.
This study was approved by the Institutional Review Board of each participating center (Asan Medical Center, 2018-0492; Ulsan University Hospital, 2019-11-037) and was performed in accordance with the ethical standards of institutional research and the Declaration of Helsinki. The need for informed consent for this study was waived, as retrospective analyses do not require consent per the Korean regulations.
Treatment
The dosing schedule of nal-IRI plus 5-FU/LV described in the NAPOLI-1 trial (80 mg/m2 irinotecan hydrochloride trihydrate salt equivalent to 70 mg/m2 irinotecan free base over 90 min, followed by 400 mg/m2 LV over 30 min and 2400 mg/m2 5-FU over 46 h, every 2 weeks) was considered standard in this analysis.11 Dose modification was allowed at the discretion of the attending physicians. Nal-IRI plus 5-FU/LV treatment continued until patients experienced intolerable toxicity or disease progression.
Evaluation
Patients were examined every 6–8 weeks using computed tomography or magnetic resonance imaging. Tumor response was graded using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. All treatment-related adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.
Statistical analysis
The ORR and disease control rate (DCR) were evaluated according to RECIST version 1.1. PFS was defined as the time from the initiation of nal-IRI plus 5-FU/LV to the time of disease progression or death, whichever occurred first. OS was defined as the time from the initiation of nal-IRI plus 5-FU/LV to death from any cause. The time to progression (TTP) was defined as the time between the initiation of specific chemotherapy and tumor progression. The ratio of TTP with nal-IRI plus 5-FU/LV to TTP with conventional irinotecan (TTPr) was calculated. Survival outcomes were estimated using Kaplan-Meier curves. A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using Statistical Package for the Social Sciences version 22.0 (IBM, Armonk, NY, USA).
Results
Patient characteristics
A total of 35 patients who received nal-IRI plus 5-FU/LV after conventional irinotecan-containing chemotherapy at Asan Medical Center and Ulsan University Hospital between January 2017 and March 2020 were identified in this analysis. Baseline characteristics of the patients are summarized in Table 1.
Table 1.
Patient baseline characteristics.
| Variables | Total N = 35 |
|---|---|
| Sex (%) | |
| Male | 16 (45.7) |
| Female | 19 (54.3) |
| Age, years, median (range) | 58 (35–73) |
| <65 | 27 (77.1) |
| ⩾65 | 8 (22.9) |
| Primary tumor site (%) | |
| Head | 22 (62.9) |
| Body | 7 (20.0) |
| Tail | 6 (17.1) |
| Disease extent (%) | |
| Metastatic | 35 (100.0) |
| Site of metastasis (%) | |
| Liver | 23 (65.7) |
| Lymph node | 16 (45.7) |
| Peritoneum | 12 (34.3) |
| Lung | 7 (20.0) |
| Bone | 3 (8.6) |
| Adrenal gland | 1 (2.9) |
| Baseline CA19-9 level (U/ml) (%) | |
| Within normal range | 1 (2.9) |
| > UNL | 22 (62.9) |
| N/A | 12 (34.3) |
| Prior surgery | 12 (34.3) |
| Prior radiotherapy | 14 (40.0) |
| Number of prior lines of chemotherapy (%) | |
| 2 | 26 (74.3) |
| 3 | 9 (25.7) |
| Prior irinotecan-containing chemotherapya | 35 (100.0) |
| Interval between the last dose of prior conventional irinotecan and the start of nal-IRI plus 5-FU/LV, months, median (range) | 7.0 (0.6–30.8) |
All patients received conventional irinotecan as a component of FOLFIRINOX.
5-FU/LV, fluorouracil/leucovorin; CA19-9, carbohydrate antigen 19-9; N/A, not available; nal-IRI, liposomal irinotecan; UNL, upper normal limit.
The median age was 58 years (range, 35–73 years) and 16 (45.7%) patients were male. Majority of the patients (n = 28, 80.0%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. Most common location of the primary tumor was the pancreatic head (n = 22, 62.9%), followed by the body (n = 7, 20.0%) and the tail (n = 6, 17.1%). All patients had metastatic disease, and the most common metastatic sites were the liver (n = 23, 65.7%), lymph nodes (n = 16, 45.7%), peritoneum (n = 12, 34.3%), and lungs (n = 7, 20.0%). Furthermore, 12 (34.3%) patients underwent prior surgery and 14 (40%) patients received prior radiotherapy. The number of lines of prior systemic chemotherapy were two (n = 26, 74.3%) and three (n = 9, 25.7%). The median interval between the last dose of prior conventional irinotecan therapy and the initiation of nal-IRI plus 5-FU/LV was 7.0 months (range, 0.6–30.8 months).
Prior conventional irinotecan therapy
Prior to nal-IRI plus 5-FU/LV, all patients had received both conventional irinotecan and gemcitabine-based chemotherapy. All patients received conventional irinotecan as a component of FOLFIRINOX (Table 2). Majority of the patients (n = 29, 82.9%) were treated with FOLFIRINOX as first-line therapy for either locally advanced or metastatic diseases.
Table 2.
Details of prior conventional irinotecan chemotherapy.
| Variables | Total N = 35 |
|---|---|
| Chemotherapy regimen including prior irinotecan (%) | |
| FOLFIRINOX | 35 (100) |
| Disease extent at the time of irinotecan initiation (%) | |
| Locally advanced, non-metastatic | 23 (62.1) |
| Metastatic | 12 (32.4) |
| Treatment line of irinotecan (%) | |
| First | 29 (82.9) |
| Second | 5 (14.3) |
| Third | 1 (2.9) |
| Duration of administration of irinotecan therapy, months, median (range) | 4.6 (0.5–16.8) |
| Cumulative dose of irinotecan therapy, mg, median (range) | 1230 (150–4650) |
| Reason of discontinuation of irinotecan (%) | |
| Disease progression | 28 (80.0) |
| Conversion surgery | 6 (17.1) |
| Adverse event | 1 (2.9) |
| TTP with irinotecan-containing chemotherapy, months, median (95% CI) | 5.7 (4.9–6.4) |
CI, confidence interval; TTP, time to progression.
At the time of FOLFIRINOX treatment, the extent of disease stage was locally advanced in 23 (62.1%) patients and metastatic disease in 12 (32.4%) patients. The median duration of prior conventional irinotecan treatment was 4.6 months (range, 0.5–16.8 months), and the median cumulative dose of conventional irinotecan was 1230 mg (range, 150–4650 mg). The best responses to prior conventional irinotecan-containing regimen were partial response (PR), stable disease (SD), and progressive disease (PD) in 6 (17.1%), 20 (57.1%), and 7 (20.0%) patients, respectively; none of the patients achieved complete response (CR). The most common reasons for discontinuation of conventional irinotecan-containing therapy were tumor progression during the treatment (n = 28, 80.0%) and completion of scheduled chemotherapy (n = 6, 17.1%).
Effectiveness of nal-IRI plus 5-FU/LV
Effectiveness outcomes of nal-IRI plus 5-FU/LV in this study cohort are summarized in Table 3.
Table 3.
Effectiveness outcomes of liposomal irinotecan plus fluorouracil/leucovorin therapy.
| Variables | Nal-IRI plus 5-FU/LV (N = 35) |
|---|---|
| Best response (%) | |
| CR | 0 (0.0) |
| PR | 1 (2.9) |
| SD | 11 (31.4) |
| PD | 21 (60.0) |
| Not evaluable | 2 (5.7) |
| Median PFS, months (95% CI) | 2.0 (1.4–2.6) |
| 6-month PFS rate (%) (95% CI) | 16.5 (7.5–36.0) |
| Median OS, months (95% CI) | 4.4 (3.0–5.7) |
| 6-month OS rate (%) (95% CI) | 37.5% (24.2–58.2) |
5-FU/LV, fluorouracil/leucovorin; CI, confidence interval; CR, complete response; Nal-IRI, liposomal irinotecan; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
According to RECIST v1.1, one (2.9%) patient achieved PR and none achieved CR, revealing an ORR of 2.9%. SD and PD was best response in 11 (31.4%) and 21 (60.0%) patients, respectively, and the DCR was 34.3%.
During a median follow-up of 9.2 months [95% confidence interval (CI) 7.8–10.5 months], the median PFS and OS were 2.0 months (95% CI: 1.4–2.6 months) and 4.4 months (95% CI: 3.0–5.7 months), respectively. The 6-month PFS and OS rates were 16.5% (95% CI: 7.5%–36.0%) and 37.5% (95% CI: 24.2%–58.2%), respectively (Figure 1).
Figure 1.
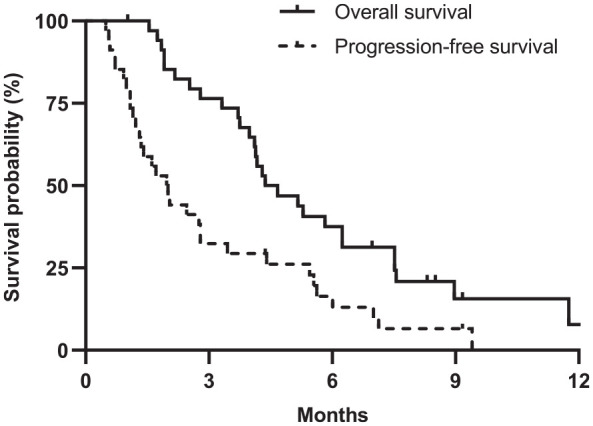
Survival outcomes with liposomal irinotecan with fluorouracil/leucovorin.
According to the progression status on previous conventional irinotecan-containing chemotherapy, there were no significant differences in terms of ORR [no progression (n = 7) versus progression (n = 28), 0% versus 3.6%, p = 1.00], median PFS [2.8 months (95% CI: 0.0–5.8) versus 2.0 months (95% CI: 1.0–3.0), p = 0.814] and OS [5.2 months (95% CI: 2.5–7.8) versus 4.4 months (95% CI: 3.6–5.2), p = 0.496] (Supplemental Figure 1).
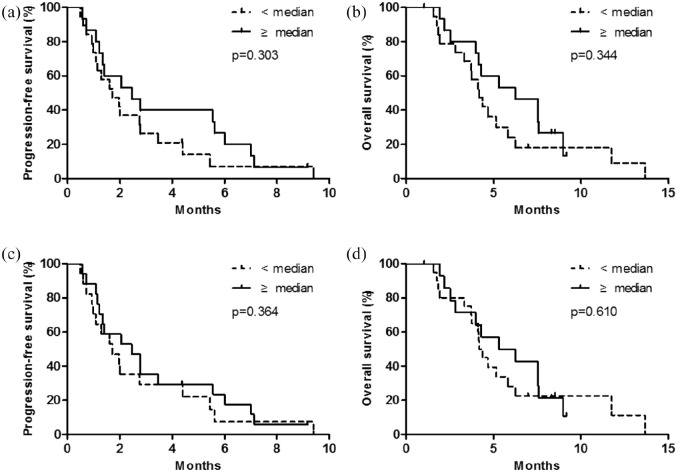
Correlation analysis between nal-IRI plus 5-FU/LV survival outcomes and prior exposure to conventional irinotecan (based on duration and cumulative dose) was performed. When patients were stratified according to the median duration of prior irinotecan therapy (<4.6 versus ⩾4.6 months), the median PFS and OS with nal-IRI plus 5-FU/LV were quantitatively better in patients with longer irinotecan exposure than in those with shorter irinotecan exposure; however, the differences were not statistically significant [PFS, 1.7 months (95% CI: 1.0–2.5) versus 2.5 months (95% CI: 0.7–4.2), p = 0.303; OS, 4.2 months (95% CI: 3.6–4.7) versus 6.2 months (95% CI: 3.2–9.3), p = 0.344; Figure 2(a) and (b)]. When stratified according to the median cumulative dose of prior irinotecan therapy (<1230 mg versus ⩾1230 mg), patients administered a higher cumulative dose showed quantitatively better median PFS [1.6 months (95% CI: 0.9–2.4 months) versus 2.5 months (95% CI: 1.5–3.5 months); p = 0.364] and OS [4.2 months (95% CI: 3.7–4.7 months) versus 5.3 months (95% CI: 1.5–9.1 months); p = 0.610] than those administered a lower cumulative dose, but the difference was not statistically significant [Figure 2(c) and (d)].
Figure 2.
Progression-free survival and overall survival with liposomal irinotecan plus fluorouracil/leucovorin according to the duration of prior conventional irinotecan therapy (a and b) and the cumulative dose of prior conventional irinotecan therapy (c and d).
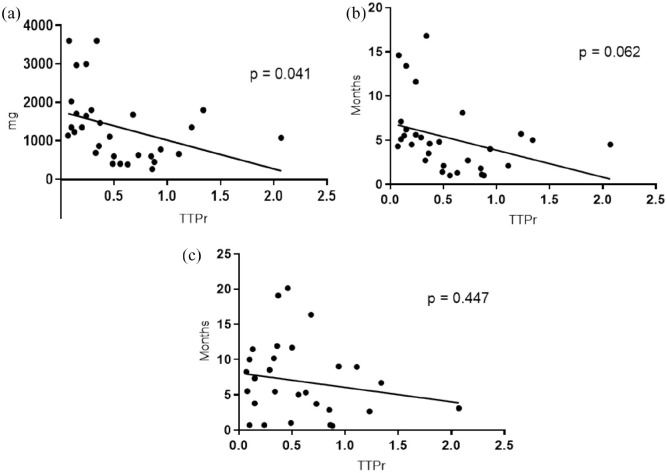
The median TTPr was 0.41 (range, 0.07–2.07), and the correlation analysis showed that the TTPr was significantly inversely correlated with the cumulative dose of prior conventional irinotecan therapy [R = −0.37, p = 0.041; Figure 3(a)]. The TTPr showed tendency of negative correlation with the duration of prior irinotecan therapy (R = −0.35, p = 0.062) and the interval between the last dose of prior irinotecan and the initiation of nal-IRI plus 5-FU/LV [R = −0.17, p = 0.447; Figure 3(b) and 3(c)].
Figure 3.
Lineal regression between the time to progression ratio and (a) the cumulative dose of prior conventional irinotecan therapy, (b) duration of prior conventional irinotecan therapy, and (c) interval between the last dose of prior conventional irinotecan therapy and the initiation of liposomal irinotecan with fluorouracil/leucovorin therapy.
Safety profiles
Treatment-emergent AEs with nal-IRI plus 5-FU/LV that occurred in >10% patients are listed in Table 4.
Table 4.
Treatment-emergent adverse events with nal-IRI plus 5-FU/LV occurring in >10% patients.
| Adverse events (Total N = 35) | ||
|---|---|---|
| Any grade (%) | Grade 3–4 (%) | |
| All, n (%) | 31 (88.6) | 11 (31.4) |
| Neutropenia, n (%) | 16 (45.7) | 7 (20.0) |
| Febrile neutropenia, n (%) | 2 (5.7) | 2 (5.7) |
| Anemia, n (%) | 12 (34.3) | 0 (0.0) |
| Thrombocytopenia, n (%) | 6 (17.1) | 1 (2.9) |
| AST/ALT elevation, n (%) | 7 (20.0) | 0 (0.0) |
| Fatigue, n (%) | 11 (31.4) | 3 (8.6) |
| Nausea, n (%) | 15 (42.9) | 1 (2.9) |
| Vomiting, n (%) | 8 (22.9) | 1 (2.9) |
| Diarrhea, n (%) | 6 (17.2) | 0 (0.0) |
5-FU/LV, fluorouracil/leucovorin; ALT, alanine transaminase; AST, aspartate aminotransferase; nal-IRI, liposomal irinotecan.
Any-grade treatment-emergent AEs with nal-IRI plus 5-FU/LV were observed in majority of the patients (n = 31, 88.6%); the most common AEs were neutropenia (n = 16, 45.7%) and nausea (n = 15, 42.9%). Precisely, 11 patients (31.4%) had grade 3–4 toxicities, and the most common grade 3–4 AEs were neutropenia (n = 7, 20.0%) and fatigue (n = 3, 8.6%). Additionally, febrile neutropenia occurred in two (5.7%) patients.
Discussion
In this retrospective analysis, we evaluated the effectiveness and toxicities of nal-IRI plus 5-FU/LV therapy in 35 Korean patients with mPAC previously treated with conventional irinotecan-containing chemotherapy. In our study, the median PFS and OS were 2.0 months and 4.4 months, respectively; these outcomes appear to be numerically worse than those reported by the NAPOLI-1 trial and other previous real-world analyses, which showed a median PFS and OS of 2.9–3.5 and 5.3–9.4 months, respectively.12–14 Current findings are in line with earlier retrospective studies which have reported reduced survival outcomes with nal-IRI plus 5-FU/LV in the patient subgroup that was previously treated with irinotecan-based chemotherapy, with a median PFS of 1.7–2.2 months and a median OS of 3.9–4.4 months.12,13 It can be speculated that these survival outcomes might be a result of the resistance developed against irinotecan or SN-38 during prior conventional irinotecan-containing chemotherapy. The impact of nal-IRI treatment on the improvement of pharmacological properties such as biodistribution, extension of the circulation time, and tumor accumulation time, might not be sufficient to overcome the resistance against irinotecan or SN-38.15,16 However, the modest effectiveness outcomes with nal-IRI plus 5-FU/LV in the current study might be also related with its use in the later lines itself,12 as all patients in the current analysis received nal-IRI plus 5-FU/LV as at least third-line therapy.
In the correlation analysis between survival outcomes with nal-IRI plus 5-FU/LV and prior exposure to conventional irinotecan (based on duration and cumulative dose), significant relationships were not noted. However, the TTPr, effectiveness indicator of nal-IRI plus 5-FU/LV in comparison with prior FOLFIRINOX, was significantly inversely correlated with the cumulative dose of prior conventional irinotecan therapy (R = −0.37, p = 0.041). This may suggest the efficacy of nal-IRI plus 5-FU/LV appears to be decreased in patients who have received conventional irinotecan-containing therapy with higher cumulative doses. Although the effectiveness outcomes shown in our patient population are modest and not justifiable to recommend nal-IRI plus 5-FU/LV for all patients who progressed on both FOLFIRINOX and gemcitabine-based chemotherapy, our findings indicate that nal-IRI plus 5-FU/LV may provide clinically meaningful outcomes in some subgroups of patients (i.e. less exposure to conventional irinotecan in terms of cumulative dose). Further studies with larger sample sizes are needed to find the subgroups who would be benefited with nal-IRI after progression on conventional irinotecan, considering the dismal prognosis and limited therapeutic options of those patients. As biomarkers such as plasma interleukin-8 or nomogram using clinically relevant variables (performance status, presence of liver metastasis, baseline albumin, neutrophil/lymphocyte ratio, carbohydrate antigen 19-9, disease stage and body mass index) have been reported for the association with survival outcomes in patients with mPAC receiving nal-IRI, these could be also helpful to predict the efficacy outcomes in patients previously treated with conventional irinotecan and future validation is needed.17,18
The safety profile of nal-IRI plus 5-FU/LV reported in this real-world study was consistent with the results of the NAPOLI-1 trial and previous trials.11 The most common grade 3–4 toxicities were neutropenia (20%) and fatigue (8.6%). The incidence of nonhematological toxicities, including diarrhea was lower than that reported in the NAPOLI-1 trial, which can be explained by the ethnic differences in the pharmacokinetics of nal-IRI in the East Asian population or a potential underestimation considering the retrospective nature of our study.19
Our study has several limitations. First, the retrospective design subjects this study to unintentional biases. Second, although our study included patients from two cancer referral centers, the number of analyzed patients was relatively small. However, our data are clinically applicable as this study provides the outcomes of the largest real-world analysis of patients with mPAC who received nal-IRI plus 5-FU/LV after conventional irinotecan-containing therapy. Third, our study included an ethnically homogeneous East Asian population of South Korea; therefore, the results are not generalizable to other populations.
Conclusion
Nal-IRI plus 5-FU/LV showed modest effectiveness and manageable toxicities for patients with mPAC previously treated with conventional irinotecan-containing chemotherapy. The cumulative dose of prior conventional irinotecan therapy may be inversely correlated with the effectiveness of nal-IRI plus 5-FU/LV.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211003053 for Clinical outcomes of liposomal irinotecan plus fluorouracil/leucovorin for metastatic pancreatic adenocarcinoma in patients previously treated with conventional irinotecan-containing chemotherapy by Kyunghye Bang, Jaekyung Cheon, Jae Ho Jeong, Hyeon-Su Im, Kyu-pyo Kim, Baek-Yeol Ryoo and Changhoon Yoo in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: The authors contributed as follows: study concepts: CY; study design: CY, KB, JC; data acquisition: all authors; data analysis and interpretation: all authors; statistical analysis: CY, KB; manuscript preparation: CY, KB, JC; manuscript editing: CY, KB, JC; and manuscript review and approval: all authors.
Conflict of interest statement: Changhoon Yoo received grants from Bayer, ONO, AstraZeneca and Servier; Consultancy and Advisory role for Bayer, Eisai, Ipsen, MSD, BMS, AstraZeneca and Servier. The other authors have no potential conflicts of interest to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Statement of ethics: This study was approved by the Institutional Review Board of each participating center (Asan Medical Center, 2018-0492; Ulsan University Hospital, 2019-11-037) and was performed in accordance with the ethics standards of the institutional research and the Declaration of Helsinki. The need for informed consent in this study was waived, as Korean regulations do not require consent for retrospective analyses.
ORCID iD: Changhoon Yoo  https://orcid.org/0000-0002-1451-8455
https://orcid.org/0000-0002-1451-8455
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kyunghye Bang, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Jaekyung Cheon, Division of Hematology-Oncology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Republic of Korea.
Jae Ho Jeong, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Hyeon-Su Im, Division of Hematology-Oncology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Republic of Korea.
Kyu-pyo Kim, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Baek-Yeol Ryoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Changhoon Yoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Republic of Korea.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 2. Hong S, Won YJ, Park YR, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat 2020; 52: 335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burris Hr, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 4. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo C, Hwang I, Song TJ, et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther Adv Med Oncol. Epub ahead of print 16 September 2020. DOI: 10.1177/1758835920953294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chae H, Jeong H, Cheon J, et al. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: multicenter retrospective analysis. Ther Adv Med Oncol. Epub ahead of print 27 May 2020. 12. DOI: 10.1177/1758835920923424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang J, Hwang I, Yoo C, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs 2018; 36: 732–7341. [DOI] [PubMed] [Google Scholar]
- 9. Kalra AV, Kim J, Klinz SG, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res 2014; 74: 7003–7013. [DOI] [PubMed] [Google Scholar]
- 10. Ko A, Tempero M, Shan Y, et al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer 2013; 109: 920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang-Gillam A, Li C-P, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 12. Glassman DC, Palmaira RL, Covington CM, et al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 2018; 18: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoo C, Im HS, Kim KP, et al. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol. Epub ahead of print 23 August 2019. DOI: 10.1177/1758835919871126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang-Gillam A, Hubner RA, Siveke JT, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur J Cancer 2019; 108: 78–87. [DOI] [PubMed] [Google Scholar]
- 15. Drummond DC, Noble CO, Guo Z, et al. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res 2006; 66: 3271–3277. [DOI] [PubMed] [Google Scholar]
- 16. Chang T, Shiah H, Yang C, et al. Phase I study of nanoliposomal irinotecan (PEP02) in advanced solid tumor patients. Cancer Chemother Pharmacol 2015; 75: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merz V, Zecchetto C, Santoro R, et al. Plasma IL8 Is a biomarker for TAK1 activation and predicts resistance to nanoliposomal irinotecan in patients with gemcitabine-refractory pancreatic cancer. Clin Cancer Res 2020; 26: 4661–4669. [DOI] [PubMed] [Google Scholar]
- 18. Chen LT, Macarulla T, Blanc JF, et al. Nomogram for predicting survival in patients treated with liposomal irinotecan plus fluorouracil and leucovorin in metastatic pancreatic cancer. Cancers 2019; 11: 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adiwijaya BS, Kim J, Lang I, et al. Population pharmacokinetics of liposomal irinotecan in patients with cancer. Clin Pharmacol Ther 2017; 102: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211003053 for Clinical outcomes of liposomal irinotecan plus fluorouracil/leucovorin for metastatic pancreatic adenocarcinoma in patients previously treated with conventional irinotecan-containing chemotherapy by Kyunghye Bang, Jaekyung Cheon, Jae Ho Jeong, Hyeon-Su Im, Kyu-pyo Kim, Baek-Yeol Ryoo and Changhoon Yoo in Therapeutic Advances in Medical Oncology