Abstract
Addition of uric acid (UA) to thrombolytic therapy, although safe, showed limited efficacy in improving patients’ stroke outcome, despite alleged neuroprotective effects of UA in preclinical research. This systematic review assessed the effects of UA on brain structural and functional outcomes in animal models of ischemic stroke. We searched Medline, Embase and Web of Science to identify 16 and 14 eligible rodent studies for qualitative and quantitative synthesis, respectively. Range of evidence met 10 of a possible 13 STAIR criteria. Median (Q1, Q3) quality score was 7.5 (6, 10) on the CAMARADES 15-item checklist. For each outcome, we used standardised mean difference (SMD) as effect size and random-effects modelling. Meta-analysis showed that UA significantly reduced infarct size (SMD: −1.18; 95% CI [−1.47, −0.88]; p < 0.001), blood-brain barrier (BBB) impairment/oedema (SMD: −0.72; 95% CI [−0.97, −0.48]; p < 0.001) and neurofunctional deficit (SMD: −0.98; 95% CI [−1.32, −0.63]; p < 0.001). Overall, there was low to moderate between-study heterogeneity and sizeable publication bias. In conclusion, published rodent data suggest that UA improves outcome following ischemic stroke by reducing infarct size, improving BBB integrity and ameliorating neurofunctional condition. Specific recommendations are given for further high-quality preclinical research required to better inform clinical research.
Keywords: ischemic stroke, uric acid, animal model, brain damage, neurofunctional deficit
Introduction
Stroke remains as a leading cause of death, permanent disability and dementia worldwide. Although age-standardised mortality rates have decreased sharply from 1990 to 2016, the decrease in age-standardised incidence has been less steep, indicating that the burden of stroke is likely to remain high.1 Major advances in the treatment of acute ischemic stroke came from vascular approaches to dissolve (thrombolysis with recombinant tissue plasminogen activator [rt-PA]) or remove (endovascular thrombectomy) the occluding clot. However, only a minority of patients benefit from such treatments.2 Although reperfusion therapies are therefore restricted, they are more clinically beneficial than neuroprotective strategies, which have failed in the translation from bench to bedside.3 Although the track report of therapeutic approaches in stroke is very poor, neuroprotectants should be revisited in the era of recanalization with thrombectomy since reperfusion would be more beneficial when carried out along with delivery of neuroprotectants.4
Uric acid (UA) is the end product of purine metabolism in humans and a major endogenous antioxidant in blood.5 Moreover, systemic infusion of high UA doses was safe and increased serum antioxidant capacity in healthy humans.6 Mice heterozygous for a disrupted urate oxidase transgene, with elevated serum UA levels, showed reduced brain damage and improved functional outcome after ischemic stroke.7 With regard to ischemic stroke patients, serum UA level at stroke onset is a predictor biomarker of better prognosis, thus pointing to a protective effect on neurological outcome,8 confirmed by systematic reviews with meta-analyses.9,10 Moreover, a pilot study showed that the administration of UA was safe, decreased oxidative stress markers and prevented an early fall of serum UA in stroke patients treated with rt-PA.11,12 The moderately sized URICO–ICTUS stroke trial showed that the addition of UA to thrombolytic therapy resulted in an increase in the rate of excellent outcome at 90 days compared to placebo (adjusted risk ratio 1.23, 95% CI [0.96, 1.56], p = 0.099), with no safety concerns.13
It is more than 20 years ago that Yu and colleagues first described the effects of UA to protect neurons against excitotoxic and metabolic insults in cell culture and against focal ischemic brain injury in vivo.14 This and other subsequent studies have been used to claim the protective effect of UA in acute ischemic stroke at the preclinical level.15 However, despite the importance of high-quality preclinical evidence to attempt to translate the concept of UA neuroprotection to patients with acute stroke, there is no published study or registered protocol of a systematic review and meta-analysis of preclinical studies showing the neuroprotective effects of UA in animal models of ischemic stroke. In the present systematic review, we set out to describe which UA interventions have been tested in ischemic stroke models of disease and the range of conditions under which UA efficacy has been tested. Furthermore, pooling aggregate data using meta-analysis will assess the overall efficacy of UA, the impact of factors relating to internal and external validity and the possible publication bias, thus giving valuable insights for translational success. Our systematic review and meta-analysis aim to provide empirical evidence to improve the rigor of the conduct and reporting of ongoing and future preclinical research on UA treatment for ischemic stroke, akin to their use in improving the conduct and reporting of UA randomised controlled trials in clinical research.
Research question. What is the effect of UA on brain structural and functional outcomes in animal models for ischemic stroke?
Methods
All methods were prespecified in a systematic review protocol for animal intervention studies (format by SYRCLE, http://www.syrcle.nl)16 that was registered and published online (12 February 2020) on the CAMARADES Preclinical Systematic Review & Meta-analysis Facility (SyRF, http://syrf.org.uk/protocols/), and which can be accessed at (https://drive.google.com/file/d/11cmtQvgc0mPAdSKeiVrQ4QgArvKdwOdU/view). The methodological approach followed specific guidelines for meta-analysis of data from animal studies.17
Literature search strategy
The search process was carried out according to the guidelines to systematically identify all relevant animal studies.18 Two reviewers (AAV and JBM) identified studies on UA in animal models of ischemic stroke from electronic searches of Medline (using PubMed interface), Embase and Web of Science, up to and including February 2020. Search syntax was (uric acid OR UA) AND (stroke OR ischaemia OR MCAO) AND (rat OR rats OR mouse OR mice OR rodent* OR rabbit*) AND (infarct* OR neurofunct* OR neurologic* OR oedema OR damage OR injury) AND (brain OR cerebral). Search fields were [Title/Abstract] in Medline (PubMed) and Embase, and [Topic] in Web of Science. There were no language restrictions. One reviewer identified duplicated/triplicated references (JBM). Results were screened independently by title and abstract by two reviewers, using predefined inclusion/exclusion criteria (AAV and JBM). Two reviewers (MCR and GT) then screened full texts of included articles independently. Other sources for study identification were the reference lists of included articles and relevant reviews.9,10,15,19 Discrepancies were resolved through discussion with the supervisor (JBS).
Study selection: Inclusion and exclusion criteria
With regard to study design, primary experimental studies with control group (receiving no treatment, vehicle, or other sham intervention), regardless of randomisation, were included. Preclinical studies in rats, mice, rabbits or other laboratory animal species used in models of ischemic stroke, regardless of age and sex, were included. UA-based interventions were included: UA (or soluble UA analogue) administration, alone or in co-treatment with medications commonly used in stroke patients (i.e. rtPA, statins, blood pressure-lowering medication, aspirin, etc.), regardless of dose, route, method and treatment schedule or other interventions (e.g. genetic modification) raising endogenous UA levels. Finally, studies analysing brain damage (i.e. infarct size, blood-brain barrier (BBB) impairment/brain oedema and/or neurofunctional outcomes) were included.
Observational studies, experimental studies without control group and reviews, were excluded. In vitro studies (i.e. cell cultures), ex vivo studies (i.e. brain slices) and studies in animal models of global cerebral ischaemia or haemorrhagic stroke, were excluded. Finally, studies reporting results without individual or aggregate data, or no statement of sample size, were excluded.
Data extraction
Two reviewers (MCR and GT) independently extracted study ID (first author, year, journal name and DOI), study characteristics for the assessment of external validity and study quality data for the assessment of internal validity.
Study characteristics included experimental groups and sample sizes, animal model (species, strain, gender, age, co-morbidities, type of ischemic stroke, duration of ischaemia and anaesthetic used), intervention (type of intervention, UA dosage, route, method and treatment schedule, co-treatment or other interventions raising endogenous UA levels), outcome measures, time points and methods of assessment (infarct size, BBB impairment/brain oedema and neurofunctional deficit).
Range of evidence was assessed by using a 13-item checklist,20 taken from the updated STAIR criteria21: (1) evidence from two or more laboratories, (2) from two or more species, (3) from animals with co-morbidities, (4) from male and female animals, (5) from both permanent and temporary models of ischaemia, (6) testing at least two doses of the drug, (7) with some doses given at least 1 h after vessel occlusion, (8) testing using a feasible route of drug delivery, (9) use of both histologic and behavioural outcomes, (10) outcome measured at least four weeks after vessel occlusion, (11) from species other than rodents, (12) interaction studies with medications commonly used in stroke patients and (13) use of relevant biomarker endpoints.
Study quality was scored to assess risk of bias by using both the former 10-item CAMARADES’ checklist,22 and an adapted checklist20 which included up to 15 relevant items from the updated STAIR criteria21: (1) peer-reviewed publication, (2) control of temperature, (3) randomisation of group allocation, (4) blinded induction of ischaemia, (5) blinded assessment of outcome, (6) avoidance of anaesthetics with marked intrinsic neuroprotective properties, (7) use of animals with co-morbidities (e.g. hypertension, diabetes), (8) sample size calculation, (9) statement of compliance with animal welfare requirements, (10) statement of potential conflicts of interest, (11) physiological monitoring during stroke induction (in addition to control of temperature, e.g. blood pressure, gases), (12) prespecified inclusion and exclusion criteria, (13) reporting of animals excluded from analysis, (14) reporting of study funding and (15) injury confirmed via laser Doppler or perfusion imaging.
Two unblinded reviewers (JBS and DH) independently collected outcome data. Infarct size (continuous variable, expressed in absolute or percent units) was considered as the primary outcome measure to stablish whether UA might have neuroprotective effects. Secondary outcome measures were BBB impairment/brain oedema (continuous variable, expressed in absolute or percent units), and neurofunctional outcomes (continuous, expressed in absolute units, or ordinal, in absolute units within the score range). For each outcome measure, the number of animals in which this was assessed, the aggregate value of effect (i.e. mean or median) and a measure of group variance were extracted. Where outcomes from the same group of animals were reported at different time points, only the last time point was extracted. Data were extracted from text and tables. When only graphic presentation was available, data were obtained by using WebPlotDigitizer 4.2 (https://apps.automeris.io/wpd/) on highly magnified images. Information was also requested directly from authors for checking extracted data or if it was unavailable in the publication.
Analysis
For infarct, BBB impairment/brain oedema and neurofunctional outcomes, we calculated a standardised mean difference (SMD) as effect size for each comparison. Since only one study reported two different measures of neurofunctional outcome (continuous variable and ordinal scale) from the same cohort of animals at the same time point, the ordinal score used in all the studies (instead of a combined summary estimate) was included in the analysis. For each outcome, comparisons were combined using random-effects modelling to account for heterogeneity, with a restricted maximum likelihood (REML) estimate of between-study variance, and results displayed in forest plots. The studies included in the analysis did not report multiple comparison experiments, with a control group serving more than one treatment group. Therefore, there was no need for adjusting the number of control animals (i.e. by division by the number of treatment groups served). Heterogeneity was described using Cochran’s Q (heterogeneity statistic), tau2 (estimation of between-study variance) and I2 (the percentage of the residual variation that is attributable to between-study heterogeneity). We used meta-regression to assess study quality score as a source of heterogeneity. When possible, the impact of individual study characteristics and study quality items on estimated effect sizes was also assessed by performing subgroup stratified meta-analysis. Bonferroni correction was used to account for the number of parameters tested within each of the two domains considered for stratifications: study characteristics and quality. Moreover, sensitivity analyses were also performed by including only expectedly homogeneous studies (carried out in male animals, without co-morbidities) in the random effects models. In the case of neurofunctional outcome, an additional sensitivity analysis was performed by using the medians instead of the means.23 We tested for the presence and extent of publication bias using funnel plots. Adjusted effect sizes were then estimated by incorporating theoretically missing studies using the trim and fill approach.24 Association between the effect sizes on infarct and neurofunctional score for studies reporting both outcomes was assessed using weighted Spearman’s correlation. Statistical analyses were performed using R (version 4.0) and R packages clickR (version 0.47), meta (version 4.12-0), dmetar (version 0.0.9), metamedian (version 0.1.5) and wCorr (version 1.9.1). All estimates included a 95% confidence interval (CI) and p values < 0.05 were considered statistically significant.
Results
Literature search
A total of 238 records were identified electronically in Medline, Embase and Web of Science databases. A detailed study selection flow chart is shown in Figure 1. Briefly, after removing duplicate and not relevant records, 18 full-text articles and four congress abstracts were assessed for eligibility. No additional record was identified in the reference lists of these articles and other relevant review articles. Subsequent reasoned exclusions rendered 16 studies included in the qualitative synthesis,7,14,25–38 and 14 studies subjected to meta-analysis.7,14,25–28,30–33,35–38 Analyses of the three outcomes were based on 19 comparisons reporting infarct size, 8 comparisons reporting BBB impairment or oedema and 14 comparisons reporting neurofunctional score.
Figure 1.
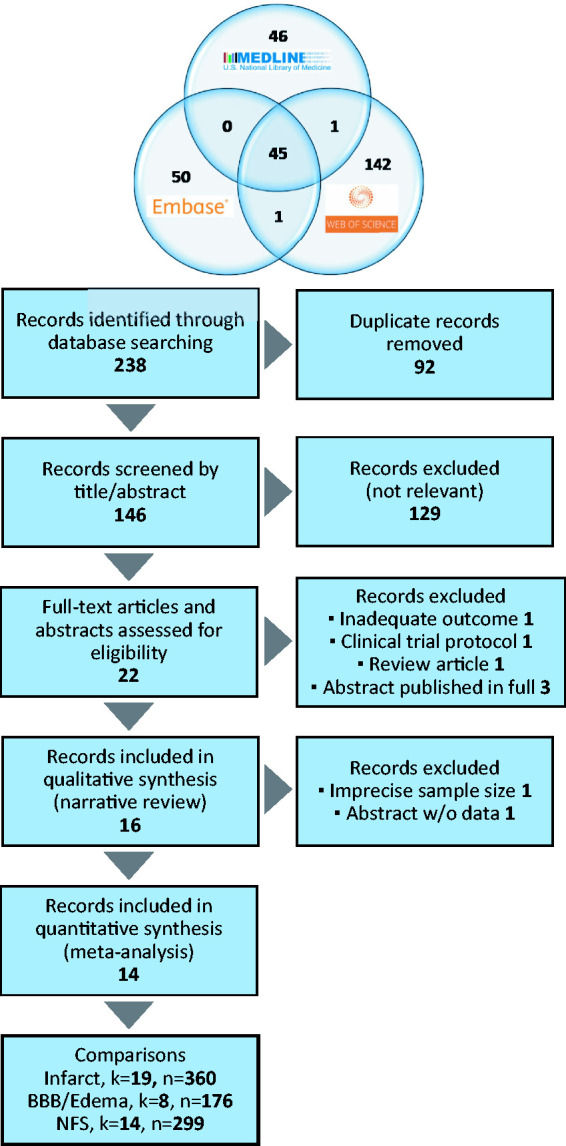
Flow diagram to depict the processes for literature search in three databases and subsequent study selection for qualitative and quantitative synthesis. K: number of comparisons; n: number of animals.
Study characteristics
A summary of the study characteristics is shown in Table 1. Five studies used C57BL/6 mice and 13 studies used four different rat strains, including two of them carried out in SHR rats. Most of the studies used adult male animals, except for two studies using females. Intraluminal filament transient middle cerebral artery (MCA) occlusion of different durations (45–120 min) was the most used ischaemia method, although in two studies thromboembolism was used instead. One study performed permanent MCA occlusion, and the ischaemia method was not specified in other, although both studies were excluded from further analysis because imprecise sample sizes and only qualitative results were reported, respectively.29,34 For the two excluded studies, we sought further information from the authors, but this was not forthcoming. Intrinsically neuroprotective anaesthetics were used almost twice than neutral ones. The most frequent intervention was intravenous UA administration, at 16 mg/kg, during or after the ischemic insult, although higher doses and intraperitoneal administration were also reported. Two studies carried out interventions raising endogenous UA levels.7,33 One study using soluble UA analogues was the one excluded because of imprecise sample sizes. UA was administered together with alteplase (rtPA) in the two studies inducing thromboembolic ischaemia. Infarct size was measured in all the studies, neurofunctional deficit in 12 studies and BBB impairment/oedema only in 5 studies. Most of the studies (10 out of 16) assessed outcomes at 24 h, but shorter and longer outcome times (up to two weeks) were also reported in two and four studies, respectively.
Table 1.
Study characteristics summary.
| Variable | n (%) |
|---|---|
| Species and strain | |
| C57BL/6 mouse | 5 (31.25) |
| Sprague–Dawley rat | 8 (50) |
| Wistar rat | 1 (6.25) |
| WKY rat | 2 (12.5) |
| SHR rat | 2 (12.5) |
| Gender | |
| Male | 15 (93.75) |
| Female | 2 (12.5) |
| Age | |
| Adult | 16 (100) |
| Aged | 0 (0) |
| Co-morbidity | |
| Hyperglycaemia | 1 (6.25) |
| Hypertension | 2 (12.5) |
| None | 15 (93.75) |
| Ischaemia method and timing | |
| Thromboembolism | 2 (12.5) |
| Intraluminal filament, 45 min | 2 (12.5) |
| Intraluminal filament, 60 min | 3 (18.75) |
| Intraluminal filament, 90 min | 4 (25) |
| Intraluminal filament, 120 min | 4 (25) |
| Permanent electrocoagulation | 1 (6.25) |
| Unknown | 1 (6.25) |
| Anaesthetic | |
| Neuroprotective | 9 (56.25) |
| Neutral | 5 (31.25) |
| Unknown | 2 (12.5) |
| UA intervention | |
| Genetic hyperuricemia | 1 (6.25) |
| IPC-induced brain UA increase | 1 (6.25) |
| Intraperitoneal UA (62.5 mg/kg), pre-ischaemia | 1 (6.25) |
| Intraperitoneal UA (62.5 mg/kg/d), 7 d post-ischaemia | 1 (6.25) |
| Intraperitoneal UA (93.5 mg/kg/d), 7 d post-ischaemia | 1 (6.25) |
| Intravenous UA (16 mg/kg), intra-ischaemia | 3 (18.75) |
| Intravenous UA (16 mg/kg), post-ischaemia | 9 (56.25) |
| Intravenous UA analogue (20 mg/kg), pre-ischaemia | 1 (6.25) |
| Intravenous UA analogue (20 mg/kg), post-ischaemia | 1 (6.25) |
| Co-treatment | |
| Alteplase (rtPA) | 2 (12.5) |
| None | 14 (87.5) |
| Outcome measure | |
| Infarct | 16 (100) |
| BBB impairment/oedema | 5 (31.25) |
| Neurofunctional deficit | 12 (75) |
| Outcome assessment time | |
| <24 h | 2 (12.5) |
| 24 h | 10 (62.5) |
| >24 h | 4 (25) |
UA: uric acid.
Range of evidence
The range of evidence met ten of a possible 13 updated STAIR criteria assessed. Fourteen studies chosen for meta-analysis came from 10 laboratories in five countries (China, Israel, Japan, Spain and USA). They used two rodent species (mostly different rat strains, but also mice). In addition to adult male healthy animals, a few studies used female animals31,32 and animals with co-morbidities like hypertension27,35 or hyperglycaemia,36 although aged animals were not used. Two UA doses were tested, given up to 180 min after ischaemia onset (60 min after reperfusion), and using feasible routes of drug delivery (mostly intravenous, but also intraperitoneal). Both structural and functional outcomes were measured, and relevant biomarker endpoints were also used in some cases, such as MRI assessment of brain injury,32,35,36 as well as serum/plasma biomarkers of oxidative stress,25,35 inflammatory response35 and angiogenesis.27 UA was tested in temporary models of ischaemia, mostly intraluminal filament MCAO, but also thromboembolism,26,37 and the only study in permanent ischaemia was excluded because of imprecise sample sizes.34 Evidence is also still lacking for outcome measurement beyond 14 days, and in species other than rodents. Finally, although UA has been studied in animals co-treated with alteplase (rtPA),26,37 no interaction studies with other medications commonly used by stroke patients such as statins, blood pressure-lowering medication and aspirin were identified.
Study quality
Table 2 shows methodological quality for each of the 14 studies included in the meta-analysis. Overall, the median (Q1, Q3) quality score was 5 (4.25, 7) on the 10-item checklist, and 7.5 (6, 10) on the extended 15-item checklist. Of note, eight studies (57.14%) reported randomisation of group allocation, but only three studies (21.43%) reported allocation concealment, although seven studies (50%) reported blinded assessment of outcome. Only five studies (35.71%) avoided the use of anaesthetics with neuroprotective properties, and animals with co-morbidities were used in three studies (21.43%). A few four studies (28.57%) reported sample size calculation. Prespecified inclusion and exclusion criteria were stated in seven studies (50%), and six of them (42.86%) reported the number of animals excluded from analysis. Finally, brain injury was confirmed via laser Doppler or perfusion imaging in seven studies (50%), and only four studies (28.57%) carried out any systemic physiological monitoring during stroke induction.
Table 2.
Study quality report.
| Author | Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | QS (0–10) | 11 | 12 | 13 | 14 | 15 | QS (0–15) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliena-Valero | 2018 | + | + | + | + | + | + | + | + | 8 | + | + | + | + | + | 13 | ||
| Cutler | 2019 | + | + | + | 3 | + | + | + | 6 | |||||||||
| Dhanesha | 2018 | + | + | + | + | + | + | 6 | + | + | + | + | 10 | |||||
| Glantz | 2005 | + | + | + | + | 4 | + | 5 | ||||||||||
| Jiménez-Xarrié | 2018 | + | + | + | + | + | + | + | + | 8 | + | + | + | + | 12 | |||
| Justicia | 2017 | + | + | + | + | + | + | + | + | 8 | + | 9 | ||||||
| Kikuchi | 2018 | + | + | + | + | + | 5 | + | 6 | |||||||||
| Ma | 2012 | + | + | + | + | + | 5 | + | + | 7 | ||||||||
| Onetti | 2015 | + | + | + | + | + | 5 | + | + | + | + | + | 10 | |||||
| Romanos | 2007 | + | + | + | 3 | + | + | + | + | 7 | ||||||||
| Vila | 2019 | + | + | + | + | + | + | + | 7 | + | + | + | + | 11 | ||||
| Ya | 2018 | + | + | + | + | + | + | + | 7 | + | 8 | |||||||
| Yu | 1998 | + | + | + | + | 4 | + | 5 | ||||||||||
| Zhang | 2017 | + | + | + | + | + | 5 | + | 6 |
Study quality (SQ) items are (1) peer-reviewed publication, (2) control of temperature, (3) randomisation of group allocation, (4) blinded induction of ischaemia, (5) blinded assessment of outcome, (6) avoidance of anaesthetics with marked intrinsic neuroprotective properties, (7) use of animals with co-morbidities (e.g. hypertension, diabetes), (8) sample size calculation, (9) statement of compliance with animal welfare requirements, (10) statement of potential conflicts of interest, (11) physiological monitoring during stroke induction (in addition to control of temperature, e.g. blood pressure, gases), (12) prespecified inclusion and exclusion criteria, (13) reporting of animals excluded from analysis, (14) reporting of study funding and (15) injury confirmed via laser Doppler or perfusion imaging.
Effect of UA on infarct size
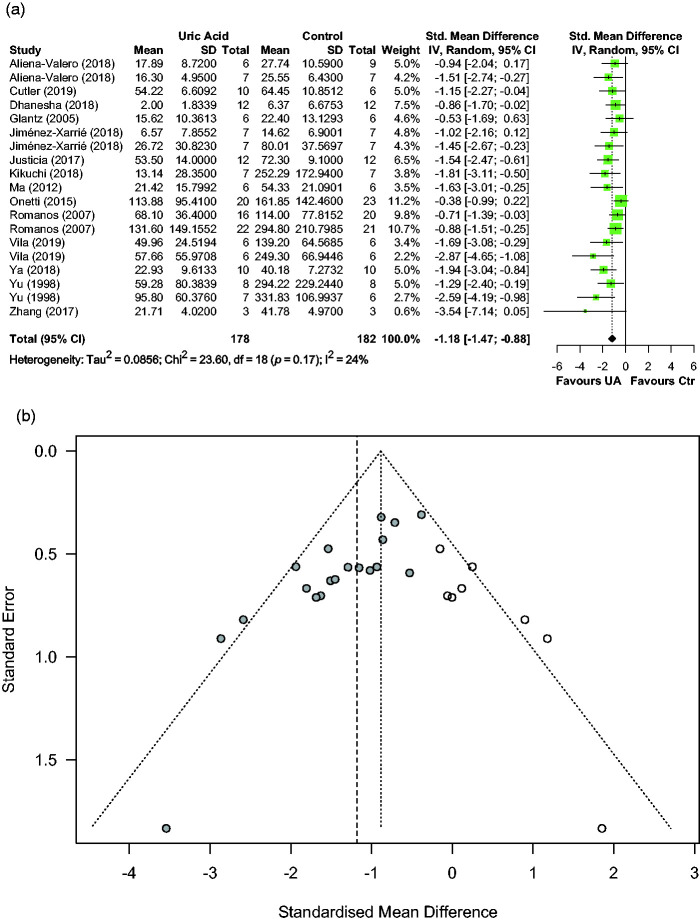
Effects of UA on infarct size were reported in fourteen articles describing 19 comparisons involving 360 animals. Median (Q1, Q3) sample sizes per comparison were 7 (6, 11) in the control group and 7 (6, 11) in the UA group. Figure 2(a) shows individual effect sizes for each of the nineteen comparisons. Overall, UA significantly reduced infarct, with a pooled SMD estimate of −1.18 (95% CI [−1.47, −0.88], p < 0.001), and with low between-study heterogeneity in the estimates (Q = 23.60, p = 0.17, I2 = 23.7%).
Figure 2.
Effect of UA on infarct size: (a) forest plot and between-study heterogeneity and (b) funnel plot showing published studies (filled circles), trim-and-fill assigned studies (open circles), and the pooled estimate of efficacy before adjusting for publication bias (dashed vertical line).
Median (Q1, Q3) quality score in the studies reporting infarct size was 7.5 (6, 10) of a possible 15. Effect of quality score as moderator of UA effect size was negligible (0.01, 95% CI [−0.11, 0.13], p = 0.86). A sizeable asymmetry was detected in the funnel plot, suggesting the presence of publication bias. Therefore, trim and fill analysis was used to impute the presence of eight missing experiments (Figure 2(b)). The resulting adjusted effect size for infarct reduction was -0.89 (95% CI [−1.25, −0.53], p < 0.001). Sensitivity analysis including only 11 homogeneous studies (12 comparisons) in the model rendered a pooled SMD estimate of −1.13 (95% CI [−1.54, −0.71], p < 0.001).
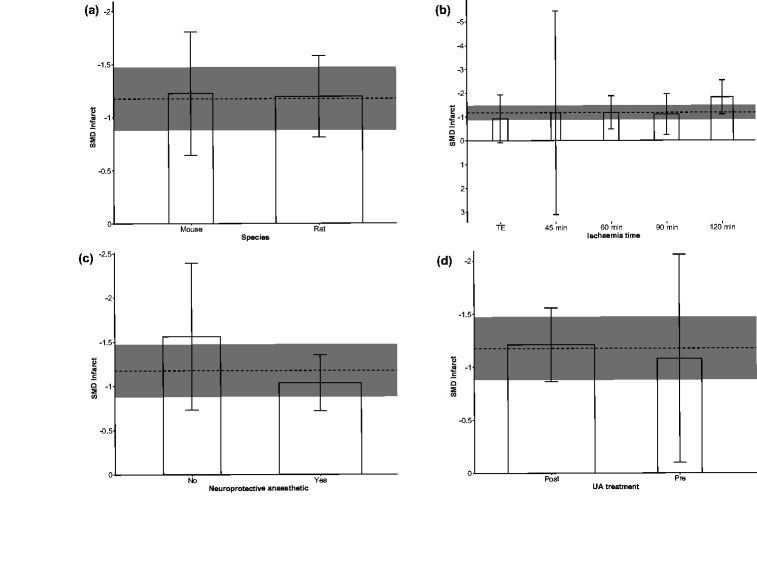
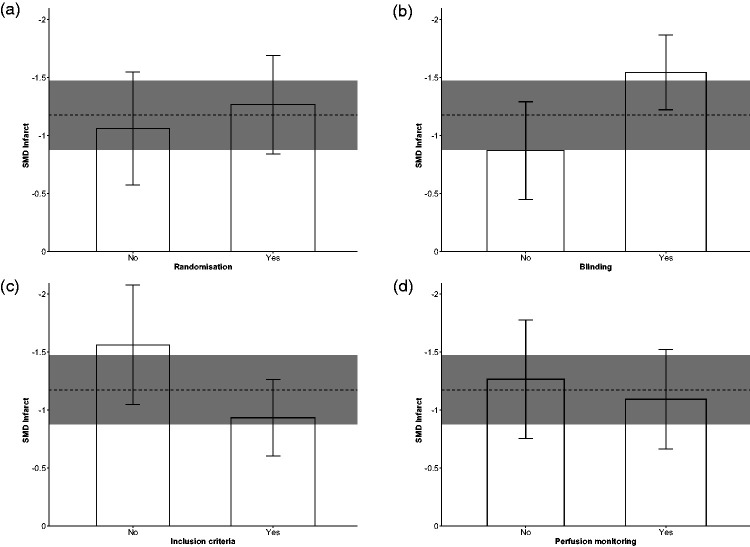
In the performed subgroup meta-analyses stratifying by study characteristics (Figure 3), none of the four considered features (species, ischaemia method/time, avoidance of neuroprotective anaesthetic and UA treatment timing) showed statistically significant effects on the pooled SMD estimate for UA-induced infarct reduction (Bonferroni adjusted p = 1.00, p = 0.52, p = 0.56 and p = 1.00, respectively). With regard to stratification in the domain of study quality (Figure 4), randomisation to treatment, prespecified inclusion/exclusion criteria and cerebral perfusion monitoring did not show statistically significant effects (p = 1.00, p = 0.068 and p = 1.00, respectively). Only studies reporting blinded assessment of infarct showed statistically significant higher UA effect size (p = 0.014) when compared to studies not reporting this quality item.
Figure 3.
Subgroup stratified meta-analysis of the effect of UA on infarct size, in the domain of study characteristics: effects of (a) species, (b) ischaemia duration, (c) use of neuroprotective anaesthetic and (d) UA treatment timing, on UA effect size. Data are effect size and 95% confidence interval (CI). The horizontal gray bar represents the 95% CI of the pooled estimate of efficacy. TE: thromboembolism; Pre: pre-ischaemic; Post: post-ischaemic.
Figure 4.
Subgroup stratified meta-analysis of the effect of UA on infarct size, in the domain of study quality: effects of (a) randomisation to treatment group, (b) blinding outcome assessment, (c) prespecified inclusion/exclusion criteria and (d) brain perfusion monitoring, on UA effect size. Data are effect size and 95% confidence interval (CI). The horizontal gray bar represents the 95% CI of the pooled estimate of efficacy.
Effect of UA on BBB impairment or oedema
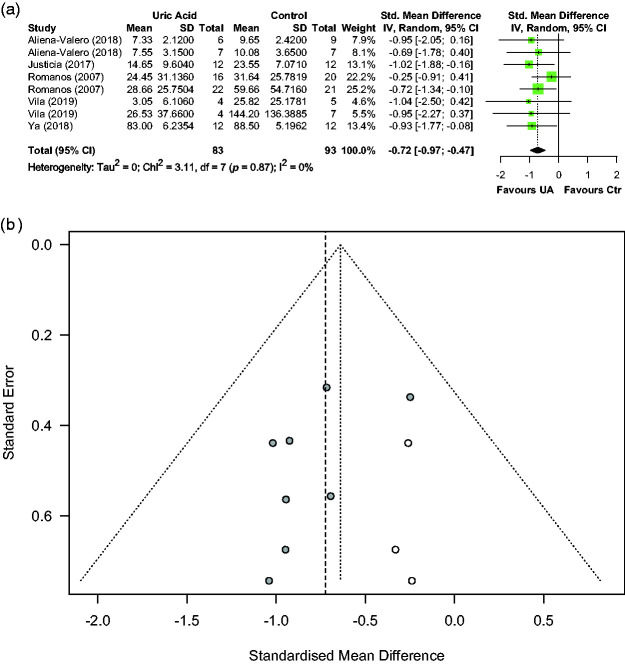
Effects of UA on BBB impairment or oedema were reported in five articles describing eight comparisons involving 176 animals. Median (Q1, Q3) sample sizes per comparison were 10.5 (7, 14) in the control group and 9.5 (5.5, 13) in the UA group. Figure 5(a) shows individual effect sizes for each of the eight comparisons. Overall, UA significantly reduced BBB impairment or oedema, with a pooled SMD estimate of −0.72 (95% CI [−0.97, −0.48], p < 0.001), and with trivial between-study heterogeneity in the estimates (Q = 3.11, p = 0.88, I2 = 0.00%). Median (Q1, Q3) quality score in the studies reporting BBB impairment or oedema was 9 (8, 11) of a possible 15. Effect of quality score as moderator of UA effect size was not statistically significant (−0.06, 95% CI [−0.17, 0.05], p = 0.22). A sizeable asymmetry was detected in the funnel plot, suggesting the presence of publication bias. Therefore, trim and fill analysis was used to impute the presence of three missing experiments (Figure 5(b)). The resulting adjusted effect size for protection of BBB impairment/oedema was −0.64 (95% CI [−0.86, −0.42], p < 0.001). Sensitivity analysis including only four homogeneous studies (four comparisons) in the model rendered a pooled SMD estimate of −0.84 (95% CI [−1.06, −0.61], p < 0.005).
Figure 5.
Effect of UA on BBB impairment/oedema: (a) forest plot and between-study heterogeneity and (b) funnel plot showing published studies (filled circles), trim-and-fill assigned studies (open circles), and the pooled estimate of efficacy before adjusting for publication bias (dashed vertical line).
Effect of UA on neurofunctional deficit
Effects of UA on neurofunctional deficit were reported in nine articles describing 14 comparisons involving 299 animals. Median (Q1, Q3) sample sizes per comparison were 7 (6.25, 15) in the control group and 7.5 (7, 12) in the UA group. Figure 6(a) shows individual effect sizes for each of the 14 comparisons. Overall, UA significantly reduced neurofunctional deficit, with a pooled SMD estimate of −0.98 (95% CI [−1.32, −0.63], p < 0.001), and with moderate between-study heterogeneity in the estimates (Q = 22.06, p = 0.054, I2 = 41.1%). Median (Q1, Q3) quality score in the studies reporting neurofunctional deficit was 10 (6, 11) of a possible 15. Effect of quality score as moderator of UA effect size was not statistically significant (−0.05, 95% CI [−0.18, 0.08], p = 0.39). A sizeable asymmetry was detected in the funnel plot, suggesting the presence of publication bias. Therefore, trim and fill analysis was used to impute the presence of four missing experiments (Figure 6(b)). The resulting adjusted effect size for neurofunctional deficit reduction was −0.78 (95% CI [−1.12, −0.43], p < 0.001). Sensitivity analysis including only seven homogeneous studies (eight comparisons) in the model rendered a pooled SMD estimate of −1.04 (95% CI [−1.43, −0.66], p < 0.001). An additional sensitivity analysis was performed by using the medians instead of the means, which were reported in five articles describing eight comparisons. The pooled estimate for the difference between the medians was −0.88 (95% CI [−1.17, −0.58], p < 0.001) (Figure 6(c)).
Figure 6.
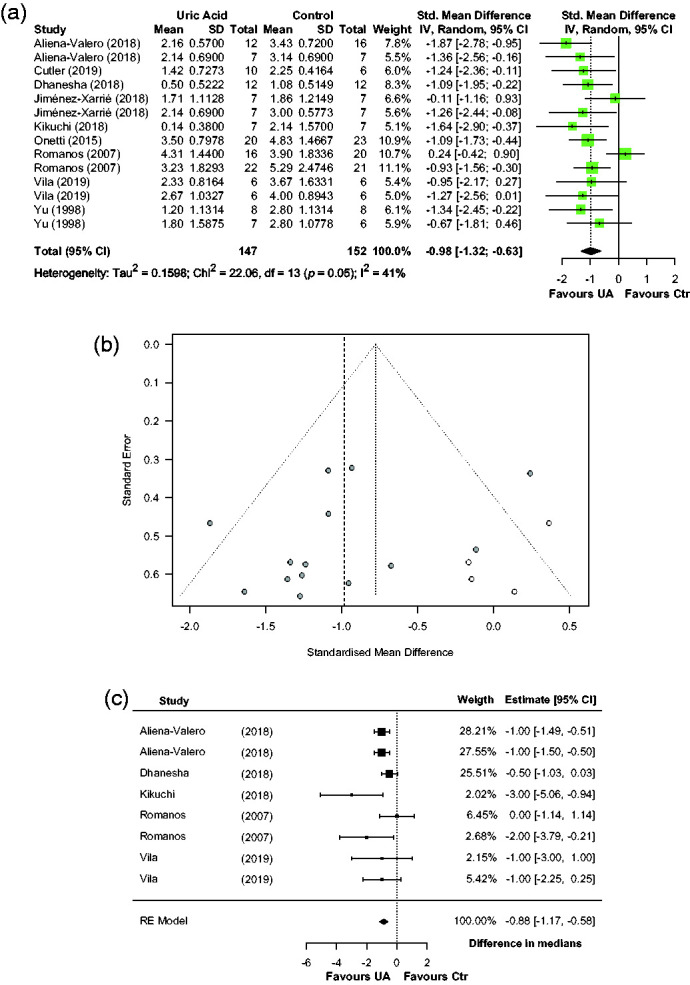
Effect of UA on neurofunctional deficit: (a) forest plot and between-study heterogeneity; (b) funnel plot showing published studies (filled circles), trim-and-fill assigned studies (open circles), and the pooled estimate of efficacy before adjusting for publication bias (dashed vertical line) and (c) forest plot from sensitivity analysis using studies reporting medians for neurofunctional score.
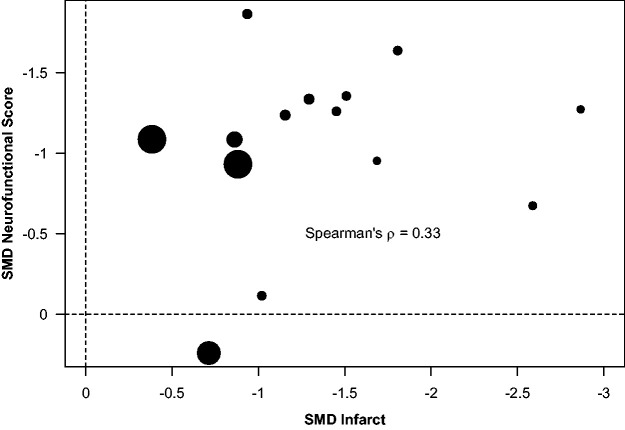
Association between infarct and neurofunctional score
We assessed the association between the effect sizes of UA for infarct and neurofunctional deficit reduction using weighted Spearman’s correlation. Results showed a moderate, although not significant, correlation between both effects (ρ = 0.33, 95% CI [−0.27, 0.84], p = 0.16) (Figure 7).
Figure 7.
Association between the effect sizes of UA for infarct and neurofunctional deficit reduction, using weighted Spearman’s correlation.
Discussion
In response to the research question stated for the present study, the results of the systematic review and meta-analysis support a beneficial effect of UA on brain structural and functional outcomes in animal models for ischemic stroke. Neuroprotective efficacy of UA was observed in terms of infarct reduction, BBB impairment/oedema reduction and neurofunctional improvement. Moreover, the meta-analysis included the assessment of the risk of bias that could impact on our encouraging estimates of pooled effect sizes.
Infarct size, which we considered as the primary outcome measure to stablish whether UA might have neuroprotective effects, was reported in the 14 studies analysed. Overall, UA reduced infarct with the highest pooled SMD estimate and with low between-study heterogeneity in the estimates. Secondary outcome measures were BBB impairment/brain oedema and neurofunctional deficit. Brain oedema, of both cytotoxic and vasogenic origins, is a leading cause of death after stroke.39 Vasogenic oedema and eventual haemorrhagic transformation are particularly relevant as part of the post-ischemic reperfusion injury contributing to brain damage and stroke outcome.40 Our results show that UA also reduced BBB impairment or oedema volume, two pathophysiologically related events, in rodents subjected to transient focal cerebral ischaemia. Of note, only five articles reported the effects of UA on BBB impairment or oedema, which were jointly analysed, with trivial between-study heterogeneity in the estimates. Finally, meta-analysis of neurofunctional outcome measures retrieved from nine articles showed that UA reduced functional impairment, with moderate between-study heterogeneity in the estimates. Sensitivity analysis performed by using the medians instead of the means, which were reported in five articles, also resulted in a pooled estimate of effect size favouring UA.
Altogether, UA rendered beneficial effects on the three analysed stroke outcomes, although a lower number of studies reporting neurofunctional outcome and a relatively higher between-study heterogeneity make this evidence less robust, when compared to the effect on infarct. Moreover, there was a modest correlation between the effect sizes of UA for infarct and neurofunctional deficit reduction, thus showing some inconsistency of improvement by UA as determined by these two outcome measures. The structural brain damage observed in rats subjected to ischemic stroke is highly correlated to deficiencies observed in composite neurologic assessments.41 In contrast, our results show that the structural–functional correlation is not so evident regarding the UA effect sizes, thus indicating that functional improvement cannot be taken for granted in the light of structural benefit. However, it should be noted that only easy to perform, but subjective in nature, composite tests were used in the nine articles subjected to correlation analysis, despite the available diversity of motor, sensory and cognitive tests.42
Our data set included results from 14 studies describing 19 comparisons involving 360 animals for infarct size, the primary outcome measure. Median sample sizes per comparison were seven animals in both control and experimental groups of primary studies. Beyond trying to reach as much statistical power as possible, there is no rule of thumb about the number of studies required for a meta-analysis.43 The present study included a number of primary articles slightly lower but comparable to the average number of studies included in other nine meta-analyses assessing the efficacy of one specific neuroprotective drug candidate in animal models of ischemic stroke (median [Q1, Q3] = 17 [10, 18.5] studies),44–52 and expectedly around half the number of studies included in meta-analyses assessing the efficacy of less specific interventions (e.g. a group of pharmacodynamically related drugs; median [Q1, Q3] = 34.5 [24.5, 44.75] studies).53–60 With regard to study quality, there is no unique consensus checklist to score it. Most authors used the 10-item CAMARADES’ checklist22 (in some cases with 9 or 11 items),46,50,51 but an extended checklist, which included up to 15 relevant items from the updated STAIR criteria,21 has been proposed and used just once, as far as we know.20 Therefore, we calculated study quality scores according to both the former and the extended checklists. In both cases, the aggregate score of the studies included in the current meta-analysis, although moderate (5 of a possible 10, and 7.5 of a possible 15), is similar to the highest aggregate scores reported in a quite representative sample of previous meta-analyses of preclinical single-drug stroke studies.20,44–52
In the present review, the aggregate study quality score for all the 14 studies reporting infarct size (7.5 of a possible 15) increased in the subsets of five studies reporting BBB impairment or oedema (9/15) and of nine studies reporting neurofunctional deficit (10/15). We did not find any relationship between study quality score and UA efficacy, regardless of the outcome measure considered. The possible impact of overall quality score on estimated effect sizes of drugs/interventions is the subject of ongoing research. Previous individual meta-analyses on neuroprotectant interventions looking at the difference in effect size (infarct or neurofunctional outcomes) according to the study quality score found varying results: a decreased effect with increasing quality,45,46,48,61,62 no clear relationship44,51,52,63,64 and even an increased effect with higher quality.49,56,59,65 Quite interestingly, a higher power meta-analysis of meta-analyses, analysing and pooling several different therapies instead of looking at only one single intervention, found no apparent difference in treatment efficacy between high- and low-quality studies.66
Most probably, not all items of the CAMARADES study quality checklist are of equal importance. The interpretation of quality data on the basis of an ordinal scale with the same weight attributed to different aspects of methodologic quality is a simplistic view of the complex entity of study quality.66 Therefore, we also assessed the impact of individual items on UA efficacy. To avoid underpowered multiple comparison with the available data set, stratified meta-analysis was carried out only for selected study characteristics (species, ischaemia method/time, avoidance of neuroprotective anaesthetic and UA treatment timing) and quality items (randomisation to treatment, prespecified inclusion/exclusion criteria, blinded assessment of outcome and cerebral perfusion monitoring). In these selected items, the strata were balanced in number of studies, and only the UA effect size in the improvement of infarct outcome, reported in all the studies, was subgroup analysed. This subgroup analysis showed that only blinded infarct assessment had a positive impact on UA effect size. In line with our results, efficacy of exercise in reducing infarct volume was also higher in studies that reported the blinded assessment of outcome.58 In contrast, other meta-analyses of erythropoietin and edaravone in experimental stroke showed lower efficacy in studies where blinded assessment was reported.46,48 Since semiautomated measurement techniques are used, infarct size seems to be a relatively robust and objective measure of outcome less prone to observer bias. In fact, a metaepidemiologic study showed no significant impact of randomisation and the blinded assessment of outcome on the effectiveness of interventions in experimental stroke studies, whereas important roles for both allocation concealment and the use of animals with relevant co-morbidities were empirically evidenced.66 Although we could not include blinded induction of ischaemia and co-morbidities in the stratified meta-analysis, a sensitivity analysis showed that excluding studies in female and co-morbid animals does not substantially affect UA efficacy. Finally, avoidance of anaesthetics with marked intrinsic neuroprotective properties did not impact on UA efficacy. Quite interestingly, a recent meta-analysis showed that most anaesthetics will have some neuroprotective effect in stroke models. This makes ‘avoidance of anaesthetics with intrinsic neuroprotective effects’ difficult or impossible.59 Instead, it is recommended that novel treatment strategies should be tested under a range of anaesthetic conditions.22
Sizeable asymmetries were observed in the funnel plots for the UA effect sizes in all the three outcome measures, suggesting the presence of publication bias. In fact, trim and fill analysis imputed the presence of missing experiments, which led to downward adjusted effect sizes. Despite searching for relevant studies in three major electronic databases, we cannot rule out the possibility of missing studies because they were published in less well-known non-indexed journals or not published at all. Research with statistically significant results is more likely to be submitted and published than work with non-significant results, a type of contradictory evidence that is commonly known with the slightly derogatory term ‘negative data’, which results in efficacy overestimation.67 Previous meta-analyses have shown that publication bias in reports of animal stroke studies leads to major overstatement of efficacy: effect sizes are inflated by around one-third, and around one-sixth of experiments remain unpublished.68 In the present meta-analysis, trim and fill method unveiled around one-fourth missing experiments, although this ratio could be overestimated because funnel plot asymmetry should not be equated with publication bias and has other possible causes.69 Nevertheless, effect sizes adjusted to account for this reporting bias keep favouring UA treatment in all the three outcome measures.
Although the present results are positive, they should be interpreted with caution due to both the limitations within the included studies and of the present study methodology. In addition to the above discussed criteria relating to the quality of evidence on the beneficial effects of UA in animal models of ischemic stroke (which should be met by every primary study), other criteria relating to the range of evidence (which might be represented across different publications) need consideration.22 Although the range of evidence obtained from diverse research groups around the world meet 10 of a possible 13 criteria, important lacks come from clear underrepresentation of both experiments in female animals and in animals with co-morbidities. Moreover, experiments in aged animals and species other than rodents (gyrencephalic species), outcome measurements after permanent ischaemia and at long term (beyond two weeks), as well as interaction studies with stroke relevant drugs (other than alteplase) were absent. One study performed permanent MCA occlusion, and the ischaemia method was not specified in other, although both studies were excluded from meta-analysis because imprecise sample sizes and only qualitative results were reported, respectively.29,34 We sought further information from the authors, but unfortunately this was not forthcoming. With regard to limitations of the meta-analysis itself, it should be noted that meta-analysis was developed as a statistical approach to providing summary estimates of efficacy from clinical trials data. As such, it is less well suited to animal studies, where experiments can be orders of magnitude smaller. In the present study, median sample sizes per comparison were around 7 in both the control and UA groups for infarct and neurofunctional outcomes, and around 10 for BBB impairment/brain oedema. These sample sizes are in line with the median group sizes of control animals (10) and treated animals (9) reported in a review assessing the methodological quality of 45 articles on neuroprotection in animal models of focal cerebral ischaemia.70 The small group sizes produce less precise estimates of the variance and, therefore, the SMD. However, SMD instead of weighted mean difference was used to merge different scales measuring the same parameter (i.e. infarct sizes expressed as percentage and absolute values) in different species. 17,57,62,71 Interpretation of SMD is less intuitive but it has allowed us to include all the studies within the meta-analysis. Additionally, some between-study heterogeneity is present secondary to the variability in design of individual studies. This has been accounted for, in part, by using a random-effect model of analysis to combine comparisons.17 Another limitation was the number of studies that met our inclusion criteria, which were insufficient to perform multivariate regression or subgroup analysis using all variables of interest. For those items analysed, available comparisons could lead to limited power to detect subgroups effects, or to spurious findings. To minimize this risk, we prespecified subgroup analyses to be conducted and corrected thresholds for significance to account for multiple testing. The limited data available meant there were insufficient comparisons to carry out any stratified meta-analysis for BBB impairment/brain oedema and neurofunctional score. Finally, as with all systematic reviews, we could only assess the impact of variables as they were reported. Some studies might have taken measures to reduce bias but not reported them. This highlights the importance of transparent reporting of study methodology to optimize the predictive value of preclinical research findings.72
The results of the present meta-analysis showing UA efficacy and its limitations have important implications for future animal studies and clinical trials aiming to improve the evidence supporting the suitability of UA for the treatment of acute ischemic stroke. The roadmap to fill the gaps in preclinical research should include improved and properly reported study quality, and focus on unmet or scarcely fulfilled criteria regarding the range of evidence. It is expected that the present results will spur further preclinical research addressing these shortcomings. To this end, in addition to single laboratory initiatives, it is proposed that a multicentre phase III type preclinical study73 would increase the translational impact of preclinical UA stroke research, which should be reassessed in a future meta-analysis update. Lessons can be learnt from the preclinical research track on interleukin-1 receptor antagonist in stroke.20,52,74 Additionally, our results can provide clues about the limited success of the URICO-ICTUS stroke trial,13 and inform the design and conducting of future clinical research. No evidence on the UA efficacy in aged rodents is available while the median age of UA-treated patients was 77 (69–82) years. Preclinical UA efficacy was investigated in a study sample containing 11% female animals versus 53% women in the clinical trial. Prevalence of co-morbidities was hypertension, 7% animals versus 69% patients; diabetes, 7% animals versus 27% patients; and dyslipidaemia, no animals versus 44% patients. This comparison highlights how the range of preclinical evidence is not in line with the clinical characteristics of target stroke patients. Time to UA i.v. administration from stroke onset was almost half in animals (90 [20, 130] min) than in patients (175 [135, 228] min). UA was administered i.v. at a dose of 16 mg/kg in animals. Patients were given 1000 mg UA i.v., based on UA effects on circulating biomarkers in previous clinical studies including healthy volunteers6 and acute stroke patients receiving rtPA.11 However, no interspecies allometric scaling75 for the conversion of the neuroprotective UA dose from rodents to humans was reported in the URICO-ICTUS protocol.76 Translation of UA dosage regimen is particularly relevant in the light of differences in UA metabolism and thus pharmacokinetics in rodents and humans. Since humans and higher primates lack a functional uricase (urate oxidase, UOX) gene,77 and thus show slower UA excretion rate and higher plasmatic UA levels than rodents, it will be of particularly relevant translational value to obtain evidence of UA efficacy from stroke models in nonhuman primates78,79 and humanized UOX deficient rodents.7 These insights into the reasons why UA efficacy was at least partially lost in translation show the potential strength of reverse translational research back into preclinical stroke models.
In conclusion, the current systematic review and meta-analysis build on prior individual publications and systematic narrative reviews claiming a neuroprotective role for UA in experimental ischemic stroke. Our results quantitatively state the overall efficacy of UA in reducing infarct, BBB impairment and oedema as well as neurofunctional deficits, according to available data from animal stroke models. However, analysis of the data set also shows limits to the UA efficacy, including some publication bias, improvable study quality and important shortcomings in the range of the evidence. Altogether, current evidence highlights the need for conducting and reporting further high-quality preclinical research in order to better inform clinical research. Specific recommendations include: (1) assessment of UA dose-response relationship, effect of repeated doses and interaction with stroke relevant drugs (other than alteplase); (2) testing in awake/freely moving animals, and under different anaesthetic conditions; (3) experiments in female animals, animals with co-morbidities, aged animals and species other than rodents (gyrencephalic species, particularly nonhuman primates) and (4) outcome measurements after permanent ischaemia, at long term (beyond two weeks), and using diverse motor, sensory and cognitive tests. Filling these gaps would increase the chances of translational success determining the suitability of UA as a treatment for human stroke.
Acknowledgements
We thank Nirav Dhanesha, Anil K. Chauhan, Enrique C. Leira, Andrew A. Pieper, Carles Justicia, Eduardo Romanos, Anna M. Planas, Elena Jiménez-Xarrié, Yara Onetti, Elisabet Vila, Francesc Jiménez-Altayó, Kiyoshi Kikuchi, Salunya Tancharoen, Y.H. Ma, Zhou Fei and Yan Qu for kindly providing information from their studies.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by RETICS research network INVICTUS+ from Spanish ‘Instituto de Salud Carlos III’ (co-financed with European Regional Development Fund), through grant RD16/0019/0008. The funding source had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the paper for publication.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Johnson CO, Nguyen M, Roth GA, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019; 18: 439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hankey GJ.Stroke. Lancet 2017; 389: 641–654. [DOI] [PubMed] [Google Scholar]
- 3.Chamorro Á, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 2016; 15: 869–881. [DOI] [PubMed] [Google Scholar]
- 4.Chamorro Á.Neuroprotectants in the era of reperfusion therapy. J Stroke 2018; 20: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker BF.Towards the physiological function of uric acid. Free Radic Biol Med 1993; 14: 615–631. [DOI] [PubMed] [Google Scholar]
- 6.Waring WS, Webb DJ, Maxwell SR.Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol 2001; 38: 365–371. [DOI] [PubMed] [Google Scholar]
- 7.Cutler RG, Camandola S, Feldman NH, et al. Uric acid enhances longevity and endurance and protects the brain against ischemia. Neurobiol Aging 2019; 75: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamorro A, Obach V, Cervera A, et al. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke 2002; 33: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 9.Lei Z, Cai J, Hong H, et al. Serum uric acid level and outcome of patients with ischemic stroke: a systematic review and meta-analysis. Neurologist 2019; 24: 121–131. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Lin Y, Liu Y, et al. Serum uric acid levels and outcomes after acute ischemic stroke. Mol Neurobiol 2016; 53: 1753–1759. [DOI] [PubMed] [Google Scholar]
- 11.Amaro S, Soy D, Obach V, et al. A pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke 2007; 38: 2173–2175. [DOI] [PubMed] [Google Scholar]
- 12.Amaro S, Obach V, Cervera A, et al. Course of matrix metalloproteinase-9 isoforms after the administration of uric acid in patients with acute stroke: a proof-of-concept study. J Neurol 2009; 256: 651–656. [DOI] [PubMed] [Google Scholar]
- 13.Chamorro Á, Amaro S, Castellanos M, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol 2014; 13: 453–460. [DOI] [PubMed] [Google Scholar]
- 14.Yu ZF, Bruce-Keller A, Goodman Y, et al. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res 1998; 53: 613–625. [DOI] [PubMed] [Google Scholar]
- 15.Amaro S, Jiménez-Altayó F, Chamorro Á.Uric acid therapy for vasculoprotection in acute ischemic stroke. Brain Circ 2019; 5: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries RBM, Hooijmans CR, Langendam MW, et al. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence-Based Preclin Med 2015; 2: e00007. [Google Scholar]
- 17.Vesterinen HM, Sena ES, Egan KJ, et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 2014; 221: 92–102. [DOI] [PubMed] [Google Scholar]
- 18.Leenaars M, Hooijmans CR, van Veggel N, et al. A step-by-step guide to systematically identify all relevant animal studies. Lab Anim 2012; 46: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Huang C, Chen J, et al. The role of uric acid as a potential neuroprotectant in acute ischemic stroke: a review of literature. Neurol Sci 2015; 36: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 20.McCann SK, Cramond F, Macleod MR, et al. Systematic review and meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke: an update. Transl Stroke Res 2016; 7: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher M, Feuerstein G, Howells DW, STAIR Group et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sena E, van der Worp HB, Howells D, et al. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 2007; 30: 433–439. [DOI] [PubMed] [Google Scholar]
- 23.McGrath S, Sohn H, Steele R, et al. Meta-analysis of the difference of medians. Biometrical J 2020; 62: 69–98. [DOI] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R.A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000; 95: 89–98. [Google Scholar]
- 25.Onetti Y, Dantas AP, Perez B, et al. Middle cerebral artery remodeling following transient brain ischemia is linked to early postischemic hyperemia: a target of uric acid treatment. Am J Physiol – Hear Circ Physiol 2015; 308: H862–H874. [DOI] [PubMed] [Google Scholar]
- 26.Romanos E, Planas AM, Amaro S, et al. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab 2007; 27: 14–20. [DOI] [PubMed] [Google Scholar]
- 27.Vila E, Solé M, Masip N, et al. Uric acid treatment after stroke modulates the krüppel-like factor 2-VEGF-A axis to protect brain endothelial cell functions: impact of hypertension. Biochem Pharmacol 2019; 164: 115–128. [DOI] [PubMed] [Google Scholar]
- 28.Ya B, Liu Q, Li H, Fang et al. Uric acid protects against focal cerebral ischemia/reperfusion-induced oxidative stress via activating Nrf2 and regulating neurotrophic factor expression. Oxid Med Cell Longev 2018; 2018: 6069150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You Y, Hu M, He Z.Protective effect of uric acid on cerebral ischemia and its regulatory action on NOS and XO. Acta Pharmacol Sin 2013; 34: 119.23202804 [Google Scholar]
- 30.Zhang B, Yang N, Lin S, et al. Suitable concentrations of uric acid can reduce cell death in models of OGD and cerebral ischemia–reperfusion injury. Cell Mol Neurobiol 2017; 37: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aliena-Valero A, López-Morales MA, Burguete MC, et al. Emergent uric acid treatment is synergistic with mechanical recanalization in improving stroke outcomes in male and female rats. Neuroscience 2018; 388: 263–273. [DOI] [PubMed] [Google Scholar]
- 32.Dhanesha N, Vázquez-Rosa E, Cintron-Perez CJ, et al. Treatment with uric acid reduces infarct and improves neurological function in female mice after transient cerebral ischemia. J Stroke Cerebrovasc Dis 2018; 27: 1412–1416. [DOI] [PubMed] [Google Scholar]
- 33.Glantz L, Avramovich A, Trembovler V, et al. Ischemic preconditioning increases antioxidants in the brain and peripheral organs after cerebral ischemia. Exp Neurol 2005; 192: 117–124. [DOI] [PubMed] [Google Scholar]
- 34.Haberman F, Tang SC, Arumugam TV, et al. Soluble neuroprotective antioxidant uric acid analogs ameliorate ischemic brain injury in mice. NeuroMolecular Med 2007; 9: 315–323. [DOI] [PubMed] [Google Scholar]
- 35.Jiménez-Xarrié E, Pérez B, Dantas AP, et al. Uric acid treatment after stroke prevents long-term middle cerebral artery remodelling and attenuates brain damage in spontaneously hypertensive rats. Transl Stroke Res 2018; 11: 1332–1347. [DOI] [PubMed] [Google Scholar]
- 36.Justicia C, Salas-Perdomo A, Pérez-de-Puig I, et al. Uric acid is protective after cerebral ischemia/reperfusion in hyperglycemic mice. Transl Stroke Res 2017; 8: 294–305. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi K, Setoyama K, Tanaka E, et al. Uric acid enhances alteplase-mediated thrombolysis as an antioxidant. Sci Rep 2018; 8: 15844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma YH, Su N, Chao XD, et al. Thioredoxin-1 attenuates post-ischemic neuronal apoptosis via reducing oxidative/nitrative stress. Neurochem Int 2012; 60: 475–483. [DOI] [PubMed] [Google Scholar]
- 39.Heo JH, Han SW, Lee SK.Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 2005; 39: 51–70. [DOI] [PubMed] [Google Scholar]
- 40.Bai J, Lyden PD.Revisiting cerebral postischemic reperfusion injury: new insights in understanding reperfusion failure, hemorrhage, and edema. Int J Stroke 2015; 10: 143–152. [DOI] [PubMed] [Google Scholar]
- 41.Peeling J, Corbett D, Del Bigio MR, et al. Rat middle cerebral artery occlusion: correlations between histopathology, T2-weighted magnetic resonance imaging, and behavioral indices. J Stroke Cerebrovasc Dis 2001; 10: 166–177. [DOI] [PubMed] [Google Scholar]
- 42.Schaar KL, Brenneman MM, Savitz SI.Functional assessments in the rodent stroke model. Exp Transl Stroke Med 2010; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valentine JC, Pigott TD, Rothstein HR.How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat 2010; 35: 215–247. [Google Scholar]
- 44.Sena E, Wheble P, Sandercock P, et al. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke 2007; 38: 388–394. [DOI] [PubMed] [Google Scholar]
- 45.MacLeod MR, Van Der Worp HB, Sena ES, et al. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 2008; 39: 2824–2829. [DOI] [PubMed] [Google Scholar]
- 46.Jerndal M, Forsberg K, Sena ES, et al. A systematic review and meta-analysis of erythropoietin in experimental stroke. J Cereb Blood Flow Metab 2010; 30: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bustamante A, Giralt D, Garcia-Bonilla L, et al. Citicoline in pre-clinical animal models of stroke: a meta-analysis shows the optimal neuroprotective profile and the missing steps for jumping into a stroke clinical trial. J Neurochem 2012; 123: 217–225. [DOI] [PubMed] [Google Scholar]
- 48.Wu S, Sena E, Egan K, et al. Edaravone improves functional and structural outcomes in animal models of focal cerebral ischemia: a systematic review. Int J Stroke 2014; 9: 101–106. [DOI] [PubMed] [Google Scholar]
- 49.Xie CL, Wang WW, Xue XD, et al. A systematic review and meta-analysis of Ginsenoside-Rg1 (G-Rg1) in experimental ischemic stroke. Sci Rep 2015; 5: 7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis CK, Laud PJ, Bahor Z, et al. Systematic review and stratified meta-analysis of the efficacy of carnosine in animal models of ischemic stroke. J Cereb Blood Flow Metab 2016; 36: 1686–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beard DJ, Hadley G, Thurley N, et al. The effect of rapamycin treatment on cerebral ischemia: a systematic review and meta-analysis of animal model studies. Int J Stroke 2019; 14: 137–145. [DOI] [PubMed] [Google Scholar]
- 52.Banwell V, Sena ES, Macleod MR.Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis 2009; 18: 269–276. [DOI] [PubMed] [Google Scholar]
- 53.Wheble PCR, Sena ES, Macleod MR.A systematic review and meta-analysis of the efficacy of piracetam and piracetam-like compounds in experimental stroke. Cerebrovasc Dis 2008; 25: 5–11. [DOI] [PubMed] [Google Scholar]
- 54.Vesterinen HM, Currie GL, Carter S, et al. Systematic review and stratified meta-analysis of the efficacy of RhoA and rho kinase inhibitors in animal models of ischaemic stroke. Syst Rev 2013; 2: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCann SK, Irvine C, Mead GE, et al. Efficacy of antidepressants in animal models of ischemic stroke: a systematic review and meta-analysis. Stroke 2014; 45: 3055–3063. [DOI] [PubMed] [Google Scholar]
- 56.Vu Q, Xie K, Eckert M, et al. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology 2014; 82: 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.England TJ, Hind WH, Rasid NA, et al. Cannabinoids in experimental stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2015; 35: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egan KJ, Janssen H, Sena ES, et al. Exercise reduces infarct volume and facilitates neurobehavioral recovery: results from a systematic review and meta-analysis of exercise in experimental models of focal ischemia. Neurorehabil Neural Repair 2014; 28: 800–812. [DOI] [PubMed] [Google Scholar]
- 59.Archer DP, Walker AM, McCann SK, et al. Anesthetic neuroprotection in experimental stroke in rodents: a systematic review and meta-analysis. Anesthesiology 2017; 126: 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García-Bonilla L, Campos M, Giralt D, et al. Evidence for the efficacy of statins in animal stroke models: a meta-analysis. J Neurochem 2012; 122: 233–243. [DOI] [PubMed] [Google Scholar]
- 61.Macleod MR, O'Collins T, Horky LL, et al. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab 2005; 25: 713–721. [DOI] [PubMed] [Google Scholar]
- 62.Gibson CL, Gray LJ, Murphy SP, et al. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab 2006; 26: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 63.Horn J, De Haan RJ, Vermeulen M, et al. Nimodipine in animal model experiments of focal cerebral ischemia: a systematic review. Stroke 2001; 32: 2433–2438. [DOI] [PubMed] [Google Scholar]
- 64.Macleod MR, O'Collins T, Horky LL, et al. Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J Pineal Res 2005; 38: 35–41. [DOI] [PubMed] [Google Scholar]
- 65.Willmot M, Gibson C, Gray L, et al. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: a systematic review. Free Radic Biol Med 2005; 39: 412–425. [DOI] [PubMed] [Google Scholar]
- 66.Crossley NA, Sena E, Goehler J, et al. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke 2008; 39: 929–934. [DOI] [PubMed] [Google Scholar]
- 67.Dirnagl U, Lauritzen M.Fighting publication bias: introducing the negative results section. J Cereb Blood Flow Metab 2010; 30: 1263–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sena ES, B, van der Worp H, Bath PMW, et al. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 2010; 8: e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 70.Van Der Worp HB, De Haan P, Morrema E, et al. Methodological quality of animal studies on neuroprotection in focal cerebral ischaemia. J Neurol 2005; 252: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 71.Moxon JV, Trollope AF, Dewdney B, et al. The effect of angiopoietin-1 upregulation on the outcome of acute ischaemic stroke in rodent models: a meta-analysis. J Cereb Blood Flow Metab 2019; 39: 2343–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Landis SC, Amara SG, Asadullah K, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012; 490: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dirnagl U, Hakim A, MacLeod M, et al. A concerted appeal for international cooperation in preclinical stroke research. Stroke 2013; 44: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maysami S, Wong R, Pradillo JM, et al. A cross-laboratory preclinical study on the effectiveness of interleukin-1 receptor antagonist in stroke. J Cereb Blood Flow Metab 2016; 36: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nair A, Jacob S.A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016; 7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amaro S, Cánovas D, Castellanos M, et al. The URICO-ICTUS study, a phase 3 study of combined treatment with uric acid and rtPA administered intravenously in acute ischaemic stroke patients within the first 4·5 h of onset of symptoms. Int J Stroke 2010; 5: 325–328. [DOI] [PubMed] [Google Scholar]
- 77.Wu X, Lee CC, Muzny DM, et al. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci U S A 1989; 86: 9412–9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cook DJ, Tymianski M.Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics 2012; 9: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu D, Chen J, Wang B, et al. Endovascular ischemic stroke models of adult rhesus monkeys: a comparison of two endovascular methods. Sci Rep 2016; 6: 31608. [DOI] [PMC free article] [PubMed] [Google Scholar]