Abstract
Classic galactosemia is a rare inborn error of galactose metabolism with a birth prevalence of about 1/30,000–60,000. Long-term complications occurring despite dietary treatment consist of premature ovarian insufficiency (POI) and neurodevelopmental impairments. We performed with the French Reference Centers for Rare Diseases a multisite collaborative questionnaire survey for classic galactosemic patients. Its primary objective was to assess their puberty, pregnancy, gonadotropic axis, and pelvic morphology by ultrasound. The secondary objective was to determine predictive factors for pregnancy without oocyte donation. Completed questionnaires from 103 patients, 56 females (median age, 19 years (3–52 years)) and 47 males (median age, 19 years (3–45 years)), were analyzed. Among the 43 females older than 13 years old, mean age for breast development first stage was 13.8 years; spontaneous menarche occurred in 21/31 females at a mean age of 14.6 years. In these 21 women, 62% had spaniomenorrhea and 7/17 older than 30 years had amenorrhea. All age-groups confounded, FSH was above reference range for 65.7% of the patients, anti-Müllerian hormone and inhibin B were undetectable, and the ovaries were small with few or no follicles detected. Among the 5 females who sought to conceive, 4 had pregnancies. Among the 47 males, 1 had cryptorchidism, all have normal testicular function and none had a desire to conceive children. Thus, spontaneous puberty and POI are both common in this population. Spontaneous menarche seems to be the best predictive factor for successful spontaneous pregnancy.
Keywords: premature ovarian insufficiency, galactosemia, puberty, fertility
Introduction
Classic galactosemia is a rare autosomal recessive inborn error of galactose metabolism with a birth prevalence in Europe of about 1/30,000–60,000. It is due to a defect in the gene encoding the galactose-1-phospate uridyltransferase enzyme (GALT) (1). The Q188R mutation explains 40–45% of cases (2) but over 340 other mutations are described for now (3). In France, the number of living patients is estimated at 250 (estimation based on data from medical services, patient associations and European prevalence as there is no neonatal screening or registry in France). Galactosemia can cause life-threatening multiorgan failure during neonatal period. Routine neonatal screening is therefore performed in some countries (4, 5), but not yet in France (6). The early manifestations consist of milk intolerance, jaundice, failure to thrive, lethargy, hepatocellular damage, renal tubular disease and cataract. A galactose-free diet promptly reverses the neonatal symptoms but fails to prevent the development of long-term complications including neurological impairments (developmental delay, particularly affecting speech, low intellectual quotient and sometimes epilepsy) (7), osteoporosis and premature ovarian insufficiency (POI) (8).
In females, hypergonadotropic hypogonadism resulting in delayed puberty, primary or secondary amenorrhea, and infertility is common (9, 10) and severely impairs quality of life (11). The age of POI onset varies widely. Many pathophysiological hypotheses have been suggested but without confirmation (12, 13, 14, 15, 16, 17, 18, 19, 20, 21). Risk factors for POI may include homozygosity for the Q188R mutation, primary amenorrhea, and higher erythrocyte levels of galactose 1-phosphate (indicating suboptimal dietary adherence). Age at dietary therapy initiation is not correlated with the severity of the other long-term manifestations. No data is available to determine whether the ovarian alterations responsible for POI occur before, around, or after birth neither if POI develops abruptly or progressively (13). However, Spencer et al. found that AMH levels were significantly lower in galactosemic patients vs controls among 2 neonates (21). In rodent models, although one study did not find any ovarian abnormality at birth (14, 22), feeding the pregnant rats with a high-galactose diet depleted the oocyte population in the embryos (17). In women, few studies on ovarian histology described a wide range from normal to streak gonads (14). Spencer et al. found a correlation between AMH levels and residual GALT Activity (21). Recent studies in mice have also demonstrated that oxidative stress secondary to galactose and its metabolites exposure could result in follicular dysfunction (23).
In males, two cohort studies showed delayed puberty (8, 24) and below-target final height, although some patients may not have completed growth. In a study on 12 male patients, hormone levels were normal but 3 patients had cryptorchidism (20). Anti-Müllerian hormone (AMH) levels were normal or elevated and inhibin B levels normal or decreased. Evidence that males with galactosemia have fathered children comes only from personal communications.
Few descriptive data are available on the patients’ puberty onset, fertility and biological markers. Predictive factors are therefore difficult to define.
The primary objective of this collaborative questionnaire survey by French Reference Centers for Rare Diseases was to collect prospective and retrospective data on puberty, pregnancy, gonadotropic axis status, pelvic morphological description by ultrasound, and the management of infertility in adults with classic galactosemia. The secondary objective was to find predictive factors for spontaneous pregnancy in women.
Patients and method
The study and questionnaire were approved by the national committee on healthcare research data (Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé, CCTIRS), the French data protection authority (Commission Nationale de l’Informatique et des Libertés, CNIL), and the ethics committee (Comité de Protection des Personnes).
Cohort of patients with classic galactosemia
Between 2009 and 2011, 10 French metabolic-disease management centers were invited to participate in the study, as well as the pediatric and adult endocrinology units and the French self-help group for patients with galactosemia (Association des Familles Galactosémiques de France). A questionnaire developed to collect background information on each patient was completed by the patient or family, depending on patient age, or by the usual physician from their medical records.
We included all listed French patients (adults and children) with confirmed classic galactosemia, in order to assess the gonadal impairments as early as it occurred.
Missing data in returned questionnaires were collected during telephone calls to the patients or families in 2011. In January 2017, each patient was contacted again by email or telephone to collect additional data regarding pregnancies during the past 6 years.
Hormone assays and pelvic ultrasonography
Between 2011 and 2013, patients who had sent back questionnaires were sent a prescription for blood tests. Females also received a prescription for a pelvic sonogram. When we had access to the medical records of the patients, we retained the data from the most recent blood test and ultrasound scan that met the criteria for the procedure.
In females, the following were assayed on a blood sample collected between the 3rd and the 6th days of the menstrual cycle or at least 1 month after hormone therapy discontinuation: luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, AMH, testosterone, and inhibin B (in postpubertal patients). Males underwent assays of LH, FSH, testosterone, AMH, and inhibin B. Assay methods were as follows: electroluminescence (Beckman Coulter Diagnostics, Brea, CA) for LH, FSH, and estradiol; enzyme immunoassay (Beckman Coulter) for AMH; fluorescence enzyme immunoassay/RIA for testosterone; and ELISA (Beckman Coulter) for inhibin B. The radiologists who performed the pelvic sonogram entered the findings into a standardized form: ovarian volume, number of follicles, uterine length. We have chosen not to have male patients undergo a spermogram because of the very uneven quality of the analyses depending on the laboratories in France.
Definitions
Delayed puberty was defined as absence of breast development at 13 years of age or absence of testes enlargement at 14 years of age (25). Primary amenorrhea was absence of menarche at 16 years of age. POI was defined by FSH level greater than 20 IU/L before 40 years of age on two different blood samples (26), combined with amenorrhea or oligomenorrhea for longer than 4 months.
Statistical methods
The statistical analyses were performed using R 3.4.2. To assess correlations between the occurrence of puberty and homozygosity or heterozygosity for the Q188R mutation, we relied on Pearson's chi-squared test with Yates’ correction. P values below 0.05 were considered significant. The distribution of FSH values in females with vs without spontaneous puberty was assessed by applying the Wilcoxon–Mann–Whitney test.
Results
Patients
Between 2009 and 2011, questionnaires were returned for 103 (68.2%) of 151 patients, 56 females and 47 males. Mutations had been identified in 40 patients (39%) among which 15 were homozygous for the Q188R mutation, 16 heterozygous for the Q188R mutation and another mutation, and 9 with two other mutations. All but 3 patients adhered to a galactose-free diet. To compensate for the diary-free diet, 70 (68%) patients took daily calcium supplements.
Complications, particularly neurological impairments, were reported for 69 patients (67%): 23% language delay, 20% of deficiencies in fine motor-skills, 9% of global developmental delay and 7% of epilepsy. Overall, the educational history was normal or showed less than 2 years of delay at school for 83 patients (58%), with a median age of 18.3 years. Among the patients older than 18 years, 10% had a university degree or the equivalent. The patients in regular employment were in lower occupational categories than their parents.
Females
Questionnaires were obtained for 56 females with a median age of 19 years at the time of the questionnaire (range 3–52 years). Among them, 17 (30.4%) provided both hormone assay and pelvic sonogram results and 18 (32.1%) only hormone assay results.
Puberty in females (data from questionnaires)
Among the 56 females, 13 were younger than 13 years of age and all were prepubertal at the time of the questionnaire (Supplementary Materials, see section on supplementary materials given at the end of this article). Among the remaining 43 females, 31 (72%) spontaneously experienced the onset of puberty, reaching Tanner stage 2 at a mean age of 13.8 years (range, 11–18). Spontaneous menarche occurred in 21/31 (67.7%), at a mean age of 14.6 years (range, 12–18.9 years) and 8/31 were given estradiol replacement therapy to induce menarche, at a median age of 17 years (range 15–21 years). Among the 21 females with spontaneous menstrual cycles, 62% had spaniomenorrhea and 7/17 aged 30 years or more had secondary amenorrhea between 30 and 40 years old. As for the 2 patients over 40 years of age, they had secondary amenorrhea at 32 and 40 years.
No correlation was found between spontaneous puberty and genotype (P = 0.14).
Final height was available for 27 patients and was equal to target height.
Hormone levels and ultrasound findings in females
Figures 1,2 and Table 1 show the biological and sonographic data in females, divided into the same two age groups : <13 years old and 13–40 years old.
Figure 1.
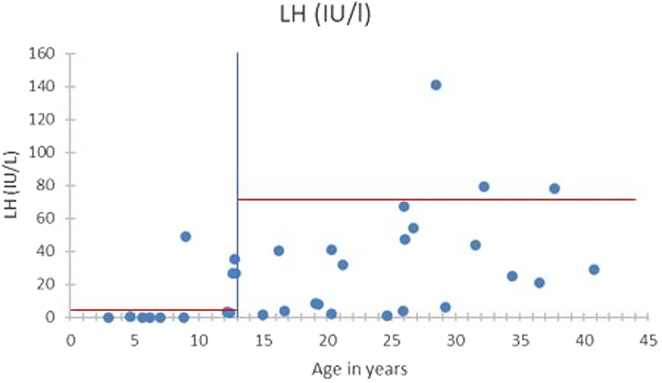
LH levels in the females according to age.
Figure 2.
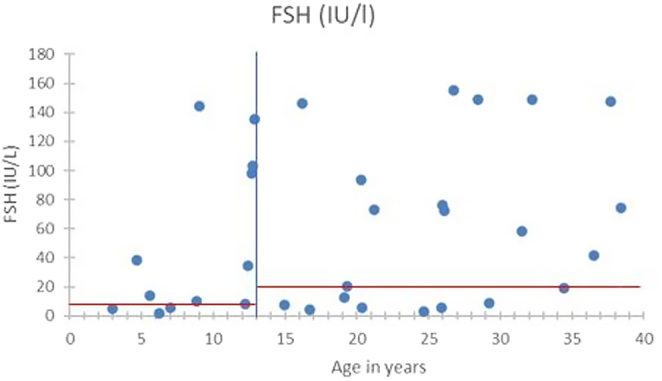
FSH levels in the females according to age.
Table 1.
Hormone assay and pelvic sonogram results for 37 women.
| Age group | No. of patients | Median | Median | Median | Median | Size of ovaries by ultrasonography |
|---|---|---|---|---|---|---|
| Median age | FSH level, IU/L (range) | LH level, IU/L (range) | Estradiol, pg/mL (range) | AMH, ng/mL (range) | ||
| No. of patients with spont. menarche | ||||||
| 0–<13 years old |
n = 10 8 years (3-12) n = 0 |
10 (1.7–144.5) (reference 0.7–6.7) |
0.1 (0–48.8) (reference <3.9) | <10 (0–45) (reference <10) |
< 10 (0–0.4) n = 8 (reference 10–200) |
<2 cm2 n = 5 (normal 2 cm2) |
| 13-40 years old |
n = 25 25 years |
Spont. men. n = 14 30.1 (3.3–155) |
Spont. men. n = 14 23 (0.8–141) |
Spont. men. n = 13 27 (0–105.3) |
Spont. men. n = 6 Undetectable |
Spont. men. n = 9 <2 cm2 n = 5 2.5–5.5 cm2 n = 4 |
| n = 14 spont. men. | Non-spont. men. n = 11 73.2 (8.6–149) (normal <20) |
Non-spont. men. n = 11 30.3 (6.1–79) (normal <70) |
Non-spont. men. n = 6 25.7 (1–75) (normal 100–1400) |
Non-spont. men. n = 5 Undetectable (normal 14–48) |
Non-spont. men. n = 3 <2 cm2 n = 2 2.5–5.5 cm2 n = 1 (normal >2 cm2) |
spont. menarche/spont. men., spontaneous menarche.
In the 10 patients under 13 years old (average age 8 years old (3–12)), none had clinical signs of puberty. However, 6 already had high FSH (median level 10 IU/L – reference range 0.7–6.7) and undetectable AMH levels, corresponding to early signs of ovarian dysfunction.
For the 25 patients aged from 13 to 40 years old, 7/14 and 10/11 patients had abnormally elevated FSH levels (reference <20 IU/mL) respectively in the subgroups with and without spontaneous menarche, with a median level of 30.1 and 73.2 IU/L (P = 0.35). AMH and inhibin B were consistently undetectable for all. Ovaries were small for most women in the two groups, mostly under the reference of 2 cm2. Only one woman had follicles detected, and is the patient who had three successful pregnancies (patient 3 in Table 2).
Table 2.
Characteristics of the four patients with successful pregnancies.
| Patient (DOB)x | 1 (09/1992) | 2 (02/1987) | 3 (07/1976) | 4 (11/1962) |
|---|---|---|---|---|
| Mutation GAL 1-P (under diet) |
Q188R/Q188R 3 mg/100 mL |
Q188R/other 0.1 mg/100 mL |
NA (classical form) NA |
NA 1.7 mg/100 mL |
| Adherence to diet | Yes | Yes | Yes | Yes |
| Puberty Menstrual cycles |
Spontaneous Short (15 days) |
Spontaneous NA |
Spontaneous Secondary amenorrhea at age 26 years old |
Spontaneous Secondary amenorrhea at age 41 years old |
| Age at testing | 19 years old | 25 years old | 36 years old | NA |
| AMH, ng/mL (normal 14-48) | 0.3 | – | <0.4 | NA |
| FSH, IU/L (normal <20) | 6 | 5.5 | 41.3 | NA |
| Estradiol, pg/mL (normal 100–1400) | 45 | 27 | <17 | NA |
| Ultrasound | RO 23·13 mm LO 19 mm Scarce follicles |
RO several follicles | RO 28·20 mm 4 follicles LO 19·13 mm 3 follicles |
NA |
| Pregnancy | ||||
| Spontaneous | 2 | 2 | 1 | 1 |
| Stimulation | 0 | 0 | 2 | 0 |
| Miscarriage | 1 (6 weeks) | 0 | 0 | 0 |
| Ovarian cryopreservation | No | Yes, at age 13 years old | No | No |
| Age at delivery | 22 years old | 19 years old/22 years old | 27 years old/32 years old/33 years old | 24 years old |
| Time to pregnancy | 4 months | 6 months | 3 months | 2 years |
DOB, date of birth; Gal 1-P, galactose 1-phosphate level in erythrocytes; LO, left ovary; NA, not available; RO, right ovary.
Reproductive history
At the time of the questionnaire, 30 women were 18 years old or more. Among them, 12 declared not having tried to conceive and 6 had a neurodevelopmental delay impairing considering parenting (7 missing data). Five tried to conceive: 4 succeeded with a total of 8 pregnancies including 7 live births (and 1 miscarriage at 6 weeks) and 1 had to resort to oocyte donation.
These 4 women all experienced a spontaneous onset of puberty, with menarche at a median age of 14.8 years. Only 2/4 had AMH level assay and were low (1 before and 1 after the pregnancies). From the 2 pregnancies in patient #1, 1 ended in early miscarriage. Patient #2 had a history of unilateral ovariectomy for cryopreservation at 14 years of age but had two spontaneous pregnancies with her remaining ovary. Patient #3 had two pregnancies after recombinant FSH stimulation at 27 and 32 years of age and a spontaneous pregnancy at 33 years of age despite permanent secondary amenorrhea. Patient #4 had a single pregnancy (Table 2).
Males
We collected questionnaires for 47 men, with a median age of 19 years old (range 3–45 years old) with 67% were 20 years old or more. Biological data (LH, FSH, testosterone, AMH and Inhibine B levels) was collected for 51% of them (24/47) (Table 3). Elements about puberty could be collected from the questionnaires for 26 men aged 12–45 years old: 25/26 had a spontaneous puberty with a median age of 13, one had a delayed puberty starting at 16 years old. Only one boy had a medical history of ectopic testis. All the patients reached their expected target height. All biological data were in the reference range for the age and puberty stage, with testosterone levels in the low range for adults however: median 4.71 ng/mL (reference range 2.5–10 ng/mL), for 10 patients >16 years old. AMH and Inhibin B levels were in the reference range, regardless of age. Every man above 18 years old declared having no parental desire and therefore no evaluation of fertility could be done.
Table 3.
Hormone assay results for men.
| Age group | No. of patients | Median | Median | Median | Median | Median |
|---|---|---|---|---|---|---|
| Median age (range) | FSH level, IU/L (range) | LH level, IU/L (range) | Testosterone, ng/mL (range) | Inhibine B, pg/mL (range) | AMH, ng/mL (range) | |
| 0 to <14 years old |
n = 18 9 years (3–13) |
0.9 (0.3-2.4) (reference 0.2–4.6) |
0.1 (0.1–5.6) (reference <0.1–7.8) |
0 (0–0.13) (reference 0–5) |
113.5 (57–227) n = 12 (reference 17–250) |
118 (53–158) n = 14 (reference: 17–193) |
| 14 to 33 years old |
n = 11 22 years (14–33) |
3.7 (1.7–8.2) (reference 1.5–12.4) |
2.9 (1.8–6.7) (reference 1.7–8.6) |
4.52 (1.03–8.5) (reference 1.2–10) |
153 (120–250) n = 8 (reference 120–350) |
Not done |
Discussion
This is the only study evaluating puberty and fertility in French males and females with classic galactosemia, compiling about 40% of the French estimated cohort. The findings confirm the high risk of POI, with high FSH levels even in the youngest females and undetectable AMH levels in most females. Despite the early FSH levels increase, two-thirds of females experienced spontaneous thelarche, which was usually followed by spontaneous menarche, albeit nearly 2 years later than in the general population (27). Overall, the proportion of females with spontaneous menarche was 55%, compared to 45% in a 2017 report (28) and 68% in Frederick study in 2018 (10).
Despite the presence of POI with frequent impairments in puberty and abnormal hormone levels, four out of five females who tried to conceive succeeded in pregnancies with low AMH levels, as the patients described by Gubbels (29). Thus, low AMH and inhibin B levels and low ultrasound follicle counts may not predict infertility in females with classic galactosemia. AMH is not a good fertility marker, even in the general population, and is generally used only to evaluate the response to stimulation (21, 30). We identified no other laboratory markers of potential use for predicting fertility in the individual patient.
Primary amenorrhea is known to predict failure of spontaneous conception in the overall population of patients with POI. In our cohort, all four women had spontaneous menarche, consistent with Gubbels study (9) but not with van Erven (28).
The impact of genotype on fertility is controversial. In our study, POI was a nearly consistent feature and, consequently, no correlation could be demonstrated between the genotype and fertility.
Ovarian cryopreservation is sometimes considered for females with classic galactosemia. This should be considered after a careful benefit-risk assessment for several reasons. The efficacy of ovarian cryopreservation is in doubt, as the ovarian lesions seem to develop very early in life, as shown by the elevated FSH and low AMH levels in patients tested during early childhood. Salto Mamsen et al. (31) described however normal follicle density in galactosemic patients under 5 years of age. High-quality oocyte counts in ovaries from females with galactosemia are low and may well be further decreased due to the tissue alterations induced by cryopreservation (16). Second, the optimal age for ovarian harvesting is unclear regarding the difficulties to obtain mature oocytes from prepubescent ovaries, however possible. Third, obtaining informed consent to cryopreservation from young patients raises ethical issues, especially in galactosemic patients with developmental delay. Fourth, fertility in females with classic galactosemia may be better than generally believed, as shown in our study, and by van Erven with 48% of female patients trying to conceive who became pregnant spontaneously within 24 months (28). Others have also reported spontaneous pregnancies including one with triplets (32). In case of failure of pregnancy, oocyte donation may be a better option than cryopreservation for galactosemic women (33). A recent study (34) supports our conclusion by showing that the relative rarity of galactosemia makes it difficult to accumulate data to determine factors defining timing of ovarian dysfunction or treatment/fertility preservation options for this group of women. However, cryopreservation could be considered for young patients under 5 with normal hormonal and follicle evaluation, regarding the high frequency of POI.
In the male patients, puberty and fertility were normal, in contrast to earlier reports (8, 20, 24). Puberty occurred at the expected age, AMH, testosterone and Inhibine B levels were normal, and a single patient had cryptorchidism. No specific recommendations exist for the clinical follow-up of boys with classic galactosemia (35). The fact that none of the men tried to conceive can probably be ascribed to the high prevalence of developmental delay, with none of the males in our cohort having a level of independence consistent with starting a family.
One limitation of our study is the retrospective collection of part of the data via a questionnaire. Questionnaire data can lack accuracy, particularly for puberty in males. In females, however, the age at menarche and presence of oligo/amenorrhea are generally reliably reported, and the data were confirmed during telephone interviews or checked in the medical files when available. There is also a bias in questionnaires as there could be significant differences between responding and nonresponding patients, and whether the questionnaire is filled by the parents of affected children or patients themselves. A second limitation is the analysis of a single blood sample. Obtaining serial blood samples would have raised logistical challenges, as the patients were disseminated throughout France. Third, the sonograms were performed by various radiologists using a variety of machines. However, for each patient, the radiologist filled a standardized form. Furthermore, the finding of scarce or absent follicles and small ovaries in every case supports the reliability of the sonogram data. Fourth, only five females tried to conceive. Factors that may have decreased the likelihood of attempting conception may include advice to this effect given during follow-up and developmental delay. Nevertheless, our findings and those reported previously (28) support a higher than previously believed fertility rate among females with classic galactosemia.
Conclusion
Our study confirms early POI in all the female patients, with however a high rate of spontaneous pregnancies. The only predictive positive factor could be spontaneous menarche, these patients should be told that the chances of spontaneous pregnancy are substantial. In those who want children but fail to conceive spontaneously, oocyte donation when available could be a better option than cryopreservation, considering the surgery risks and the ethical issues. Further work is needed to elucidate the mechanisms of the fluctuating POI seen in females and to assess fertility in males with classic galactosemia.
Supplementary Material
Declaration of interest
Isabelle Flechtner, Magali Viaud, Dulanjalee Kariyawasam Marie Perrissin-Fabert, Maud Bidet, Anne Bachelot, Philippe Touraine, Philippe Labrune, Pascale de Lonlay, Michel Polak, Galactosemia Study group declare that they have no conflict of interest. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
Galactosemia Study Group. Individual contributors: Professor HUET Frédéric, service de Pédiatrie, CHU Dijon – Doctor BRUEL Henri, service de Pédiatrie, Groupe Hospitalier Le Havre – Doctor BARTH Magalie, service de Génétique, CHU Angers; Professeur FEILLET François, service des Maladies Métaboliques, CHU Nancy-Brabois; Doctor DOBBELAERE Dries, service de Pédiatrie, CHRU Lille; Doctor HABES Dalila, service d’Hépatologie pédiatrique, CH Bicêtre; Professor RIGALLEAU Vincent, service des Maladies Métaboliques, CHU Bordeaux; Doctor LAMIREAU Delphine, service de Pédiatrie, CHU Bordeaux; Doctor MIGNOT Brigitte, service de Pédiatrie, CHU Besançon; Docteur MOLLET-BOUDJEMLINE Alix, CHU Clamart.
References
- 1.Bosch AM.Classical galactosaemia revisited. Journal of Inherited Metabolic Disease 2006. 29 516–525. ( 10.1007/s10545-006-0382-0) [DOI] [PubMed] [Google Scholar]
- 2.Tyfield LA.Galactosaemia and allelic variation at the galactose-1-phosphate uridyltransferase gene: a complex relationship between genotype and phenotype. European Journal of Pediatrics 2000. 159 (Supplement 3) S204–S. ( 10.1007/pl00014404) [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Gozalbo ME, Haskovic M, Bosch AM, Burnyte B, Coelho AI, Cassiman D, Couce ML, Dawson C, Demirbas D, Derks T.et al. The natural history of classic galactosemia: lessons from the GalNet registry. Orphanet Journal of Rare Diseases 2019. 14 86. ( 10.1186/s13023-019-1047-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schweitzer-Krantz S.Early diagnosis of inherited metabolic disorders towards improving outcome: the controversial issue of galactosaemia. European Journal of Pediatrics 2003. 162 (Supplement 1) S50–S. ( 10.1007/s00431-003-1352-2) [DOI] [PubMed] [Google Scholar]
- 5.Loeber JG.Neonatal screening in Europe; the situation in 2004. Journal of Inherited Metabolic Disease 2007. 30 430–438. ( 10.1007/s10545-007-0644-5) [DOI] [PubMed] [Google Scholar]
- 6.Honeyman MM, Green A, Holton JB, Leonard JV.Galactosaemia: results of the British Paediatric Surveillance Unit Study, 1988–90. Archives of Disease in Childhood 1993. 69 339–341. ( 10.1136/adc.69.3.339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes J, Ryan S, Lambert D, Geoghegan O, Clark A, Rogers Y, Hendroff U, Monavari A, Twomey E, Treacy EP.Outcomes of siblings with classical galactosemia. Journal of Pediatrics 2009. 154 721–726. ( 10.1016/j.jpeds.2008.11.052) [DOI] [PubMed] [Google Scholar]
- 8.Schweitzer S, Shin Y, Jakobs C, Brodehl J.Long-term outcome in 134 patients with galactosaemia. European Journal of Pediatrics 1993. 152 36–43. ( 10.1007/BF02072514) [DOI] [PubMed] [Google Scholar]
- 9.Gubbels CS, Land JA, Rubio-Gozalbo ME.Fertility and impact of pregnancies on the mother and child in classic galactosemia. Obstetrical and Gynecological Survey 2008. 63 334–343. ( 10.1097/OGX.0b013e31816ff6c5) [DOI] [PubMed] [Google Scholar]
- 10.Frederick AB, Zinsli AM, Carlock G, Conneely K, Fridovich-Keil JL.Presentation, progression, and predictors of ovarian insufficiency in classic galactosemia. Journal of Inherited Metabolic Disease 2018. 41 785–790. ( 10.1007/s10545-018-0177-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch AM, Grootenhuis MA, Bakker HD, Heijmans HSA, Wijburg FA, Last BF.Living with classical galactosemia: health-related quality of life consequences. Pediatrics 2004. 113 e423–e. ( 10.1542/peds.113.5.e423) [DOI] [PubMed] [Google Scholar]
- 12.Gitzelmann R, Steinmann B.Galactosemia: how does long-term treatment change the outcome? Enzyme 1984. 32 37–46. ( 10.1159/000469448) [DOI] [PubMed] [Google Scholar]
- 13.Kaufman FR, Xu YK, Ng WG, Silva PD, Lobo RA, Donnell GN.Gonadal function and ovarian galactose metabolism in classic galactosemia. Acta Endocrinologica 1989. 120 129–133. ( 10.1530/acta.0.1200129) [DOI] [PubMed] [Google Scholar]
- 14.Xu YK, Ng WG, Kaufman FR, Lobo RA, Donnell GN.Galactose metabolism in human ovarian tissue. Pediatric Research 1989. 25 151–155. ( 10.1203/00006450-198902000-00015) [DOI] [PubMed] [Google Scholar]
- 15.Prestoz LL, Couto AS, Shin YS, Petry KG.Altered follicle stimulating hormone isoforms in female galactosaemia patients. European Journal of Pediatrics 1997. 156 116–120. ( 10.1007/s004310050568) [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Hale GE, Hughes CL.Galactose metabolism and ovarian toxicity. Reproductive Toxicology Elmsford 2000. 14 377–384. ( 10.1016/s0890-6238(0000096-4) [DOI] [PubMed] [Google Scholar]
- 17.Bandyopadhyay S, Chakrabarti J, Banerjee S, Pal AK, Bhattacharyya D, Goswami SK, Chakravarty BN, Kabir SN.Prenatal exposure to high galactose adversely affects initial gonadal pool of germ cells in rats. Human Reproduction 2003. 18 276–282. ( 10.1093/humrep/deg058) [DOI] [PubMed] [Google Scholar]
- 18.Menezo YJR, Lescaille M, Nicollet B, Servy EJ.Pregnancy and delivery after stimulation with rFSH of a galatosemia patient suffering hypergonadotropic hypogonadism: case report. Journal of Assisted Reproduction and Genetics 2004. 21 89–90. ( 10.1023/b:jarg.0000027020.28592.7b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forges T, Monnier-Barbarino P, Leheup B, Jouvet P.Pathophysiology of impaired ovarian function in galactosaemia. Human Reproduction Update 2006. 12 573–584. ( 10.1093/humupd/dml031) [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Gozalbo ME, Gubbels CS, Bakker JA, Menheere PPCA, Wodzig WKWH, Land JA.Gonadal function in male and female patients with classic galactosemia. Human Reproduction Update 2010. 16 177–188. ( 10.1093/humupd/dmp038) [DOI] [PubMed] [Google Scholar]
- 21.Spencer JB, Badik JR, Ryan EL, Gleason TJ, Broadaway KA, Epstein MP, Fridovich-Keil JL.Modifiers of ovarian function in girls and women with classic galactosemia. Journal of Clinical Endocrinology and Metabolism 2013. 98 E1257–E. ( 10.1210/jc.2013-1374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy HL, Driscoll SG, Porensky RS, Wender DF.Ovarian failure in galactosemia. New England Journal of Medicine 1984. 310 50. ( 10.1056/nejm198401053100114) [DOI] [PubMed] [Google Scholar]
- 23.Thakur M, Shaeib F, Khan SN, Kohan-Ghadr HR, Jeelani R, Aldhaheri SR, Gonik B, Abu-Soud HM.Galactose and its metabolites deteriorate metaphase II mouse oocyte quality and subsequent embryo development by disrupting the spindle structure. Scientific Reports 2017. 7 231. ( 10.1038/s41598-017-00159-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waggoner DD, Buist NR, Donnell GN.Long-term prognosis in galactosaemia: results of a survey of 350 cases. Journal of Inherited Metabolic Disease 1990. 13 802–818. ( 10.1007/BF01800204) [DOI] [PubMed] [Google Scholar]
- 25.Sørensen K, Aksglaede L, Petersen JH, Juul A.Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. Journal of Clinical Endocrinology and Metabolism 2010. 95 263–270. ( 10.1210/jc.2009-1478) [DOI] [PubMed] [Google Scholar]
- 26.De Vos M, Devroey P, Fauser BCJM.Primary ovarian insufficiency. Lancet 2010. 376 911–921. ( 10.1016/S0140-6736(1060355-8) [DOI] [PubMed] [Google Scholar]
- 27.Gaudineau A, Ehlinger V, Vayssière C, Jouret B, Arnaud C, Godeau E. Age at onset of menarche: results from the French Health Behaviour in School-Aged Children Study. Gynecologie, Obstetrique and Fertilite 2010. 38 385–387. ( 10.1016/j.gyobfe.2010.01.014) [DOI] [PubMed] [Google Scholar]
- 28.van Erven B, Berry GT, Cassiman D, Conolly G, Forga M, Gautschi M, Gubbels CS, Hollak CEM, Janssen MC, Knerr I.et al. Fertility in adult women with classic galactosemia and primary ovarian insufficiency. Fertility and Sterility 2017. 108 168–174. ( 10.1016/j.fertnstert.2017.05.013) [DOI] [PubMed] [Google Scholar]
- 29.Gubbels CS, Kuppens SMI, Bakker JA, Konings CJAM, Wodzig KW, de Sain-van der Velden MGM, Menheere PP, Rubio-Gozalbo ME.Pregnancy in classic galactosemia despite undetectable anti-Müllerian hormone. Fertility and Sterility 2009. 91 1293.e13–1293.e16. ( 10.1016/j.fertnstert.2008.12.031) [DOI] [PubMed] [Google Scholar]
- 30.Fraisse T, Ibecheole V, Streuli I, Bischof P, de Ziegler D.Undetectable serum anti-Müllerian hormone levels and occurrence of ongoing pregnancy. Fertility and Sterility 2008. 89 723.e9–723.. ( 10.1016/j.fertnstert.2007.03.084) [DOI] [PubMed] [Google Scholar]
- 31.Mamsen LS, Kelsey TW, Ernst E, Macklon KT, Lund AM, Andersen CY.Cryopreservation of ovarian tissue may be considered in young girls with galactosemia. Journal of Assisted Reproduction and Genetics 2018. 35 1209–1217. ( 10.1007/s10815-018-1209-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noelmans L, Jacquemyn Y, De Naeyer S, Eyskens F.Pregnancy and galactosaemia. Journal of Obstetrics and Gynaecology 2006. 26 812–814. ( 10.1080/01443610600987001) [DOI] [PubMed] [Google Scholar]
- 33.Sauer MV, Kaufman FR, Paulson RJ, Lobo RA.Pregnancy after oocyte donation to a woman with ovarian failure and classical galactosemia. Fertility and Sterility 1991. 55 1197–1199. ( 10.1016/s0015-0282(1654376-3) [DOI] [PubMed] [Google Scholar]
- 34.Thakur M, Feldman G, Puscheck EE.Primary ovarian insufficiency in classic galactosemia: current understanding and future research opportunities. Journal of Assisted Reproduction and Genetics 2018. 35 3–16. ( 10.1007/s10815-017-1039-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welling L, Bernstein LE, Berry GT, Burlina AB, Eyskens F, Gautschi M, Grünewald S, Gubbels CS, Knerr I, Labrune P.et al. International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. Journal of Inherited Metabolic Disease 2017. 40 171–176. ( 10.1007/s10545-016-9990-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a