Abstract
Background:
There is paucity of data on objectively measured lung function abnormalities in Nigerian children using diagnostic testing methods such as spirometry. Such assessments could prompt early diagnosis and therapeutic interventions.
Methods:
This was a cross sectional study among children aged 6 to 12 years in South-Eastern Nigeria. We selected participants from one school using a multistage stratified random sampling technique. A structured respiratory questionnaire was administered to obtain necessary data. The lung functions of the children were measured by spirometry. We used Lower Limits of Normal (LLN) based on GLI reference equations for African-American and mixed ethnicities to define abnormal spirometry. We studied the association between the exposures and lung function using logistic regression/chi-squared tests.
Results:
A total of 145 children performed acceptable and repeatable tests. There were 73 males (50.3%), mean age of 9.13 years (+1.5) and age range 6 to 12 years. Frequency of respiratory symptoms was cough- 64 (44.1%) and wheeze in 19 (13.1%). Using GLI for African-Americans, fifty-five (37.9%) children had abnormal spirometryobstructive pattern in 40 (27.6%) and restrictive pattern in 15 (10.3%). The two references showed significant differences in interpretation of abnormality (χ2 = 72.86; P < .001). Respiratory symptom-wheeze was an independent determinant of abnormal lung function in this population.(OR = 0.31; 95%CI: 0.10–0.94; P = .04)
Conclusion:
There is a high burden of respiratory symptoms and abnormal spirometry among these children. The need for objective evaluation of lung function especially for children with respiratory symptoms is evident.
Keywords: Asthma, epidemiology, medical diagnosis, pulmonology or respiratory disorders
Introduction
The World Health Organization’s (WHO) strategy for prevention and control of Chronic Respiratory Diseases (CRD) promotes improved surveillance to map the magnitude of CRD and analyze the risk factors with reference to disadvantaged populations.1 One of the standard indicators for this surveillance is lung function measurements. Lack of requisite equipment including spirometers, and limited expertise within Africa are hindrances both in performing lung function tests and in the interpretation of results.2-5 Therefore, many children with CRD such as asthma may remain undiagnosed. This limitation increases morbidity and mortality amongst children, with recognized negative impact on long term outcomes in adulthood.3,6-10 Recognition of impaired lung function in childhood may be an opportunity for a potentially effective intervention, such as early referral for specialized care with prompt treatment.2,7-9 Optimizing lung function in childhood is thus an important public health priority.9-11
In Nigeria and most other parts of sub-Saharan Africa (SSA), there are several existing factors that potentially reduce lung function in children. These range from intrauterine and early life exposures leading to low birth weight and childhood respiratory infections, to air pollution from household use of unclean cooking fuels, and ambient air pollution. Others include socioeconomic factors like poor education, housing, and malnutrition.1,10-13 The implication of such demographics would be that the burden of impaired lung function among children in this part of the world may be higher than is reported for children in developed countries.
There are limited studies that have evaluated the lung function of children in Nigeria. Most reports have focused on comparisons of mean differences in spirometric indices based on demographics and health status.14,15 Few have interpreted their findings using globally accepted reference standards like the Global Lung Initiative (GLI-2012). The GLI-2012 has been shown to be applicable in children and adults (3-95 years) from different countries as it eliminates age, gender, ethnic, and height bias in lung function interpretation.16 This study was therefore conducted to determine the prevalence of abnormal spirometry pattern among primary school aged children in a Nigerian city using the Global Lung Initiative (GLI-2012) as the reference standard. Association of spirometry pattern with the presence of respiratory symptoms, socio-demographic, and environmental exposures was also explored with interpretation using the GLI references for “African Americans” and “Others” (other races).
Methods
Study design and study population
This was a cross sectional school-based study conducted in Awka, an urban city in Anambra state, South Eastern Nigeria from February 2017 to March 2017. The study population comprised children aged between 6 and 12 years attending public schools within the city. Of the four schools approved by the Education Ministry for this study, one school was purposively selected based on the centrality of its location in the city and heterogeneity of its population.
Sampling
The sample size was 140 participants, calculated based on an estimated prevalence of abnormal spirometry of 10% with a 5% precision at 95% confidence interval.6 Sample size was increased to 160 to include a possible 20% attrition due to poor spirograms. We selected participants using a multi-stage stratified random sampling technique. The stratification was based on class grades, age, and gender of the children. We included only children whose parents provided written consent, and who gave personal assent, where applicable. Children with chest or posture deformity, recent surgery, acute respiratory infection with ongoing cough or chronic infectious respiratory diseases like tuberculosis were excluded. The exposure variables assessed were Environmental Tobacco Smoke (ETS) exposure (smoking history of household or family members), biomass exposure from cooking fuels, parental monthly income, and presence of respiratory symptoms like cough and wheezing. The main outcome variables were the forced expiratory volume in the first second (FEV1), the Forced Vital Capacity (FVC) and the ratio of the FEV1/FVC measured by spirometry.
Data collection
We addressed the parents during the termly Parents/Teachers meeting, explaining the research aims, objectives, tools, and benefits. A structured respiratory questionnaire adapted from International Study of Asthma and Allergies in Childhood (ISAAC) phase three core questionnaire was used to obtain information on the exposure variables from consenting parents.17 Questions included wheeze in the past 12 months, presence of cough associated with cold in the past 12 months and previous diagnosis of asthma. The questionnaires were self-administered with interviewer assistance when required.
We performed general physical and respiratory system examination and measured weights and heights using standard methods. Body mass index (BMI) was calculated using the formula- Weight (kg)/Height (m2) and categorized according to the Centre for Disease Control (CDC) growth charts (BMI for age): Underweight BMI was defined as BMI less than the fifth percentile for age and sex, Overweight BMI as BMI above the 85th but below the 95th percentile for age and sex. Normal BMI was BMI between the fifth and 85th percentile for age and sex. We performed spirometry according to the American Thoracic Society/European Respiratory Society standards, using battery operated new diagnostic design (ndd) EasyOneTM diagnostic world spirometer.18 Testing was performed by an experienced pediatrician with international certification in spirometry (European Spirometry Driving License). With the child standing and wearing a nose clip, a minimum of three blows were performed with at least two acceptable and repeatable blows, obtained from a maximum of eight maneuvers. To ensure quality control, we performed daily calibration checks using a three liter syringe at the beginning of every test day and after testing eight individuals respectively. All tests were sent via a secure Internet transfer TEAMVIEWER® for independent verification for quality by a European Respiratory Society Spirometry Train-the-Trainer certified spirometry trainer prior to final acceptance and inclusion into the database.
Data management and analysis
Data entry and cleaning were done using Epidata Secure software Database prior to importation into Microsoft excel spreadsheet. Required data (Participant ID, Age, height, ethnicity, FEV1, and FVC) were then uploaded into the Global Lung Initiative (GLI-2012) online calculator to derive the spirometry Lower Limit of Normal (LLN) values and requisite z-scores using the prediction reference equations for African Americans. This was used for identification and interpretation of spirometry abnormalities.11 The lower limit of normal LLN is defined as the z-score < −1.645.11,19
Using the Global Lung Index (GLI) 2012 reference equations for African Americans, normal lung function pattern was defined by FEV1, FVC, and FEV1/FVC all > LLN. Obstructive lung function was determined by the ratio of Forced expiratory volume in the first second (FEV1) to Forced Vital Capacity (FVC) that was below the lower limit of normal (LLN) with Forced Vital Capacity above LLN (FEV1/FVC <LLN with FVC > LLN), while restrictive lung function abnormalities were suggested by values less than the LLN for the FVC (FVC < LLN with FEV1/FVC > LLN). Those with FEV1/FVC <LLN and FVC < LLN who could have a possible restrictive/obstructive disorder were classified as having possible obstruction.11 GLI 2012 reference equations for “Others” was also used to determine the relationship of its interpretation with the reference equation for African Americans.
The data was analyzed using the Statistical Package for Social Sciences (SPSS) software version 21. Categorical variables were summarized as frequencies while numerical variables were described using means. Data on birth weight and parental income were excluded from the analysis because of missing values. Variables were tested using Chi squared tests to determine statistically significant levels of association (P < .05) with abnormal spirometry pattern. Binary logistic regression was used to assess for independent determinants of abnormal spirometry patterns. The multivariate model was based on a priori factors and included variables such as ETS, use of kerosene as cooking fuel and respiratory symptoms known to influence lung function. Outcome of interest was “any abnormal lung function.” Statistical significance was set at P < .05 at 95% confidence interval.
Ethical considerations
Ethical approval was obtained from the Central Ethics Committee of the Nnamdi Azikiwe University, Awka (NAU/CEC/STA/002), and the Nnamdi Azikiwe University Teaching Hospital Ethics Committee (NAUTH/CS/66/VOL.9/134/2016/105) prior to the commencement of the study. Written approval for the study was also received from the State Basic Education board.
Results
There were a total of 204 recruited participants. Only 188 met inclusion criteria and were enrolled into the study.
Spirometry tests were performed on them with a mean of three tests each and a maximum of eight attempts per child. In line with estimated sample size, 145 children (77.1%) who performed acceptable and repeatable tests according to ATS/ERS criteria, were included in the final analysis.
Demographic characteristics
Of the 145 participants included in the final analysis, 73(50.3%) were males. Their ages ranged from 6 to 12 years with a mean age of 9.13 ± 1.5 years. Only 68 (46.9%) reported family income, of which 41 (60.3%) earned less than N10,000 a month (<1USD/day). Table 1 shows the demographic characteristics, anthropometrics, and lung function parameters of the study participants.
Table 1.
Demographic characteristics, anthropometry, and mean lung function parameters of study population.
| Variable | Male n = 73 | Female n = 72 | Total n = 145 |
|---|---|---|---|
| Age (years) | |||
| 6 | 3 | 2 | 5 |
| 7 | 7 | 8 | 15 |
| 8 | 11 | 20 | 31 |
| 9 | 22 | 9 | 31 |
| 10 | 19 | 16 | 35 |
| 11 | 8 | 16 | 24 |
| 12 | 3 | 1 | 4 |
| Mean age in years (SD) | 9.14 (1.4) | 9.13 (1.5) | 9.13 (1.5) |
| Mean weight in kg (SD) | 29.71 (5.8) | 30.17 (6.8) | 29.9 (6.3) |
| Mean height in cm (SD) | 135.47 (8.9) | 135.89 (9.9) | 135.7 (9.4) |
| Body mass index (BMI) | |||
| Mean BMI (SD) | 16.08 (1.8) | 16.15 (2.1) | 16.11 (1.96) |
| Normal BMI = n | 67 | 64 | 131 |
| Underweight BMI = n | 6 | 6 | 12 |
| Overweight BMI = n | 0 | 2 | 2 |
| Mean FEV1 z-score (SD) GLI others | −1.76 (1.2) | −1.49 (1.2) | −1.63 (1.2) |
| Mean FVC z-score (SD) GLI others | −1.19 (1.4) | −1.16 (1.5) | −1.18 (1.4) |
| Mean FEV1/FVC z-score (SD) GLI others | −1.2 (1.2) | −0.79 (1.8) | −0.99 (1.5) |
| Mean FEV1 z-score (SD) GLI African Americans | −0.66 (1.2) | −1.27 (1.1) | −0.96 (1.2) |
| Mean FVC z-score (SD) GLI African Americans | −0.08 (1.4) | −0.88 (1.2) | −0.48 (1.3) |
| Mean FEV1/FVC z-score (SD) GLI African Americans | −0.99 (1.4) | −0.79 (1.6) | −0.89 (1.5) |
Abbreviations: SD, standard deviation; n, number of persons.
Environmental exposures and respiratory symptoms of the children
Fifteen (10.3%) of the children were exposed to ETS at home. Most (110 = 75.9%) of the children used kerosene as their primary cooking fuel while only 41(28.3%) used firewood (28/41 of these also used kerosene). Only one mother smoked tobacco during pregnancy. Sixty-five (44.8%) of the children had respiratory symptoms. There had been at least one lifetime episode of wheezing in 19 (13.1%) of the children, of which 18/19 (94.7%) also had history of cough. Sixty-four (44.1%) of the 145 children had occasional cough exacerbated by one or more of exposure to cold (49 = 33.8%), dust (36 = 24.8%) or exercise (10 = 6.9%). Cough occurred mainly at night in 21 (14.5%). However, only one (0.7%) of these children had a previous diagnosis of asthma. The mean lung function z-scores stratified by children with and without respiratory symptoms, showed a mean(SD) FEV1 z-score, mean(SD) FVC z-score and mean (SD)FEV1/FVC z-scores respectively of −0.90(1.1), −0.46(1.2) and −0.73(1.7) for the children without respiratory symptoms [cough ± wheeze]. For those with these respiratory symptoms, the mean(SD) FEV1 z-score, mean(SD) FVC z-score, and mean(SD) FEV1/FVC z-scores were respectively −1.05(1.3), −0.49(1.5) and −1.08(1.1).
Abnormal spirometry parameters and patterns
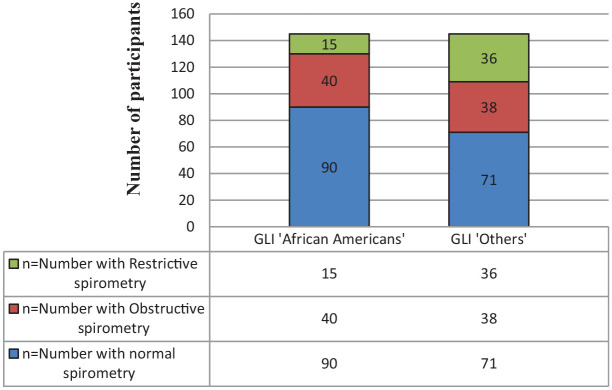
Fifty-five (37.9%) of the children had abnormal spirometry parameters (any or more than one of FEV1, FVC, and FEV1/FVC ratio being below LLN). FEV1 below LLN was the most common abnormal parameter in 35 (24.1%). The prevalence of abnormal lung function is as follows: 22 (15.2%) had obstructive patterns (FEV1/FVC < LLN), 15 (10.3%) had patterns suggestive of restriction. The prevalence of abnormal spirometry pattern is shown in Figure 1. Additionally 11 children (7.6%) had isolated low FEV1 and seven (4.8%) children had spirometry patterns suggestive of a mixed obstructive/restrictive (FEV1/FVC <LLN and FVC < LLN). These were classified as having obstructive patterns bringing the frequency of obstructive spirometry to 27.6%.11
Figure 1.
Spirometry patterns of the school aged children 6 to 12 years sampled in Awka using two reference interpretations.
χ2 = 72.86; P < .001.
Factors associated with abnormal spirometry pattern in the study population
Table 2 describes the association between spirometry pattern, respiratory symptoms, and environmental exposures. More than half of those who reported lifetime wheeze had abnormal spirometry pattern. (P = .05) About a third of those that reported cough and use of kerosene as main cooking fuel had abnormal spirometry but the relationship was not statistically significant.
Table 2.
Relationship of respiratory symptoms and environmental exposures with spirometry pattern in school aged children aged 6 to 12 years in Awka, Nigeria.
| Variables (number) | Normal spirometry pattern n (%) | Abnormal spirometry pattern n (%) | Statistics X2 | P value |
|---|---|---|---|---|
| ETS at home (15) | 9 (60) | 6 (40) | 0.03 | .86 |
| Use of Kerosene (110) | 69 (62.7) | 41 (37.3) | 0.15 | .71 |
| Use of firewood (41) | 30 (73.2) | 11 (26.8) | 2.36 | .13 |
| Lifetime wheeze (19) | 8 (42.1) | 11 (57.9) | 3.70 | .05 |
| Any cough* (64) | 40 (62.5) | 24 (37.5) | 0.01 | .92 |
| Exercise induced cough** (10) | 6 (60) | 4 (40) | 0.02 | .89 |
| Cold induced cough** (49) | 31 (63.3) | 18 (36.7) | 0.05 | .83 |
| Dust induced cough** (36) | 13 (36.1) | 23 (63.9) | 0.07 | .80 |
Any cough signifies all that have current cough associated with cold, exercise, or dust exposure.
Multiple responses allowed.
However, when the spirometry abnormalities were categorized, there was a significant relationship of underweight BMI and dust induced cough with restrictive pattern of spirometry (P < .05). Table 3. Though more of the males 24/73 (32.9%) than females 16/72 (22.2%) had obstructive spirometry and more females 9/72 (12.5%) than males 6/73 (8.2%) had restrictive spirometry, gender was not significantly associated with abnormal spirometry. Other variables tested were not significantly associated with abnormal spirometry. (Table 3)
Table 3.
Association of selected epidemiological variables with obstructive and restrictive spirometry abnormalities.
| Variables | Obstructive pattern |
Restrictive pattern |
||
|---|---|---|---|---|
| X 2 | P value | X 2 | P value | |
| Gender (male) | 2.06 | .15 | 0.72 | .40 |
| Underweight BMI | 0.78 | .38 | 7.46 | .01 |
| ETS at home | 0.28 | .60 | 0.24 | .62 |
| Use of Kerosene | 0.02 | .89 | 0.17 | .68 |
| Use of firewood | 0.76 | .38 | 1.45 | .23 |
| Lifetime wheeze | 0.94 | .33 | 2.70 | .10 |
| Any cough* | 1.87 | .17 | 3.44 | .06 |
| Dust induced cough | 2.86 | .09 | 4.28 | .04 |
Abbreviations: BMI, body mass index; ETS, environmental tobacco smoke.
Any cough signifies all that have current cough associated with cold, exercise, or dust exposure.
Spirometry patterns of respondents using GLI 2012 for “African Americans” and “Others”
When GLI 2012 for “others” was used for the interpretation of measured lung function parameters, seventy-four (51%) of the children had abnormal spirometry patterns (any of or combination of FEV1, FVC, and FEV1/FVC below LLN) with FEV1 below LLN being the most common abnormal parameter in 59 (40.7%). Thirty eight children (26.2%) had obstructive patterns while 36 (24.8%) had patterns suggestive of restriction. The discordance with GLI 2012 for “African Americans” is shown in Figure 1.
The two references showed significant differences in interpretation of abnormality (χ2 = 72.86; P < .001) and restrictive spirometry (P < .001). Twenty one of the 36 children interpreted as having restrictive spirometry patterns in “Others” were normal with “African American” reference interpretation. (P < .001).
Determinants of abnormal lung function
In the logistic regression, our model could correctly predict 22% of abnormal lung function. Respiratory symptom-wheeze was the only independent determinant of abnormal lung function in this population.(OR = 0.31; 95%CI: 0.10–0.94; P = .04) Table 4. This remained constant even with adjustment for age and gender.
Table 4.
Determinants of abnormal lung function in school aged children aged 6 to 12 years in Awka, Nigeria.
| Variables | Odds ratio | 95%CI | P-value |
|---|---|---|---|
| ETS | 1.07 | 0.34, 3.42 | .91 |
| Wheeze | 0.31 | 0.10, 0.94 | .04 |
| Cough | 1.29 | 0.59, 2.82 | .53 |
| Use of kerosene | 0.92 | 0.37, 2.27 | .85 |
| Age | 0.93 | 0.72, 1.19 | .57 |
| Gender | 0.82 | 0.40, 1.67 | .26 |
Abbreviations: ETS, environmental tobacco smoke; CI, confidence interval.
Discussion
This study has shown that performing field spirometry screening to determine lung function of apparently healthy children as part of the surveillance for CRDs is feasible in the sub-Saharan African setting. It has the capacity to identify previously unrecognized respiratory morbidity in childhood which provides opportunity for early identification and administration of potentially effective intervention to mitigate future morbidity and mortality. Using the GLI-2012 reference equations, we identified abnormalities with spirometry patterns, with a high prevalence of respiratory symptoms and significant relationships between the presence of respiratory symptoms and abnormal spirometry (restriction). Such has been previously reported in adults.20 We also confirmed that underweight and restrictive spirometry pattern were related, amongst these children.
Our main finding based on the globally accepted GLI-2012 spirometry reference standards for interpretation shows that up to a third of apparently healthy primary school children had abnormal spirometry patterns with a substantial proportion reporting respiratory symptoms (cough-42.8% and wheeze-13.1%). The presence of cough precipitated by exposure to dust was significantly associated with presence of abnormal spirometry with a borderline relationship between lifetime history of wheeze and abnormal spirometry.
We found the frequency of obstruction in this population using standard definitions to be 15.2% and when those with isolated low FEV1 and possible mixed abnormalities were included as probable mild obstruction, we had a total of 27.6% with obstructive pattern. This high frequency of obstruction is consistent with findings in Poland where obstructive spirometry pattern was found in 20.3% of children aged 4 to 18 years who had respiratory symptoms.21 In our study, obstructive pattern was not associated with either respiratory symptoms or environmental exposure which infers that obstructive spirometry among these African children may be surreptitious, highlighting the need for screening. On the other hand, it may suggest that respiratory symptoms are poorly recognized or reported among these African children. This high frequency of obstructive spirometry among these children may also indicate that the prevalence of obstructive lung disease or asthma that has been reported among Nigerian children may have been underestimated.22,23 This is plausible because most estimates of asthma prevalence were based on respiratory symptom questionnaires which may not be indicative of airway obstruction or asthma diagnosis. The use of respiratory questionnaires may also be prone to recall and comprehension bias and these observations call for greater rigor with the inclusion of spirometry in the estimation of the prevalence of asthma in children.16,21
The high rate of under-diagnosis of asthma in the context of a high prevalence of obstructive lung disease in this present study is a source of concern. Many children who also had symptoms of triggered cough and wheeze did not have a diagnosis of asthma. It implies a high burden of unrecognized respiratory morbidity among these children which bodes for a poor outcome as these children are left untreated. The Global Initiative for Asthma (GINA) recognizes that undiagnosed and untreated asthma in children increases the risk of emergency room visits, asthma mortality, and lung function decline.24 Under-diagnosis of asthma in these children may also indicate a low level of health literacy in the general population and poor knowledge of doctors regarding asthma diagnosis as previously reported.3
The choice of reference equation in defining spirometry pattern may account for the wide disparity in the prevalence of obstructive spirometry between this present study and a previous study among children aged 5 to 11 years in Northern Nigeria (4.6%).19 While our interpretation of obstructive pattern was dependent on the FEV1/FVC ratio < LLN using GLI 2012, Thacher et al19 based their assessment on the ratio of FEV1 to FEV6 less than 85% predicted based on African Americans National Health and Nutrition Examination Survey (NHANES III) reference equation. Using percentage predicted as Thacher et al19 did, has been criticized because it could lead to significant misclassification of obstructive pattern in children.16,25 This is because a single standard deviation is used in the calculation of percentage predicted but ideally, this should vary dependent on age and outcome.25 However, in LLN calculation, the same cut off for z-scores has been found to be applicable across all ages, gender groups, and spirometric pulmonary function indices.25
The restrictive pattern found in 7.5% of these children is difficult to interpret due to a lack of validation of GLI references in the local population and it has been shown that lung volume is a factor of ethnicity.16,26,27 Much higher prevalence of restrictive patterns in children have been previously reported in the United States and in Malawi, a developing country.28,29 However these were in children with known co-morbidities.28,29 We cautiously note that measurement of total lung capacity (TLC) and residual volume which confirm a restrictive pattern were not done and what we have reported is probable restriction. Furthermore, some children with a probable restrictive pattern may have air trapping from obstructive lung diseases particularly when the FEV1 is below the LLN in addition to LLN FVC, with normal ratios.30 Consequently, the FEV1/FVC ratio may be normal resulting in a wrong interpretation as restrictive lung disease.30
The effects of undernutrition on restrictive lung function as documented in our study, has been previously reported. While some authors have found a significant relationship between undernutrition and restrictive spirometry as in our study, others have found no relationship.31-33 Some have attributed this relationship to decreased lung growth and respiratory muscle mass. Biomass exposure has also been implicated as a risk factor for increase in both respiratory symptoms and low FVC.13,34 In Ecuador, Rinne et al34 found that children aged 7 to 15 years, living in homes that use biomass fuel and who were exposed to ETS had significantly lower FVC and these children were more affected than female adults when appropriate statistical comparison was done. However, our study found no significant relationship of exposure to ETS and biomass with abnormal spirometry.
When we interpreted our findings using GLI 2012 for “others,” we found significant disparities especially in the interpretation of restrictive spirometry. The prevalence of reduced FVC while using GLI “others” was much higher at 24.8% compared to the 7.5% with African American references. The use of references for African Americans in our study was predicated by the non-representation of Sub Saharan African data in GLI 2012.
There is some evidence that GLI reference equations for African Americans could be applied to African children, as GLI references would ideally be validated in local populations as much as possible, especially in Africa.26,35,36 In a study on adult Nigerians by Obaseki et al,26 prevalence of reduced FVC was 70.4% for men and 72.8% for women when using NHANES values for white Americans, 17.8% for men and 14.4% for women using NHANES equations for African Americans, and 15.5% for men and 20.5% for women using the Global Lung Function Initiative 2012 equations for African Americans. In a study in Malawi, Meghji et al27 reported the prevalence of spirometric restriction to be 38.6% using National Health and Nutrition Examination Survey III reference ranges and 9.0% using local reference ranges. This further highlights the need for locally derived references for objectivity.
The association we found between the presence of respiratory symptoms and abnormal spirometry in children has been previously reported.6,19,32 This is important because it suggests that the presence of cough precipitated by dust, and wheeze in children should serve as a prompt for screening spirometry to exclude abnormal patterns which could be either obstructive or restrictive. Failure to identify abnormal spirometry pattern in children is a missed opportunity for early intervention to improve both short term and long outcomes.
The relationship between gender and abnormal spirometry in children has not been consistent. While some authors have found obstructive lung disease to be more common among female children, we reported more males with obstructive pattern.15,19 Although female children are usually more exposed to biomass,19,24 some studies have reported a higher prevalence of obstructive lung disease in males during the pre-pubertal period with a reversal at puberty that has been attributed to the effect of hormones.37 The mean age of the children in this study was within the pre-pubertal age and could be the reason for our finding. However relationship between abnormal spirometry and gender in this study was not significant.
Major strengths of this study include the strict quality control in the spirometry measurements and interpretation, and in the use of GLI LLN which is widely applicable across age groups.
We also acknowledge some limitations. First, it was conducted in only one school which was purposively selected based on the heterogeneity of the population and centrality of its location in the city. The results may thus not be generalizable to the entire population in the city. Secondly, the high frequency of abnormal spirometry and presence of respiratory symptoms may also be indicative of a shared exposure among the children based on the location of the school. As stated earlier, inability to measure TLC makes the prevalence of restrictive spirometry only speculative. We also note that the evaluation of environmental exposures and history of respiratory symptoms are prone to recall bias and cultural peculiarities in providing information that could occur in many African settings.6,19
Conclusion
This study has shown a high burden of abnormal spirometry in these apparently healthy children that portends increased morbidity and mortality if not adequately addressed. There is thus a need to sustain the WHO strategy on CRD surveillance for children for the purpose of early diagnosis and analysis of the risk factors for the purpose of intervention. The study has also provided valuable data on lung function indices among children which can be used in future systematic analyzes knowing the inherent challenges of collecting population-specific reference data in low- and middle-income settings. Information from this study is also expected to influence physician practice by bringing to the fore the need for objective evaluation of lung function especially for children with respiratory symptoms.
Acknowledgments
Pan African Thoracic Society-Methods in Epidemiologic, Clinical, and Operations Research (PATS-MECOR) course. This project was developed as part of the program. Lindsay Zurba for independent verification of spirograms and PATS (Masekela R, Zurba L, Gray D) for Spirometer equipment loan, Wairimu Kimani, and Thandile.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research of this article: BREATHE Small Grants Award 2016 received through Pan African Thoracic Society Methods in Epidemiologic Clinical and Operations Research (PATSMECOR).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s Note: Abstract presented at the 2018 ATS. C104. The modern pediatric pulmonary function lab: Am J Respir Crit Care Med 2019;199:A5680. Internet address: www.atsjournals.org
Author contributions: CIN: Conceptualization, Data curation, Data analysis, Funding acquisition, Methodology, Data collection, Project administration, Writing – original draft, Writing – review & editing.
OBO: Conceptualization, Supervision, Data analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
BMA: Conceptualization, Data curation, Data analysis, Funding acquisition, Methodology, Writing – review & editing.
ACA: Data analysis, Supervision, Methodology, Writing – original draft, Writing – review & editing.
JCE-I: Project administration, Methodology, Writing – original draft, Writing – review & editing.
BIA: Methodology, Supervision, Writing – review & editing.
ORCID iDs: Chizalu Ifeyinwa Ndukwu  https://orcid.org/0000-0003-1286-823X
https://orcid.org/0000-0003-1286-823X
Adaeze C Ayuk  https://orcid.org/0000-0003-3325-3488
https://orcid.org/0000-0003-3325-3488
References
- 1. World Health Organization. Strategy for prevention and control of chronic respiratory diseases. 2002. World Health Organization Management of Noncommunicable Diseases Department Chronic Respiratory Diseases and Arthritis. Accessed September 18, 2019. https://www.who.int/respiratory/publications/crd_strategy/en/
- 2. Bousquet J, Khaltaev N, eds. The World Health Organization. Global Surveillance, Prevention and Control of Chronic Respiratory Disease. A Comprehensive Approach. World Health Organization; 2007. [Google Scholar]
- 3. Ayuk AC, Uwaezuoke SN, Ndukwu CI, Ndu IK, Iloh KK, Okoli CV. Spirometry in asthma care: a review of the trends and challenges in pediatric practice. Clin Med Insights Pediatr. 2017;11:1179556517720675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dombkowski KJ, Hassan F, Wasilevich EA, Clark SJ. Spirometry use among pediatric primary care physicians. Pediatrics. 2010;126:682-687. [DOI] [PubMed] [Google Scholar]
- 5. Masekela R, Zurba L, Gray D. Dealing with access to spirometry in Africa: A commentary on challenges and solutions. Int J Environ Res Public Health. 2018;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Constant C, Sampaio I, Negreiro F, et al. Respiratory disease screening in school-aged children using portable spirometry. J Pediatr (Rio J). 2011;87:123-130. [DOI] [PubMed] [Google Scholar]
- 7. Grimwood K, Chang AB. Long-term effects of pneumonia in young children. Pneumonia (Nathan). 2015;6:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014:49(5):430-434. [DOI] [PubMed] [Google Scholar]
- 9. Reyfman PA, Washko GR, Dransfield MT, Spira A, Han MK, Kalhan R. Defining impaired respiratory health. A paradigm shift for pulmonary medicine. Am J Respir Crit Care Med. 2018;198:440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marciniuk D, Ferkol T, Montes de Oca M, Rabe K, Zar H. Respiratory disease in the world. Realities of today- opportunities for tomorrow. 2013 Forum of International Respiratory Societies. Afr J Respir Med 2014;9:4-13. Accessed September 18, 2019. https://www.openaccessjournals.com/articles/AJRM%20Mar%2014%20pp%204-13.pdf [Google Scholar]
- 11. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948-968. [DOI] [PubMed] [Google Scholar]
- 12. Grigg J. Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:564-569. [DOI] [PubMed] [Google Scholar]
- 13. Oguonu T, Obumneme-Anyim IN, Eze JN, Ayuk AC, Okoli CV, Ndu IK. Prevalence and determinants of airflow limitation in urban and rural children exposed to cooking fuels in South-East Nigeria. Paediatr Int Child Health. 2018;38:121-127. [DOI] [PubMed] [Google Scholar]
- 14. Kuti BP, Oladimeji OI, Kuti DK, Adeniyi AT, Adeniji EO, Osundare YJ. Rural-urban disparity in lung function parameters of Nigerian children: effects of socio-economic, nutritional and housing factors. Pan Afr Med J. 2017;28:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akhiwu HO, Aliyu I. Spirometric values in healthy Nigerian school children aged 6-11 years. J Adv Med Med Res. 2017;22:1-8. [Google Scholar]
- 16. Quanjer PH, Stanojevic S, Cole TJ, et al. Multiethnic reference values for spirometry for the 3-95 year age range: the Global Lung Function 2012 equations. Eur Respir J. 2012;40:1324-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. International Study of Asthma and Allergies in Childhood (ISAAC). Tools and Manuals. Accessed July 29, 2016. http://isaac.auckland.ac.nz/phases/phasethree/corequestionnaire_6-7.pdf [PubMed]
- 18. Miller MR, Hankinson J, Brusasco V, et al.; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338. [DOI] [PubMed] [Google Scholar]
- 19. Thacher JD, Emmelin A, Madaki AJK, Thacher TD. Biomass fuel use and the risk of asthma in Nigerian children. Respir Med. 2013;107:1845-1851. [DOI] [PubMed] [Google Scholar]
- 20. Abbasi IN, Ahsan A, Nafees AA. Correlation of respiratory symptoms and spirometric lung patterns in a rural community setting, Sindh, Pakistan: a cross sectional survey. BMC Pulm Med. 2012;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peradzynska J, Krenke K, Szylling A, Krenke R. The influence of reference values on the interpretation of lung function in children; comparison of GLI 2012 and Polish 1998 reference values. In: Pokorsi M, ed. Pulmonary Function. Advances in Experimental Medicine and Biology. Vol. 858. Springer; 2015:31-38. [DOI] [PubMed] [Google Scholar]
- 22. Falade AG, Olawuyi JF, Osinusi K, Onadeko BO. Prevalence and severity of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema in 6- to 7-year-old Nigerian primary school children: the international study of asthma and allergies in childhood. Med Princ Pract. 2004;13:20-25. [DOI] [PubMed] [Google Scholar]
- 23. Ozoh OB, Aderibigbe SA, Ayuk AC, et al. The prevalence of asthma and allergic rhinitis in Nigeria: a nationwide survey among children, adolescents and adults. PLoS One. 2019;14:e0222281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2020. Accessed June 29, 2020. www.ginasthma.org
- 25. Stanojevic S, Wade A, Stocks J. Reference values for lung function: past, present and future. Eur Respir J. 2010;36:12-19. [DOI] [PubMed] [Google Scholar]
- 26. Obaseki DO, Erhabor GE, Awopeju OF, et al. Reduced forced vital capacity in an African population. Prevalence and risk factors. Ann Am Thorac Soc. 2017;14:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meghji J, Nadeau G, Davis KJ, et al. Noncommunicable lung disease in Sub-Saharan Africa. A community-based cross-sectional study of adults in Urban Malawi. Am J Respir Crit Care Med. 2016;194:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkins SMM, Taylor AL, Sillau SH, Mitchell MB, Rausch CM. Restrictive lung function in paediatric patients with structural congenital heart disease. J Thorac Card Surg. 2014;148:207-211. [DOI] [PubMed] [Google Scholar]
- 29. Mwalukomo T, Rylance SJ, Webb EL, et al. Clinical characteristics and lung function in older children vertically infected with human immunodeficiency virus in Malawi. J Pediatric Infect Dis Soc. 2016;5:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vijayasekaran D, Subramanyam L, Balachandran A, Shivbalan S. Spirometry in clinical practice. Indian Pediatr. 2003;40:626-632. [PubMed] [Google Scholar]
- 31. Do JG, Park C, Lee Y, Yoon KJ. Association between underweight and pulmonary function in 282,135 healthy adults: a cross-sectional study in Korean population. Sci Rep. 2019;9:14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park CH, Yi Y, Do JG, Lee YT, Yoon KJ. Relationship between skeletal muscle mass and lung function in Korean adults without clinically apparent lung disease. Medicine (Baltimore). 2018;97:e12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lelijveld N, Kerac M, Seal A, et al. Long-term effects of severe acute malnutrition on lung function in Malawian children: a cohort study. Eur Respir J. 2017;49:1601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rinne S, Rodas EJ, Bender BS, Rinne ML. Relationship of pulmonary function among women and children to indoor air pollution from biomass use in rural Ecuador. Respir Med. 2006;100:1208-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madanhire T, Ferrand RA, Attia EF, Sibanda EN, Rusakaniko S, Rehman AM. Validation of the global lung initiative 2012 multi-ethnic spirometric reference equations in healthy urban Zimbabwean 7-13 year-old school children: a cross-sectional observational study. BMC Pulm Med. 2020;20:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Obaseki DO, Erhabor GE, Gnatiuc L, Adewole OO, Buist SA, Burney PG. Chronic airflow obstruction in a Black African population: results of BOLD study, Ile-Ife, Nigeria. COPD. 2016;13:42-49. [DOI] [PubMed] [Google Scholar]
- 37. Fawken J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm. the EPICure study. Am J Respir Crit Care Med. 2010;182:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]