Abstract
Aim
To find a cut-off ratio of estradiol/metaphase II oocyte (E2/M2) ratio and to evaluate the correlation with patients’ characteristics, embryo morphokinetics using EmbryoScope™ and IVF cycle outcomes.
Material and methods
For this retrospective cohort study, records of all fresh cycles that were cultured and scored by EmbryoScope™ were evaluated. The peak E2/M2 ratio was calculated on the day of human chorionic gonadotropin (hCG) administration and correlated to embryo morphokinetic quality and cycle outcomes. A receiver operating characteristics analysis was calculated for the E2/M2 ratio and clinical pregnancy rates.
Results
A total of 2461 oocytes were collected from 319 patients. Receiver operating characteristics analysis revealed a cutoff of 204 as a discriminative point to predict clinical pregnancy with a sensitivity of 69.5% and specificity of 62.1% (P < 0.001). E2/M2 > 204 group were older, had higher E2 concentration, fewer M2 oocytes despite elevated gonadotrophin doses. E2/M2 ratio ≤ 204 was correlated with higher fertilization rate, better embryo quality, higher pregnancy and live birth rates, and more frozen embryos.
Conclusion
E2/M2 ratio < 204 yielded the best probability to achieve good quality embryos with good morphokinetic scores and better pregnancy outcomes and may be used to predict IVF cycle outcomes. Advanced maternal age and low ovarian response received higher concentrations of gonadotrophins, which resulted in higher E2/M2 ratio. Milder stimulation to those patients may improve their cycle outcomes.
Keywords: estradiolmetaphase II oocyte (E2/M2) ratio, pregnancy rate, embryo morphokinetics, time-lapse incubator
Introduction
A goal of in vitro fertilization (IVF) is to stimulate the ovary to produce several oocytes while avoiding ovarian hyperstimulation syndrome (OHSS) (1). Careful evaluation of the patient during treatment is crucial for its success. It is well-established that a very high estradiol (E2) ratio is associated with poor cycle outcomes due to impaired endometrial receptivity (2). This might cause long-term pregnancy complications due to mal-placentation (3). However, elevated E2/total oocyte ratio is not believed to affect embryo quality and development based on donor oocyte treatment cycles (2).
Several studies have reported the ideal ratio of peak E2 concentration to collected oocytes (E2/oocyte) in agonist down-regulation protocols vs antagonist cycles (4, 5) and reported that the E2/oocyte ratio is an important predictive parameter for IVF success. Loumaye et al. reported the highest pregnancy rate in long down-regulation cycles, in which the E2/oocyte ratio was 70–140 pg/mL per oocyte (4). An increased E2/oocyte ratio correlated with decreased implantation and pregnancy rates (6). Orvieto et al. found that E2/oocyte ratio ranging from 100 to 200 pg/mL in antagonist protocols offers the best likelihood of a positive outcome (7).
In contrast with the above studies that examined the ratio of E2 concentration per total number of oocytes collected, the current study examined the E2/metaphase II (mature oocyte (M2)) ratio on ovulation trigger day. We decided to evaluate the ratio between E2 and mature oocytes to eliminate the impact of immature oocytes that do not contribute to the outcome of the cycle. We evaluated embryo morphokinetics in a time-lapse incubator in terms of the treatment protocol, basic clinical parameters and their effect on IVF cycle outcomes. The outcomes were clinical pregnancy rates, live birth rates and miscarriage rates. The secondary outcomes included follicular phase duration, gonadotropin amounts used, E2 concentrations on the day of hCG administration, number of oocytes retrieved, number of mature oocytes, fertilization rate, number of top-quality embryos and numbers of embryos transferred or cryopreserved.
Materials and methods
Patients
In this retrospective cohort study, records of all patients with embryos cultured in a time-lapse incubator during the period January 1, 2016 to December 31, 2017 were evaluated. Exclusion criteria were non-fresh transfers due to endometrial polyps, premature progesterone elevation and the use of donor oocytes.
Treatment protocol
Treatment protocol (agonist or antagonist) and type and doses of gonadotropins were prescribed on a case-by-case basis according to patient characteristics and clinician preferences and judgment. The initial dose of gonadotropin was individualized for each patient according to age, basal FSH concentrations, antral follicle count, BMI, and previous response to ovarian stimulation. All treatments were conducted as previously described (1, 8, 9, 10, 11). Patients were allocated to agonist (decapeptyl 0.1 mg, Ferring) protocol or antagonist (cetrorelix 0.25 mg, Merck-Serono, Israel). Patients underwent controlled ovarian stimulation with one of the following methods: (1) recombinant follicle stimulating hormone alone (Gonal-F, Merck-Serono, Israel or Puregon, MSD, Israel), (2) highly purified human menopausal gonadotropin alone (Menopur, Ferring) or (3) a combination of both. Estrogen and progesterone concentrations were routinely measured at every follow-up visit, including the day of hCG (Ovitrelle Merck-Serono) administration.
Oocyte retrieval, embryo culture and transfer and pregnancy determination
Oocyte retrieval was conducted under anesthesia proximally 36 h after hCG was administered. The fertilized oocytes were placed in the EmbryoScope™ (Unisense FertiliTech, Aarhus, Denmark) for up to 5 days at 37°C, in 5.8% CO2 and 5% O2. Images were acquired of each embryo every 10 min in seven focal planes, starting from second polar body extraction up to 120 h after fertilization, to determine the exact timing of cell divisions (12). Embryos were scored by Known Implantation Data (KID) score and an Alfa ESHRE score, as well as the common morphology grade (13, 14) A maximum of two embryos were transferred on day 3 or one on day 5 of embryonic development. Embryo quality was also evaluated on the day of transfer, as previously described (15). A top-quality embryo was described as one with four to five blastomeres on day 2 or more than six equal-size blastomeres and ≤20% fragmentation.
We calculated the E2/M2 ratio by dividing the total E2 concentration on the day of hCG triggering by the total number of M2 oocytes collected.
For luteal phase support, patients received 300 mg micronized progesterone (Endometrin®, Ferring) in three divided doses, daily. The E2/M2 ratio was calculated and used as the main parameter to evaluate clinical outcomes.
Pregnancy was assessed with β-hCG test 14 days after embryo transfer. A clinical pregnancy was confirmed when a gestational sac with fetal heart beat was visible on ultrasound examination after 6 weeks of pregnancy.
Data collection
The following parameters were obtained from patients’ files for each cycle: baseline parameters of age, BMI, cause of infertility, treatment protocol, basal FSH and LH concentrations, basal E2 and progesterone concentrations and endometrial thickness at the beginning of the cycle. Cycle characteristics included total days of stimulation, total amount of gonadotrophins, number of oocytes retrieved, number of M2, normal fertilization (2PN), number of transferred embryos, number of embryos available to freeze, embryo grade and morphokinetic scores and morphokinetic parameters by hours from fertilization. Cycle outcomes included biochemical pregnancy test and clinical pregnancy rates, miscarriage rate and live birth rate.
Statistical analysis
Statistical analysis was performed using the SPSS software package (SPSS Inc.). Comparisons were analyzed using Student’s t-test or Mann–Whitney U-test, each when appropriate. All data were included and no outliers were omitted from the analysis. Proportions were compared using Chi-Square test or Fisher exact test. P-values less than 0.05 were considered significant. We used a multivariate logistic regression analysis model to rule out confounders. A receiver operating characteristics (ROC) analysis was performed to determine the most efficient cut-off values for the E2/M2 oocyte ratio that would discriminate between successful and unsuccessful IVF/ICSI-ET outcomes.
The STROBE guidelines for retrospective cohort study were followed.
Results
A total of 2461 oocytes were collected from 319 patients undergoing 449 oocyte retrievals. Table 1 presents the demographic and clinical characteristics of the patients.
Table 1.
Patient characteristics and cycle outcomes (n = 319).
| Characteristic | Mean ± s.d. |
|---|---|
| Age (years) | 35.4 ± 5.9 years |
| BMI (kg/m2) | 25.9 ± 5.5 |
| Cause of infertility % | |
| Anovulation | 5 |
| Male factor | 33 |
| Mechanical factor | 12 |
| Unexplained | 39 |
| Other | 11 |
| Antagonist protocol (%) | 71 |
| Agonist protocol (%) | 29 |
| E2 concentrations on hCG day (pg/L) | 1553 ± 970 |
| P concentrations on hCG day (ng/L) | 0.68 ± 0.65 |
| E2/M2 ratio (pg/L per M2) | 273 ± 198 |
| E2/M2 ratio with agonist protocol (pg/L per M2) | 275 ± 139 |
| E2/M2 ratio with antagonist protocol (pg/L per M2) | 265 ± 158 |
| Endometrial thickness on hCG day (mm) | 9.5 ± 2.3 |
| Stimulation days, mean + s.d. | 10.1 ± 2.7 |
| Oocytes at OPU, mean + s.d. | 9.8 ± 6.5 |
| M2 oocytes at OPU, mean + s.d. | 6.9 ± 4.6 |
| Normal fertilized embryos, mean + s.d. | 5.5 ± 3.7 |
| Biochemical pregnancy | 147 (38.1%) |
| Clinical pregnancy | 144 (37.3%) |
E2/M2 cutoff for prediction of pregnancy outcomes
Using ROC analysis, we found that an E2/M2 ratio cutoff of 204 can be a good predictor of cycle results. E2/M2 ≤ 204 can predict clinical pregnancy with a sensitivity of 69.5%, while E2/M2 ratio > 204 predicts cycle failure with a specificity of 62.1% (P < 0.001).
We divided the cohort into two groups according to the E2/M2 cutoff of 204. Women in the group with E2/M2 ≤ 204 were younger, received a lower dose of gondotropins, had lower peak E2 concentrations, more oocytes collected per cycle, more mature oocytes, more normal fertilizations per cycle, higher mean ESHRE score, more embryos available to freeze and higher pregnancy and live birth rates, as compared to the group with E2/M2 > 204 (Table 2).
Table 2.
Cycle characteristics and results based on E2/M2 ratio cutoff of 204.
| Variable | E2/M2 ≤ 204, n = 150 | E2/M2 > 204, n = 234 | P-value |
|---|---|---|---|
| Age, years | 34.3 ± 6.1 | 36.6 ± 5.6 | 0.002 |
| Basal FSH (IU/L) | 8.9 ± 3.1 | 8.7 ± 2.9 | NS |
| Basal LH (IU/L) | 5.7 ± 2.3 | 6.1 ± 2.8 | NS |
| Basal E2 (pg/L) (median 25–75%) | 45 (30–119) | 42 (28–78) | NS |
| Basal Progesterone (ng/L) | 0.3 (0.1–0.7) | 0.3 (0.15–0.6) | NS |
| BMI (kg/m2) | 26.2 ± 6.1 | 25.6 ± 5.3 | NS |
| Endometrial thickness (mm) | 9.7 ± 2.3 | 9.6 ± 2.2 | NS |
| Protocol % | P = 0.90 | ||
| Agonist | 38 (28.4%) | 66 (29.6%) | |
| Antagonist | 96 (71.6%) | 157 (70.4%) | |
| Total days of stimulation | 10.1 ± 2.6 | 9.9 ± 2.8 | NS |
| Total gonadotropin dose (iu) | 2070 ± 1200 | 2525 ± 1448 | 0.02 |
| E2 concentration at hCG (pg/L) | 1270 ± 689 | 1529 ± 786 | 0.001 |
| Number oocytes/cycle | 10.8 ± 5.4 | 7.7 ± 2.8 | 0.0001 |
| Number M2/cycle | 9.4 ± 5.1 | 5.3 ± 3.4 | 0.0001 |
| Normal fertilizations/cycle | 6.6 ± 3.7 | 4.1 ± 2.4 | 0.0001 |
| Transferred embryos | 1.8 ± 0.7 | 1.8 ± 0.7 | NS |
| Number of embryos to freeze | 3.2 ± 1.9 | 2.5 ± 1.8 | 0.007 |
| Grade (mean) | 1.8 ± 0.6 | 1.9 ± 0.6 | 0.005 |
| KID score (mean) | 3.3 ± 1.6 | 3.3 ± 1.6 | NS |
| ESHRE score (mean) | 1.6 ± 1.1 | 1.4 ± 1.1 | 0.005 |
| KID 4–5 (%) | 56.7 | 56.4 | NS |
| ESHRE 2–3 (%) | 53.9 | 49.2 | 0.038 |
| Pregnancy outcome/transfer (%) | |||
| Biochemical pregnancy | 45.3 | 34 | 0.032 |
| Clinical | 45.3 | 32.3 | 0.013 |
| Miscarriage rate (%) | 13/150 = 8.6 | 36/234 = 15.3 | 0.05 |
| Live birth rate/transfer (%) | 36.7 | 18.7 | 0.0001 |
The group with E2/M2 ≤ 204 had more M2 compared to the group with E2/M2 > 204 (9.4 ± 5 vs 5.3 ± 3.4; P = 0.0001).
Women in the group with E2/M2 ≤ 204 were younger. We evaluated the impact of age on E2/M2 ratio using univariate analysis. Younger patients demonstrated significantly lower E2/M2 ratios (Fig. 1A). Among women younger than 35 years, 49% had E2/M2 ratio ≤ 204, while only 35% of women 35–42 years of age had E2/M2 ratio ≤ 204 and among women older than 43 years, only 17% had E2/M2 ratio ≤204 (Fig. 1B).
Figure 1.
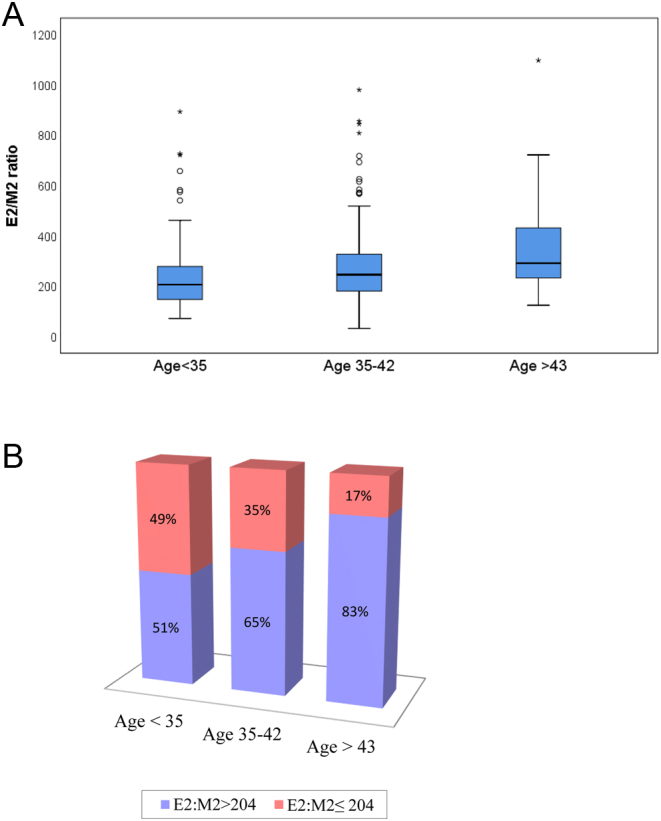
Effect of age on E2/M2 ratio. (A) Mean E2/M2 ratio per age group. (B) Changes in E2/M2 ratios per age group. Age <35 vs Age 35–42; P = 0.039. Age <35 vs Age >43; P < 0.0001. Age 35–42 vs Age >43; P = 0.025.
Multivariate analysis to evaluate pregnancy outcomes
A multivariate analysis to predict E2/M2 ≤ 204 that included female age, total dose of gonadotropins, basal FSH concentrations, duration of treatment and cause of infertility revealed that only female age was a significantly predictive factor, with odds ratio (OR) 0.94, 95% CI 0.9–1.0; P = 0.046.
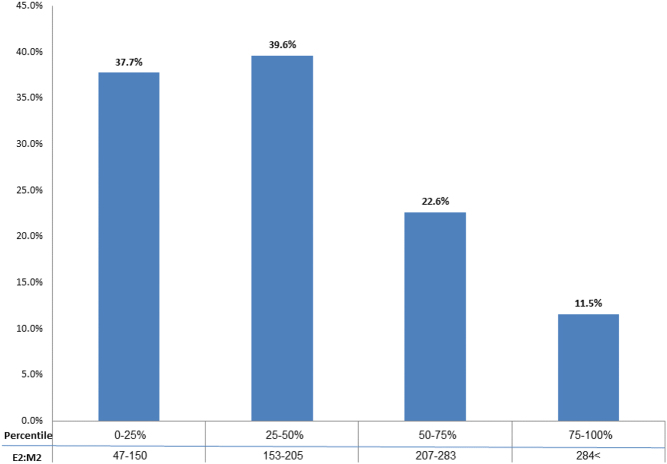
We then divided the E2/M2 ratios into quartiles (47–150, 153–205, 207–283, 284 and above) and clinical pregnancy rates were calculated for each quartile. The first and second quartile E2/M2 ratios had pregnancy rates of 42%, while pregnancy rates in the third and fourth quartiles were lower at 32% (P = 0.035). E2/M2 in the first and second quartiles had significantly higher chances of achieving pregnancy, as compared to the third and fourth quartiles (OR 6.5, 95% CI = 4.6–8.7; P < 0.0001 and OR 5.1, 95% CI = 3.6–7.0; P < 0.0001, respectively; Fig. 2).
Figure 2.
Pregnancy rate by quartile of E2/M2 ratios. Comparable pregnancy rates between Q1 and Q2. *P < 0.001- Q1 vs Q3 and Q4, Q2 vs Q3 and Q4, Q3 vs Q4.
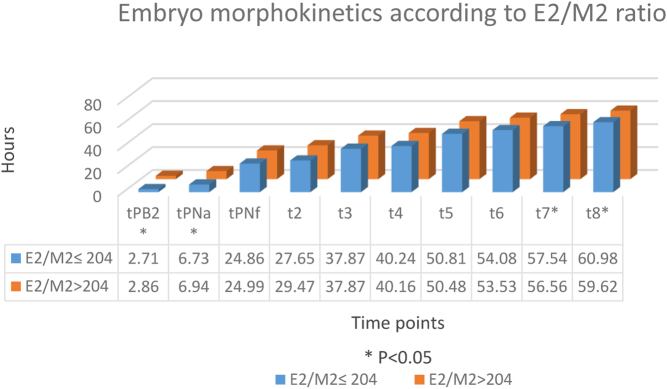
Morphokinetic differences and embryo quality relative to E2/M2
The morphokinetics of the embryos relative to E2/M2 ratio ≤ 204 are shown in Fig. 3. Among oocytes in the E2/M2 ≤ 204 group, the second polar body (tPB2) was extracted significantly faster than in the E2/M2 > 204 group. The second meiosis and two pronuclei (tPNa) also occurred more quickly in the group of E2/M2 ≤ 204 group. Afterwards, cell divisions during the cleavage stage occurred sooner and were significantly slower at t7 and t8 among patients with the lower ratio (Fig. 3).
Figure 3.
Embryo morphokinetics according to E2/M2 ratio. Values are cumulative time, in hour.
Significantly different embryo quality was observed between the two groups. For E2/M2 ≤ 204, better embryos were obtained in terms of grading (1.8 ± 0.6 vs 1.9 ± 0.6; P = 0.005) and ESHRE score (1.6 ± 1.1 vs 1.4 ± 1.1; P = 0.005; Table 2). Moreover, fewer embryos were suitable for freezing when the E2/M2 was > 204 (3.2 ± 1.9 vs 2.5 ± 1.8; P = 0.007).
Pregnancy outcomes
Both biochemical pregnancy rate (45% vs 34%; P = 0.032) and clinical pregnancy rate (45 vs 32; P = 0.013) were significantly higher in the group of E2/M2 ≤ 204 compared with E2/M2 >204. Miscarriage rate was significantly higher in the E2/M2 > 204 group (8.6% vs 5.4%; P = 0.05; Table 2).
Discussion
Oocyte and embryo quality are important factors affecting the success rate of IVF. The effects of E2 on the endometrium during the proliferative phase are well-recognized (16, 17, 18). In contrast, the role of E2 concentration during the follicular phase and specifically the E2 to M2 ratio is less clear. Our study reports significantly lower pregnancy and delivery rates in the group of E2/M2 > 204. Advanced age, lower response to gonadotropins administered, higher total E2 and fewer M2 were observed in the group of patients with E2/M2 > 204. In agreement with our results, Sandoval reported that elevated peak E2/mature oocyte ratio is associated with a lower IVF pregnancy outcome in GnRH antagonist ICSI cycles (19). However, they did not evaluate embryo morphokinetics and evaluated only antagonist cycles in a small cohort of 162 cycles.
Conflicting data are reported regarding the possible correlations between peak E2 concentrations to oocyte and embryo quality, pregnancy rates and cycle outcomes. Sharara et al. did not find an effect of higher E2 concentrations on cycle outcomes. However, other studies reported that high E2 concentrations are correlated with poorer outcomes, including abnormal implantation, placentation and pregnancy outcomes (4, 7, 20).
The current study assessed the effect of the E2/M2 oocyte ratio on IVF treatments rather than the absolute E2 concentrations, taking in consideration embryo morphokinetic development based on EmbryoScope. We found that in the group of E2/M2 ≤ 204, maternal age was significantly lower but the difference between the group was not clinically significant. The younger patients received lower doses of gonadotropins and ended with lower E2/M2 ratios, which correlated with better cycle outcomes. The group of E2/M2 > 204 were treated with higher doses of gonadotrophins, resulting in higher E2 concentrations but fewer oocytes and mature oocytes.
The question whether different gonadotrophin doses for poor ovarian response will alter the IVF outcome has been studied. An open-label multicenter randomized trial by Youssef et al. reported similar ongoing pregnancy rates with mild ovarian stimulation as a conventional ovarian stimulation strategy (21). Furthermore, higher E2/M2 ratio was demonstrated in the group with advanced maternal age. This may be explained by the impaired follicular metabolism in the older women. Pacella et al. demonstrated that women with either reduced ovarian reserve or advanced maternal age have different follicular cell metabolism, follicular fluid metabolites and progesterone production. This environment may be responsible for impaired oocyte development and subsequent embryo development. They reported higher E2 and progesterone concentrations in the follicular fluids of older patients despite significantly fewer oocytes collected in that group. They also found that cumulus cells from older women did not display the changes in metabolic and steroidogenic activity that were observed in young women with normal ovarian reserves, suggesting altered or lack of differentiation in response to the LH surge (22). In our study, the group of E2/M2 > 204 were patients with advanced maternal age and reduced ovarian response. They demonstrated altered ovarian response with higher E2 and fewer oocytes, which may explain the higher E2/M2 ratio found in our study. The higher dose of gonadotropins did not overcome these changes and might have contributed to the elevated E2 without an increase in M2.
Both Loumaye (4) and Orvieto (7) demonstrated that higher E2/total oocyte ratios correlated with negative outcomes. Loumaye reported that an E2/oocyte ratio of 70–140 pg/mL in long down-regulation protocol correlated with a significantly better pregnancy rate (4). Orvieto et al. (7) reported that in GnRH antagonist protocol, E2/oocyte ratio should reach the 100–200 pg/mL range to achieve the best IVF outcomes. Vaughan et al. (23) reported clinical pregnancy rates were highest in patients with E2/oocyte ratios in the range of 250–750 pmol/L/oocyte and declined as this ratio increased, independent of patient age. They reported OR of 3.4 (95% CI 2.67–4.34; P < 0.001) for clinical pregnancy, when the E2/oocyte ratio was 250–750 pmol/L/oocyte vs E > 1500 pmol/L/oocyte.
Despite the similarities to the above studies, we found that maternal age had a significant impact on E2/M2 ratio. Moreover, in contrast to those reports, our study measured E2 to mature oocyte ratio. In our opinion, this analysis is more accurate since only M2 oocytes may have an impact on fertilization and cycle outcomes. Moreover, it is known that immature oocytes contribute less E2 to the total circulating concentration of estrogen. Therefore, the total E2/oocyte ratio may not be an accurate predictor of cycle outcomes. We found that the E2/M2 ratio can be used to predict cycle outcomes and embryo quality and that a ratio >204 predicted lower rates of fertilization, fewer good quality embryos and fewer embryos to freeze and more positive pregnancy test and clinical pregnancies. In terms of pregnancy outcomes, Hu et al. (24) reported a higher miscarriage rate among patients undergoing IVF treatments when E2/follicle ratio exceeded 553 pg/mL. They assumed this was a result of an unfavorable endometrium. Based on our results, we assume that the higher miscarriage rate could be due to an unfavorable endometrium but also due to lower embryo quality, as significantly lower embryo quality was found in the group with higher E2/M2 ratio. This is also reflected in that fewer embryos were available to freeze in the higher E2/M2 group (3.2 ± 1.9 vs 2.5 ± 1.8, respectively; P = 0.007).
Our study showed a significant difference between the groups according to E2/M2 ratios in terms of cell-cycle division. As shown, when E2/M2 ≤ 204, oocytes completed the second meiosis significantly faster. However, those oocytes were slower during cell division throughout the cleavage stage in t7-t8. There is a consensus among researchers that embryo kinetic parameters are predictive of the likelihood of achieving pregnancy. Considering the higher pregnancy rates and number of good quality embryos in the E2/M2 ≤ 204 group, our data support the assumption that slower cell division correlates with a longer time for cell repair, which results in higher quality embryos. Motato et al. timed cell cycles and found that the duration of the second and third cell cycles was longer in embryos that developed by blastocysts, as compared with embryos arresting before blastocyst stage. This may indicate that a minimum period of time in these cell stages is required for orderly, efficient DNA synthesis as part of the cell cycle (25).
Desai et al. found that specific, early cell-cycle kinetics were predictive of embryo developmental capacity to blastocyst but did not correlate to embryo ploidy. In contrast, late kinetic parameters appeared to be associated with greater likelihood of euploidy (26). This may explain the better pregnancy rate and lower miscarriage rate in the group of E2/M2 ≤ 204, in which slower cleavage was observed.
The strength of our study is that to the best our knowledge this is the first study to explore the correlation between E2/M2 ratios and embryo morphokinetics. We evaluated a high number of oocytes and followed the patients until delivery. However, the study is limited due to its retrospective nature.
In conclusion, our results demonstrated that higher E2/M2 ratio correlated with advanced maternal age and lower ovarian response, poorer quality embryos and ended with reduced IVF outcomes. Lower E2/M2 ratios produced higher quality embryos, which developed relatively slower and resulted in a better pregnancy rate. Whether milder ovarian stimulation may produce lower E2 concentration and improve IVF outcomes among patients with poor ovarian response by that is still to be determined.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Ethics approval
This retrospective chart review study involving human participants was conducted in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee of Hillel Yafe Medical center approved this study.
Author contribution statement
All authors contributed substantially to this work. The authors collectively developed the original concept of this study. Conceptualization was done by Nardin Aslih, Yuval Atzmon, Einat Shalom-Paz. Data curation by Nardin Aslih, Diana Poltov-Mashenko, Yuval Atzmon, Einat Shalom-Paz. Formal analysis by Nardin Asih, Einat Shalom-Paz. Investigation was done by Nardin Aslih, Mediea Michaeli, Einat Shalom-Paz. Methodology by Nardin Aslih, Oshrit Laibovitz, Einat Shalom-Paz. Project administration was done by Nardin Aslih, Einat Shalom-Paz. Resources were provided by Nardin Aslih. Supervision was done by Einat Shalom-Paz. Visualization was done by Yuval Azmon, Einat Shalom-Paz. Original draft was written by Nardin Aslih. Review and editing was done by Nardin Aslih, Einat Shalom-Paz, Adrian Ellenbogen. All authors contributed to critical discussion and reviewed and approved the final version of the manuscript for submission.
Acknowledgement
We want to thank our nurse, Mrs Tali Amar, our study coordinator for all her help.
References
- 1.Albano C, Smitz J, Camus M, Riethmüller-Winzen H, Van Steirteghem A, Devroey P.Comparison of different doses of gonadotropin-releasing hormone antagonist cetrorelix during controlled ovarian hyperstimulation. Fertility and Sterility 1997. 67 917–922. ( 10.1016/s0015-0282(9781407-0) [DOI] [PubMed] [Google Scholar]
- 2.Simón C, Cano F, Valbuena D, Remohí J, Pellicer A.Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Human Reproduction 1995. 10 2432–2437. ( 10.1093/oxfordjournals.humrep.a136313) [DOI] [PubMed] [Google Scholar]
- 3.Farhi J, Ben-Haroush A, Andrawus N, Pinkas H, Sapir O, Fisch B, Ashkenazi J.High serum oestradiol concentrations in IVF cycles increase the risk of pregnancy complications related to abnormal placentation. Reproductive Biomedicine Online 2010. 21 331–337. ( 10.1016/j.rbmo.2010.04.022) [DOI] [PubMed] [Google Scholar]
- 4.Loumaye E, Engrand P, Howles CM, O’Dea L.Assessment of the role of serum luteinizing hormone and estradiol response to follicle-stimulating hormone on in vitro fertilization treatment outcome. Fertility and Sterility 1997. 67 889–899. ( 10.1016/s0015-0282(9781402-1) [DOI] [PubMed] [Google Scholar]
- 5.Ludwig M, Katalinic A, Diedrich K.Use of GnRH antagonists in ovarian stimulation for assisted reproductive technologies compared to the long protocol. Meta-analysis. Archives of Gynecology and Obstetrics 2001. 265 175–182. ( 10.1007/s00404-001-0267-2) [DOI] [PubMed] [Google Scholar]
- 6.Yang JH, Chen HF, Lien YR, Chen SU, Ho HN, Yang YS.Elevated E2: oocyte ratio in women undergoing IVF and tubal ET. Correlation with a decrease in the implantation rate. Journal of Reproductive Medicine 2001. 46 434–438. [PubMed] [Google Scholar]
- 7.Orvieto R, Zohav E, Scharf S, Rabinson J, Meltcer S, Anteby EY, Homburg R.The influence of estradiol/follicle and estradiol/oocyte ratios on the outcome of controlled ovarian stimulation for in vitro fertilization. Gynecological Endocrinology 2007. 23 72–75. ( 10.1080/09513590601137137) [DOI] [PubMed] [Google Scholar]
- 8.Barbieri RL, Hornstein MD.Assisted reproduction-in vitro fertilization success is improved by ovarian stimulation with exogenous gonadotropins and pituitary suppression with gonadotropin-releasing hormone analogues. Endocrine Reviews 1999. 20 249–252. ( 10.1210/edrv.20.3.0363) [DOI] [PubMed] [Google Scholar]
- 9.Huirne JAF. Lambalk CB, Van Loenen ACD, Schats R, Hompes PGA, Fauser BCJM, Macklon NS.Contemporary pharmacological manipulation in assisted reproduction. Drugs 2004. 64 297–322. ( 10.2165/00003495-200464030-00005) [DOI] [PubMed] [Google Scholar]
- 10.Olivennes F, Alvarez S, Bouchard P, Fanchin R, Salat-Baroux J, Frydman R.The use of a GnRH antagonist (cetrorelix) in a single dose protocol in IVF-embryo transfer: a dose finding study of 3 versus 2 mg. Human Reproduction 1998. 13 2411–2414. ( 10.1093/humrep/13.9.2411) [DOI] [PubMed] [Google Scholar]
- 11.The ganirelix dose-finding study group. A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle. Human Reproduction 1998. 13 3023–3031. ( 10.1093/humrep/13.11.3023) [DOI] [PubMed] [Google Scholar]
- 12.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J.The use of morphokinetics as a predictor of embryo implantation. Human Reproduction 2011. 26 2658–2671. ( 10.1093/humrep/der256) [DOI] [PubMed] [Google Scholar]
- 13.Racowsky C, Stern JE, Gibbons WE, Behr B, Pomeroy KO, Biggers JD.National collection of embryo morphology data into society for assisted reproductive technology clinic outcomes reporting system: associations among day 3 cell number, fragmentation and blastomere asymmetry, and live birth rate. Fertility and Sterility 2011. 95 1985–1989. ( 10.1016/j.fertnstert.2011.02.009) [DOI] [PubMed] [Google Scholar]
- 14.Petersen BM, Boel M, Montag M, Gardner DK.Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on Day 3. Human Reproduction 2016. 31 2231–2244. ( 10.1093/humrep/dew188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose BI.Approaches to oocyte retrieval for advanced reproductive technology cycles planning to utilize in vitro maturation: a review of the many choices to be made. Journal of Assisted Reproduction and Genetics 2014. 31 1409–1419. ( 10.1007/s10815-014-0334-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh D, Sengupta J.Another look at the issue of peri-implantation oestrogen. Human Reproduction 1995. 10 1–2. ( 10.1093/humrep/10.1.1) [DOI] [PubMed] [Google Scholar]
- 17.Edgar DH.Oestrogen and human implantation. Human Reproduction 1995. 10 2–4. ( 10.1093/humrep/10.1.2) [DOI] [PubMed] [Google Scholar]
- 18.de Ziegler D.Hormonal control of endometrial receptivity. Human Reproduction 1995. 10 4–7. ( 10.1093/humrep/10.1.4) [DOI] [PubMed] [Google Scholar]
- 19.Sandoval JS, Steward RG, Chen C, Li YJ, Price TM, Muasher SJ.High Peak estradiol/mature oocyte ratio predicts lower clinical pregnancy, ongoing pregnancy, and live birth rates in gnrh antagonist intracytoplasmic sperm injection cycles. Journal of Reproductive Medicine 2016. 61 11–16. [PubMed] [Google Scholar]
- 20.Sharara FI, McClamrock HD.High estradiol levels and high oocyte yield are not detrimental to in vitro fertilization outcome. Fertility and Sterility 1999. 72 401–405. ( 10.1016/S0015-0282(9900293-9) [DOI] [PubMed] [Google Scholar]
- 21.Youssef MA, van Wely M, Al-Inany H, Madani T, Jahangiri N, Khodabakhshi S, Alhalabi M, Akhondi M, Ansaripour S, Tokhmechy R. et al. A mild ovarian stimulation strategy in women with poor ovarian reserve undergoing IVF: a multicenter randomized non-inferiority trial. Human Reproduction 2017. 32 112–118. ( 10.1093/humrep/dew282) [DOI] [PubMed] [Google Scholar]
- 22.Pacella L, Zander-Fox DL, Armstrong DT, Lane M.Women with reduced ovarian reserve or advanced maternal age have an altered follicular environment. Fertility and Sterility 2012. 98 986–94.e1. ( 10.1016/j.fertnstert.2012.06.025) [DOI] [PubMed] [Google Scholar]
- 23.Vaughan DA, Harrity C, Sills ES, Mocanu EV.Serum estradiol:oocyte ratio as a predictor of reproductive outcome: an analysis of data from >9000 IVF cycles in the Republic of Ireland. Journal of Assisted Reproduction and Genetics 2016. 33 481–488. ( 10.1007/s10815-016-0664-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu D, Dong X, Xiong M, Xiong T, Huang B, Zeng D, Ai J, Zhang H.Can the peak E2/follicle ratio be a quantitative indicator of pregnancy outcomes following assisted reproductive cycles? A retrospective study. International Journal of Clinical and Experimental Medicine 2015. 8 10964–10970. [PMC free article] [PubMed] [Google Scholar]
- 25.Motato Y, de los Santos MJ, Escriba MJ, Ruiz BA, Remohí J, Meseguer M.Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system. Fertility and Sterility 2016. 105 376.e9–38. ( 10.1016/j.fertnstert.2015.11.001) [DOI] [PubMed] [Google Scholar]
- 26.Desai N, Goldberg JM, Austin C, Falcone T.Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy? Fertility and Sterility 2018. 109 665–674. ( 10.1016/j.fertnstert.2017.12.025) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a