Abstract
Post-stroke neurological deficits and mortality are often associated with vascular disruption and neuronal apoptosis. Galectin-3 (Gal3) is a potent pro-survival and angiogenic factor. However, little is known about its protective role in the cerebral ischemia/reperfusion (I/R) injury. We have previously shown significant up-regulation of Gal3 in the post-stroke rat brain, and that blocking of Gal3 with neutralizing antibody decreases the cerebral blood vessel density. Our current study demonstrates that intracerebral local delivery of the Gal3 into rat brain at the time of reperfusion exerts neuroprotection. Ischemic lesion volume and neuronal cell death were significantly reduced as compared with the vehicle-treated MCAO rat brains. Gal3 increased vessel density and neuronal survival after I/R in rat brains. Importantly, Gal3-treated groups showed significant improvement in motor and sensory functional recovery. Gal3 increased neuronal cell viability under in vitro oxygen–glucose deprivation conditions in association with increased phosphorylated-Akt, decreased phosphorylated-ERK1/2, and reduced caspase-3 activity. Gene expression analysis showed down regulation of pro-apoptotic and inflammatory genes including Fas-ligand, and upregulation of pro-survival and pro-angiogenic genes including Bcl-2, PECAM, and occludin. These results indicate a key role for Gal3 in neuro-vascular protection and functional recovery following ischemic stroke through modulation of angiogenic and apoptotic pathways.
Keywords: Middle cerebral artery occlusion, ischemic brain injury, cytokine, Akt-apoptosis pathway.
Introduction
Ischemic stroke remains the second leading cause of mortality following heart disease, and is a major cause of long-term disability worldwide.1,2 At present, only a few drugs are available for effective stroke treatment.1–6 A better understanding of the molecular mechanisms and potential drug targets may aid in enhancing post-stroke brain regeneration and improving functional recovery following ischemic stroke.
Post-stroke outcome is often determined by cerebral vascular integrity and cellular dynamics in the ischemic tissue microenvironment.5–9 Cytokines including Galectin-3 (Gal3) are critical regulators of cell survival, apoptosis, migration, and angiogenesis, which modulate post-stroke brain damage and repair.10–20 These cytokines are involved in activation of downstream protein kinase-mediated signaling cascades that modulate the functions of many cell types including neuronal cells.21 Particularly, activation of the phosphoinositide-3-kinase/serine-threonine kinase Akt (PI3K/Akt) pathway promotes cell proliferation, survival, and angiogenesis in the brain and cerebral ischemia.22–26 The PI3K/Akt pathway inhibits pro-apoptotic mechanisms, and genes that increase thrombogenicity and disrupt vascular permeability.25–28 Interestingly, extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), which are members of the mitogen-activated protein kinase family are involved in both cell survival and cell death events through suppressing PI3/Akt signaling.29–35 Indeed, studies have demonstrated that the over-activation of ERK1/2 contributes to cell death.31–34 The caspases, a family of intracellular proteases are activated in response to apoptotic stimuli.36–45 Activated and cleaved caspase-3 promotes DNase-mediated nuclear DNA fragmentation, a phenomenon observed in ischemic brain infarction.46,47 Thus, targeting the apoptotic pathway may be a key approach to reduce cerebral ischemia/reperfusion injury.
Gal3 is a member of the lectin family with β-galactoside carbohydrate recognition domains, and is localized to cytoplasm and nucleus, and is also found in secreted form. It plays an important role in cell–cell adhesion, cell–matrix interactions, macrophage activation, and tumor metastasis. Gal3 is a strong anti-apoptotic and pro-angiogenic cytokine that modulates paracrine and autocrine stimulation. Gal3 enhances the aggressiveness of tumors by increasing cell proliferation, survival, cell motility, metastatic activity and angiogenesis, which is thoroughly reviewed.47–50 Gal3 is one of the major active components of the low oxygen/ischemic environment.51,52 We have shown significant up-regulation of Gal3 in the post-stroke rat brain. Furthermore, blocking of Gal3 with neutralizing antibody decreased the cerebral blood vessel density.50 Further, we also have previously demonstrated that Gal3 enhances the ability of endothelial cells to form pro-angiogenic structures that requires endothelial cell proliferation and survival.19 The role of Gal3 is relatively well defined in tumor growth, and to some extent in different models of ischemic injury.53–63 Gal3 is shown to be associated with aging-related microglia activation and cognitive impairment in the rhesus monkey.64 However, so far no studies have systematically investigated the potential use of Gal3 in reducing post-stroke brain damage and in increasing functional recovery.
The aim of this study was to determine the protective effects of Gal3 against cerebral ischemia/reperfusion injury, and to examine its role in improving the neurological functions in a pre-clinical middle cerebral artery occlusion rat model. We further tested the hypothesis that the beneficial effects of Gal3 are mediated through activation of the PI3K/Akt signaling pathway associated with pro-survival and angiogenic processes. Our results show that Gal3 administration exerts protective effects by significantly decreasing the post-stroke brain infarct volume and improving functional recovery in association with increased vessel and neuronal cell density through regulating apoptotic pathway molecules.
Materials and methods
Animals and focal cerebral ischemic model
Animal housing and caring were carried out according to the Guide for the Care and Use of Laboratory Animals (NIH). Experiments have been reported in compliance with the ARRIVE guidelines. All animal experiments and surgical procedures were approved by the Animal Care and Use Committee of the University of Wisconsin-Madison. We used spontaneously hypertensive rats (SHR), as more than 70% of the stroke patients are hypertensive. Furthermore, hypertension is the single most important risk factor for stroke.65 Focal ischemia was induced in adult SHRs (270–320 g; Charles River, Wilmington, MA) by transient middle cerebral artery occlusion (MCAO) as described in our previous studies.65 Briefly, under isoflurane anesthesia, a 3–0 monofilament nylon suture with a blunted tip was introduced into the left external carotid artery (ECA) lumen and then advanced into the left internal carotid artery (ICA) lumen to block middle cerebral artery (MCA) blood flow (Doccol Corp., Sharon, MA, USA). After 1 h of occlusion, the suture was withdrawn to restore blood flow. The occlusion and reperfusion were confirmed by measuring the regional cerebral blood flow with a laser-Doppler flowmeter. The sham-operated rats were subjected to the same procedures without the intravascular filament occlusion. Body temperatures were maintained at 36.5–37.0°C with a heating blanket during the surgery. The sham-operated rats and ischemic rats were euthanized at days 1 and 3 after reperfusion (n = 10 at each time point) by intra-cardiac perfusion with 4% paraformaldehyde. Brains tissues were obtained from ipsi and contralateral hemisphere for further analysis.
Experimental groups
A total of 60 adult SHR were randomly assigned into 6 groups (3 groups for 1 day reperfusion, and 3 groups for 3 day reperfusion). For day 1 reperfusion time, three groups included (1) control group (n = 10); (2) MCAO +saline group (n = 10); (3) MCAO+Gal3 (5 mg/kg body weight) (n = 10). For day 3 reperfusion time, three groups included (4) control group (n = 10); (5) MCAO +saline group (n = 10); (6) MCAO+Gal3 (5 mg/kg body weight) (n = 10).
Intra-cerebral administration of Gal3
Recombinant rat galectin-3 was obtained from R&D systems (Minneapolis, MN USA). Gal3 was administered at the start of reperfusion. Briefly, three to four months animals were subjected to 60 min MCAO and at the start of reperfusion, animals were administered with Gal3 (5 mg/kg body weight). Gal3 was resuspended in 8–10 µl of saline solution. Thirty rats were stereotaxically injected (over 30 min) into cerebral cortex (bregma: 1.0 mm anterior, and 2.0 mm lateral, and 1.5 mm below the pia) with Gal3. The remaining 10 rats were injected with the same quantity of saline solution without adding Gal3, and were used as controls.
Histologic analysis of infarct volume
The extent of brain damage was evaluated by the measurement of the cerebral infarct volume at post-reperfusion time of one day. Brain infarct volume was measured by 2,3,5-triphenyltetrazolium chloride (TTC) staining of 2-mm coronal sections of brains as previously described.66 The volume of the ischemic lesion was computed by numeric integration of data from serial sections with regard to the sectional interval. The percent of infarct area of the entire brain represented the degree of cerebral infarction. Healthy brain tissues were stained red, while the unstained (white) area was considered to be the infract area. Areas of red and white staining were measured using a computer color multimedia image analysis system (Image J, NIH, USA). The percent of infarction was calculated by the equation: % infarct area = (infarct area/total area of slice) ×100 as previously described.67
Assessment of the effects of Gal3 on functional recovery
In control and Gal3-treated animals, behavioral training and tests were performed on three consecutive days before MCAO and at one and three days after ischemia/reperfusion. Neurobehavioral deficits were determined by rotarod test, beam walk test, and adhesive tape removal test.68–71
The rotarod test that determines motor coordination consists of a rod separated into four compartments (Harvard Apparatus, Holliston, MA, USA). In the rotarod motor test, rats were first habituated to a stationary rod (4 cm rod diameter). After habituation, they were exposed to a rotating rod accelerating from four to eight revolutions per minute (r/min) over a 5-min period. The time at which the animal fell off the rod was recorded automatically with stop watches connected to detectors. The latency to fall was recorded up to 5 min. The adhesive removal test is well established to assess sensorimotor function impairments after ischemic stroke. Two pieces of adhesive materials were alternatively attached to the forepaws with equal pressure before each trial. The rats were placed in a transparent plastic box and the times to feel/contact (time when the rats react to the presence of adhesive materials), and to remove each adhesive material were recorded for five trials with a maximum of 2 min. Motor coordination and balance were assessed by the beam walking test on a narrow beam (30 × 1.3 cm) test. Animals were placed in one corner of the beam and allowed to walk across the beam from one end to the other for three times. The time taken to walk across a narrow beam to a safe platform and the number of foot falls were recorded. In all animals, behavioral tests were performed by an investigator who was blinded to the experimental groups.
Measuring CD31-positive vessels/vascular density
Immunohistochemistry was carried out as we have previously reported.12 Briefly, brain coronal sections of 5 μm thickness were washed and incubated with a blocking solution (3% normal goat serum and 0.1% Triton X-100 in TBS) for 30 min at room temperature, followed by incubation overnight with primary antibodies at 4°C. Sections were then incubated with secondary antibodies for 1 h. The primary antibody anti-CD31 (a marker of endothelial cells) (Santa Cruz Biotechnology, Dallas, TX, USA) was used. Alexa Fluor 488 and 594 conjugated secondary antibodies (Molecular Probes, Eugene, OR, USA) were used according to the primary antibodies. Nuclei were identified by staining with 4′,6-diamidino-2-phenylindole (DAPI). Negative controls were performed by omitting primary antibodies. Images were acquired using a fluorescence microscope (BZ-X800, Keyence, Itasca IL, USA). Immunofluorescence staining of CD31, the marker for endothelial cells, was carried out in coronal sections. Number of blood vessels was identified by counting CD31-positive vessels. The total number of vessels/vascular density per region of interest (ROI) was determined. To characterize the size distribution of CD31-positive vessels, the lengths of all vessels in each ROI were measured using ImageJ software (NIH). The levels of pro-angiogenic markers, vascular endothelial growth factor (VEGF), and its receptor, kinase insert domain protein receptor (KDR/VEGFR2) in saline or Gal3 treated post-stroke brains were examined using primary antibodies, anti-VEGF (Santa Cruz Biotechnology, Dallas, TX, USA), and anti-KDR/VEGFR2 (Abcam, Cambridge, MA USA) using immunofluorescence staining. Images were acquired using a fluorescence microscope (BZ-X800, Keyence, Itasca IL, USA), and fluorescent intensities were measured using NIH Image J software.
Assessment of degenerated neurons and apoptotic cells in post-stroke rat brains
The beneficial effects of Gal3 may be due to decreased apoptosis. We next assessed the degenerating neurons that are undergoing progressive functional loss and death, using Fluoro-jade B staining (Histo-Chem, Jefferson, AR, USA) according to a previously published protocol.72 After deparaffinization and rehydration by rinsing in distilled water for 2 min, the slides were incubated in 0.06% potassium permanganate (VWR International, Strasbourg, France) for 15 min. Slides were then rinsed in deionized water and immersed in 0.001% Fluoro-jade B made by adding 4 ml of a 0.01% stock solution of Fluoro-Jade B to 96 ml of 0.1% acetic acid for 30 min. Then they were washed and dried for 15 min. Sections were cleared in xylene and mounted on microscope slides with Fluoromount (Diagnostic Biosystems, Pleasanton, CA, USA). To examine the levels of active caspase-3, an apoptosis inducer, immunohistochemistry was carried out as we have previously reported.12 Briefly, brain coronal sections were blocked, followed by incubation with primary and secondary antibodies. The following primary antibodies were used: anti-NeuN (neuronal marker) (Sigma-Aldrich, St. Louis, MO, USA), anti-caspase-3 (apoptotic marker) (Cell Signaling Technology, Danvers, MA, USA). Alexa Fluor 488 and 594 conjugated secondary antibodies (Molecular Probes, Eugene, OR, USA) were used according to the primary antibodies. Nuclei were identified by staining with 4′,6-diamidino-2-phenylindole (DAPI). Negative controls were performed by omitting primary antibodies. Images were acquired using a fluorescence microscope (BZ-X800, Keyence, Itasca IL, USA). To further assess the apoptotic cell death, the TUNEL reaction (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labelling) that detects DNA strand breaks, a hallmark of apoptosis was carried out on paraffin-embedded brain coronal sections according to manufacturer’s instructions (Millipore, Burlington MA, USA). Intensely TUNEL-positive cells in six ROI for each group were counted using NIH image J software.
Cell culture
We used mouse brain-derived Neuro-2a cell line that exhibits neuronal like characteristics (American Type Culture Collection ATCC, Manassas, VA, USA). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM with glucose, 4.5 g/ml and L-glutamine, GIBCO-BRL) containing 10% fetal calf serum (FCS) and 100 I.U penicillin and 100 µg/ml streptomycin (Life Technologies, Gaithersburg, MD, USA) and were grown in a 5% CO2 incubator at 37°C for 18 h in normoxic conditions (21% O2).
In vitro ischemia induction by oxygen and glucose deprivation
To create ischemic conditions, the cell culture medium was replaced with glucose-free medium (DMEM with L-glutamine and no glucose, GIBCO), and cells were transferred to a humidified incubator (Serico CB, Binder GmBH, Tuttlingen, Germany) flushed with a gas mixture of 95% N2, and 5% CO2 at 37°C for 8–16 h. Oxygen levels were maintained at 1%. Control cells were incubated for 8 h in 5% CO2 and 21% O2 in a media identical to the OGD media except for the addition of glucose. One set of cells were treated with Gal3 (1 μg/ml). Following OGD, cells were re-oxygenated by adding glucose-supplemented (4.5 g/ml) DMEM complete medium, and placing in a normoxic incubator at 37°C in 5% CO2 and 21% O2 for 4 h.
Immunofluorescence staining
Control and OGD exposed and re-oxygenated cells grown on coverslips were fixed with 3.7% paraformaldehyde for 15 min at room temperature. Cells were then blocked and permeabilized by incubating in PBS containing 2% dry milk and 0.1% Triton X-100 for 1 h. Cells were incubated with the primary anti-Ki67 (Santa Cruz Biotechnology) and anti-Caspase-3 (Cell Signaling Technology, Danvers, MA, USA) for 1 h at 37°C. Cells were washed three times with PBS, then incubated with Alexa Fluor-conjugated secondary antibody for 1 h at 37°C. Subsequently, cells were washed, stained with nuclear stain DAPI for 5 min, washed and mounted on microscope slides with Fluoromount (Diagnostic Biosystems) to preserve fluorescence. Stained cells were viewed and the images were acquired using a fluorescence microscope (BZ-9000 Keyence).
Cell viability and proliferation assays
The viability and proliferation of Neuro-2a cells were determined using both live/dead (Invitrogen) and MTT cell viability assays (Biotium, Fremont, CA, USA) according to manufacturer’s instructions. In live/dead cell assay, propidium iodide (PI) uptake and retention of calcein-AM were assessed. Cells were seeded into 96-well cell culture plates at a density of 5 ×104 cells per well and were exposed to OGD/R with or without Gal3. After 2–4 h of re-oxygenation, cells were incubated with 1 μg/ml calcein-AM and 10 μg/ml PI at 37°C for 30 min. Cells were rinsed with the PBS buffer, visualized, and images were collected using a fluorescence microscope (BZ-9000, Keyence). Cells were counted using NIH Image J software. Cell mortality was expressed as the ratio of PI-positive cells to the sum of calcein-positive and PI-positive cells. Cell proliferation was evaluated by a colorimetric assay for mitochondrial reductase catalyzed reduction of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT). To determine the effects of Gal3 on Neuro-2a cell proliferation/viability, 5 × 103 cells per well were plated in triplicate on 96-well plates and cultured for 18 h in 37°C incubator with 5% CO2. The absorbance of released purple formazan that indicates cell viability was measured at 570 nm in a microtiter plate reader (Spectramax Plus 384, Molecular Devices, Sunnyvale, CA, USA). Results are presented as mean values ± SD of triplicates.
Western blot analysis
Whole cell lysates were extracted using RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing a protease inhibitor cocktail (Sigma-Aldrich). Proteins (30 µg) were separated by SDS-PAGE and transferred to a membrane. The membranes were blocked using 5% fat-free milk and incubated with respective primary antibodies against caspase-3, phospho-ERK1/2, ERK1/2, phospho-Akt (Ser473), and Akt (1:1000; Cell Signaling Technology), or control mouse monoclonal anti-β-actin (Sigma-Aldrich) antibody overnight at 4°C. The membranes were then incubated with secondary anti-rabbit/mouse IgG horseradish peroxidase (HRP)-conjugated antibody (Cell Signaling Technology). Finally, membranes were processed using the ECL system, and were then scanned and analyzed by Odyssey Infrared Imaging System, and Image Studio (LI-COR Biotechnology, Lincoln, NE, USA). Results are presented as mean values ± SD of triplicates.
Pathway focused gene expression profiling by quantitative real-time RT-PCR
Total RNA was isolated using an RNeasy Mini Kit. cDNA was synthesized from 2 μg total RNA by using the reverse transcriptase kit from Qiagen (Valentia, CA, USA). A PCR array (Qiagen) that profiles the expression of 84 genes encoding apoptosis and endothelial cell biology associated genes was used according to the manufacturer’s protocol. Briefly, the cDNA generated from 2 μg of total RNA was combined with SYBR green qPCR master mix. Equal aliquots of this mixture were added to each well containing pre-dispensed gene specific primer sets. qRT-PCR analysis was performed in an ABI 7000 Quantitative-PCR machine (Applied Biosystems, Grand Island, NY, USA). Relative quantification was performed using standard curves generated for each gene-specific primer pairs. The values obtained from each set of primers were normalized to endogenous control genes and used to determine relative expression levels.
Semi-quantitative RT-PCR
cDNAs synthesized from total RNA (2 μg) using superscript-II reverse transcriptase (Invitrogen) were used as templates for RT-PCR. PCR amplification was carried out as previously described.12 Further, validation of expression levels of BCL2, Fas ligand, Occludin, and PECAM1 in Neuro2a cells was carried out by semi-quantitative PCR, and agarose gel electrophoresis was used to visualize and quantitate the mRNA levels. The housekeeping gene β-actin was used as an internal control to quantitate the mRNA levels. Gels were then scanned by Odyssey Infrared Imaging System and band densities were determined using Image Studio (LI-COR Biotechnology). Results are presented as mean values ± SD of triplicates.
Statistical analysis
The data were analyzed using Graph Pad Prism version 4.0. Values are presented as means ± SD for the indicated analyses. Statistical significance was determined by the unpaired non-parametric t-test. Behavioral data were analyzed using nonparametric Kruskal–Wallis test and the Mann–Whitney U test with Bonferroni corrections. To compare mean values in two separate groups for infarct volume, we used an unpaired non-parametric t-test. A probability value of P < 0.05 was considered to be statistically significant for all statistical tests.
Results
Gal3 reduces brain damage induced by focal cerebral ischemia
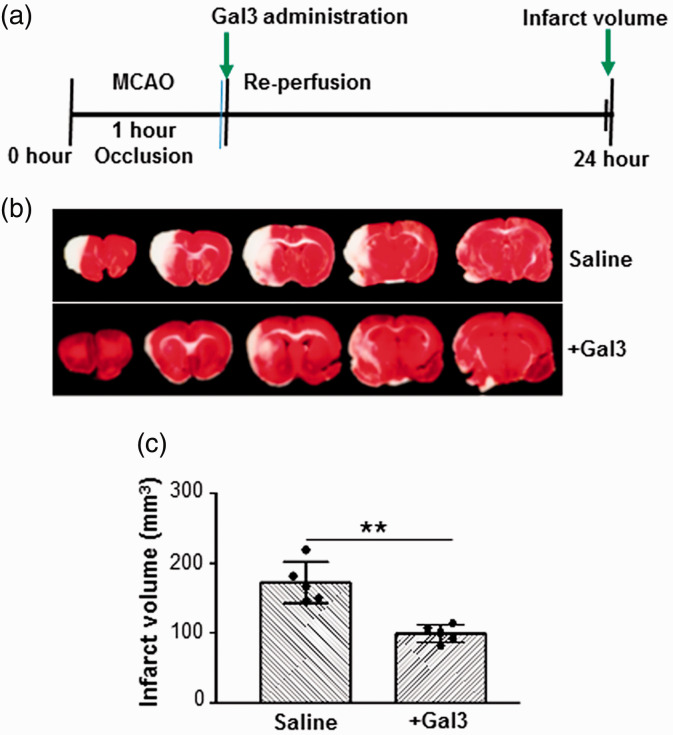
We examined the protective effects of Gal3 on cerebral ischemia/reperfusion injury in a rat MCAO model. Following 60-min transient MCAO and 1 day of reperfusion, there were significant differences in brain infarct volumes between rats in the MCAO control-saline group and MCAO Gal3-treated groups. TTC staining of brain sections from the frontal pole showed the degree of brain damage at 24-h reperfusion. The control rats exhibited larger infarcts (193 ± 57 mm3) as compared to Gal3-treated rats (99 ± 13 mm3). Intra-cerebral administration of Gal3 at the onset of reperfusion significantly reduced the post-stroke brain infarct volume by 49% as compared to saline-injected rats (P < 0.01). Schematic diagram of experimental approach is shown in Figure 1(a). TTC-stained sections from representative rats injected with either saline or Gal3 are shown in Figure 1(b). Quantitation of percent change in brain infarct volume is shown in Figure 1(c). This result suggests that Gal3 alleviates brain damage caused by ischemia/reperfusion.
Figure 1.
Gal3 reduces brain damage induced by focal cerebral ischemia. (a) Schematic representation of experimental design in rat MCAO model. (b) Representative photographs of coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride (TTC) showing reduced infarct volume in Gal3-treated animals as compared to control saline-treated animals. (c) Quantitation of cerebral infarct volume in brains was analyzed using Image J program. The infarct volume is significantly lower in the Gal3-treated group compared with the control saline group. The infarct volume is expressed as the percentage of the contra lateral hemispheric area (N = 10). Data are expressed as mean ± SD, **P < 0.01 control/saline MCAO group vs. Gal3/MCAO group.
Gal3 attenuates functional deficits in post-stroke rats
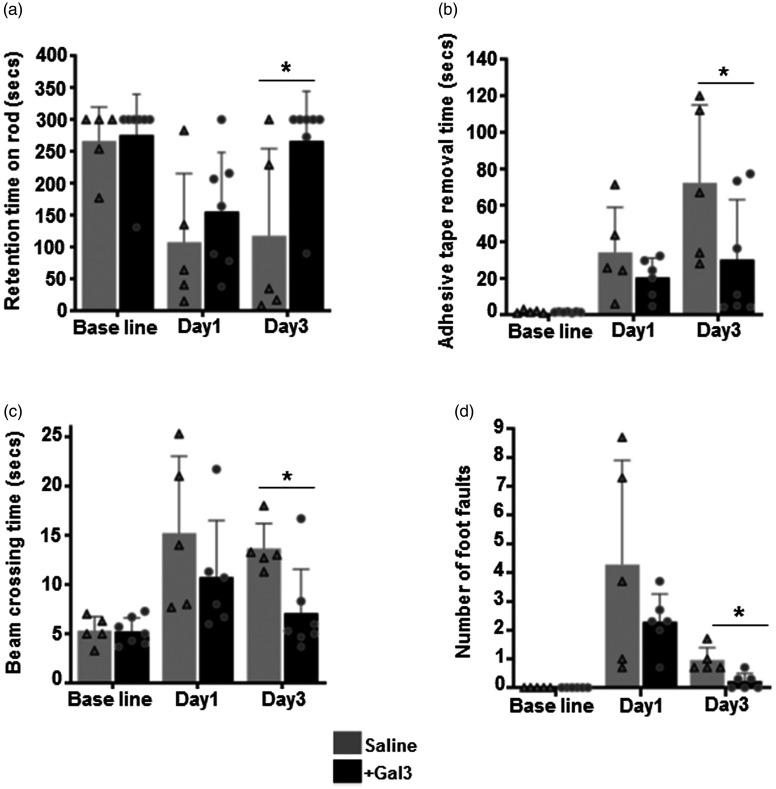
We evaluated several behavioral parameters to examine the effect of Gal3 in attenuating the neurological deficits after transient MCAO. Saline-treated control rats exhibited neurological dysfunction at day 1 to day 3 after ischemia/reperfusion as measured by rotarod test, adhesive removal test, and beam walk test. Gal3 administration significantly improved the motor and sensory recovery in all three functional tests compared with the control saline group (P < 0.05). First, motor coordination and learning were measured by rotarod test. On day 1 and day 3 of ischemia/reperfusion, a significant improvement in locomotor coordination in the accelerated rotarod test was seen in Gal3-treated rats (Figure 2(a)). Sensory motor functions were evaluated with the adhesive tape removal test. Performance for the left paw was not significantly different between two groups. Gal3-treated rats as compared to saline-treated rats, exhibited significantly decreased adhesive tape removal time for the right paw (Figure 2(b)). In beam balance test, rats treated with Gal3 showed significantly (P < 0.05) improved performance in both time taken to cross the beam and decreased foot faults (Figure 2(c) and (d)). These observations demonstrate that Gal3 not only reduces ischemic stroke-mediated brain infarct but also improves the recovery of neurological functions.
Figure 2.
Gal3 administration significantly improves post-ischemic functional recovery. (a–d) Treatment of Gal3 at the time of reperfusion significantly improved the post-ischemic motor function recovery compared with control saline-treated group at day1 and day3 after MCAO and reperfusion. Rats were tested by an investigator blinded to the study group (a). Rotarod test that measures the motor coordination and motor learning indicated by the latency time to fall from a cylinder rotating at 8 r/min. *P<0.05. (b) Adhesive removal test that measures the time taken by rats to remove small adhesive tapes from forepaws. *P<0.05, compared with control rats. (c) Time taken to cross the beam in the beam walk test on days 1 and 3 of reperfusion following transient MCAO. *P<0.05, compared with control rats. (d) Number of foot faults during the beam walk test on days 1 and 3 of reperfusion following transient MCAO (N=10). *P<0.05, compared with control rats.
Gal3 reduces the number of degenerating neurons and apoptotic cells in post-stroke rat brains
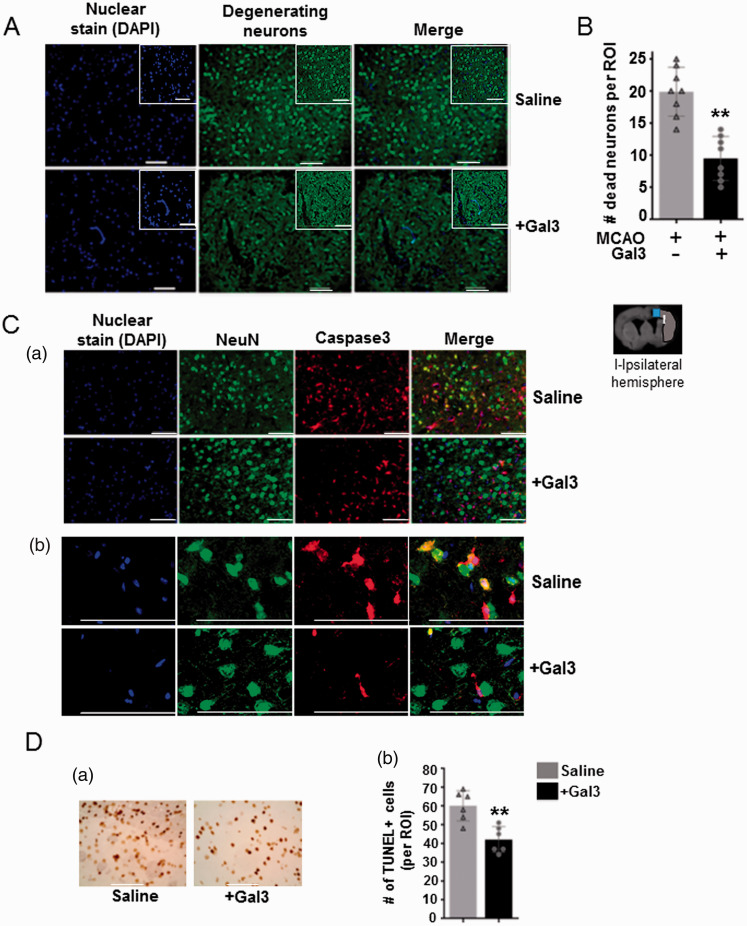
We further examined the number of degenerating neurons in post-stroke rat brains using Fluoro jade B staining. We observed a large number of degenerating neurons positive for Fluoro-Jade B staining in the ischemic hemisphere at three days after MCAO and reperfusion. However, Gal3 treatment significantly decreased the number of Fluoro-Jade B positive degenerating neurons in post-stroke rat brains. Representative images in Figure 3(a) show the degenerating neurons in ischemic cerebral hemisphere from control and Gal3-treated rats. The number of degenerating neurons in the Gal3-treated group was reduced by 52.2%, when compared to the vehicle/saline-treated group (P < 0.01) (Figure 3(b)). We next examined if Gal3 decreases I/R induced expression of caspase-3, a key mediator of apoptosis in neuronal cells. Co-immunofluorescence staining with neuronal marker NeuN and caspase-3 revealed increased number of caspase-3-positive neuronal cells in control ischemic rat brains at day 3 after MCAO, which was significantly decreased in Gal3-treated brains. Immunofluorescence of NeuN (neuronal marker, in green) and cleaved caspase-3 (apoptosis marker, in red) demonstrated a co-localization between both markers (Figure 3(Ca) (100× magnification); Figure 3(Cb) (400× magnification)). Further, TUNEL assay demonstrated significantly decreased number of apoptotic cells in Gal3-treated post-stroke rat brains as compared to saline-treated animal brains (P < 0.05), Figure 3(d). Representative images showing TUNEL-positive cells in ischemic cortex at day 3 after ischemia-reperfusion in control and Gal3 treated rat brains are shown in Figure 3(Da). Quantitation of TUNEL-positive cells in ischemic cortex at day 3 after ischemia–reperfusion in control and Gal3-treated rat brains in six randomly chosen region of interest (N = 6) are shown in Figure 3(Db). *P < 0.05, compared with control rats. These results indicate that Gal3 protects the neuronal cells from further degeneration/death in the peri-infarct region of the brain after cerebral ischemia.
Figure 3.
Neuroprotective effect of Gal3 treatment in rat brains against ischemia/reperfusion injury. (a) Gal3 reduces the number of degenerating/apoptotic neuronal cells in post-stroke rat brains. Representative images of Fluoro-jade B labeled degenerating neurons in ischemic cerebral hemisphere from control and Gal3-treated rats. (b) Quantification of the number of degenerating neurons reveals a two-fold decrease in the number of apoptotic/degenerating neuronal cells in Gal3-treated rat brains compared with control saline-treated rat brains (**P<0.01). (c) Representative immuno-fluorescence images showing co-localization of cleaved caspase-3 (apoptosis marker, in red) and NeuN (neuronal marker, in green) in ischemic cortex at day 3 after ischemia–reperfusion in control and Gal3-treated rat brains. (Ca) 300× magnification, (Cb) 1000× magnification. Nuclei were identified by staining with 4′,6-diamidino-2-phenylindole (DAPI). (Da) Representative images showing TUNEL-positive cells in ischemic cortex at day 3 after ischemia–reperfusion in control and Gal3-treated rat brains. (Db) Graph showing TUNEL-positive cells in ischemic cortex at day 3 after ischemia–reperfusion in control and Gal3-treated rat brains in six randomly chosen region of interest (N = 6); *P<0.05, compared with control rats.
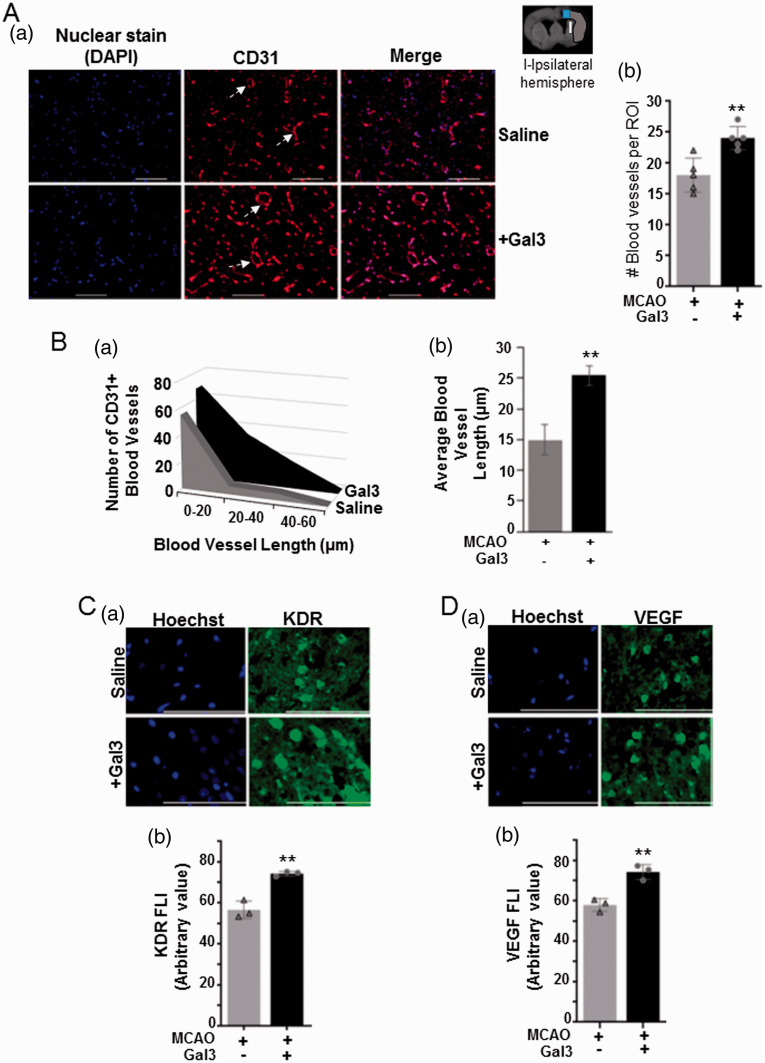
Gal3 increases vessel density and pro-angiogenic factor expression in the ischemic hemisphere of post-stroke rat brains
The blood-vessel density in control and Gal3-treated ischemic rat brains were detected by immunofluorescence staining of CD31, an endothelial cell marker as shown by white arrow (Figure 4(Aa)). The vessel density was increased about 2‐fold in Gal3-injected rat brains at day 3 of ischemia/reperfusion as compared to saline control (P< 0.01) (Figure 4(Ab)). Furthermore, in order to characterize the size distribution of the CD31-positive cells in both control and Gal3-treated ischemic rat brains, the length of vessels was measured. As compared to the control group, in Gal3-treated brains, there was an increased number of blood vessels ranging in length 0–20 µm, 20–40 µm, and 40–60 µm, across the distribution (Figure 4(Ba)). Additionally, the average length of all CD31-positive vessels was increased by about 40% in Gal3-treated ischemic rat brains as compared to saline-treated control brains (P < 0.01) (Figure 4(Bb)). In addition, immunofluorescence staining showed significant increase in the levels of pro-angiogenic factors KDR/VEGFR2 (Figure 4(Ca) and (Cb)) and VEGF (Figure 4(Da) and (Db)) in the peri-infarct area of Gal3-treated post-stroke brains (1000× magnification). These results show that Gal3 may improve angiogenic events through an increase in pro-angiogenic factors and existing endothelial cell survival leading to increased vascular coverage in the post-stroke rat brain.
Figure 4.
Gal3 enhances angiogenic effects and pro-angiogenic marker expression in rat brains following ischemia/reperfusion injury. (a) Representative images of immuno-fluorescence stained CD31-positive vessels in the ischemic cerebral hemisphere of control saline and Gal3-treated rats. White arrow indicates the blood vessels (Aa). Number of vessels was counted in randomly selected regions of interest (ROI). Quantification data are presented in the graph (Ab). Data represent means ± SD in three animal brains (N = 3); **P<0.01 vs. control. (b) Characterization of the size distribution of CD31-positive vessels in the peri-infarct area of post-stroke brains of rats treated with either saline or Galectin 3 (Gal3). The lengths of all vessels in each region of interest (ROI) were measured using ImageJ software (NIH). As compared to the control group, in Gal3-treated post-stroke brains, there was an increased number of blood vessels ranging in length 0–20 µm, 20–40 µm, and 40–60 µm, across the distribution (Ba). The average length of all CD31-positive vessels was increased by about 40% in Gal3-treated post-stroke rat brains as compared to saline-treated control brains (Bb). N = 4 (**P<0.01). (c–d) Representative immunofluorescence stained images of KDR/VEGFR2 (Ca) and VEGF (Da) in the ischemic hemisphere are shown for both saline and Gal3 treated rat brains. A graphical representation of the average fluorescence intensity across all regions of interest for KDR/VEGFR2 (Cb) and VEGF (Db) are presented. N = 3 (**P<0.01).
Gal3 provides protection against oxygen–glucose deprivation/re-oxygenation injury in neuro-2a cells
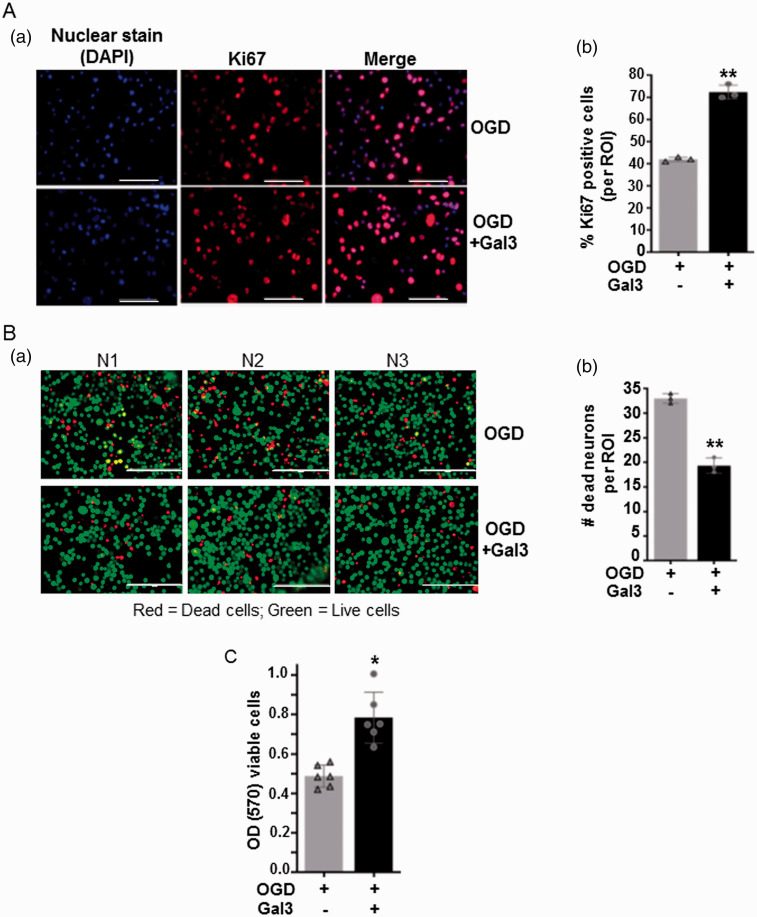
We next examined the effect of Gal3 on the proliferation and survival of neuronal cells under OGD/R that mimics ischemic stroke conditions in vitro. Pre-treatment of Gal3 before OGD/R significantly increased the number of proliferating cells as compared to untreated cells, as shown by the increased number of cells positive for nuclear antigen Ki67, a marker for proliferating cells (Figure 5(Aa)). The percent of Ki67-positive cells increased from 40% in control to about 70% in Gal3 pre-treated cells (Figure 5(Ab)). We also used live/dead cell assay to examine the protective effects of Gal3 under OGD/R. After 8 h of OGD and 4 h of re-oxygenation, Gal3 pretreatment significantly decreased the number of PI-stained dead cells compared with untreated cells. Gal3 pretreatment increased cell viability significantly up to 30–40% (P< 0.05) (Figure 5(Ba) and (Bb)), indicating in vitro neuroprotective effects of Gal3. Furthermore, the results of MTT assay that determines mitochondrial reductase activity, a measure of cell viability showed that pre-treatment with Gal3 of cells exposed to OGD/R significantly increased cell viability by about 40% (P < 0.05) (Figure 5(c)).
Figure 5.
Gal3 increases number of proliferating and viable neuronal cells under in vitro ischemia. (Aa) Cell proliferation was examined by immunofluorescence staining with anti-Ki67, a marker of cell proliferation. Representative micrograph of neuronal cells exposed to ischemia/re-oxygenation with or without Gal3 pretreatment is shown. (Ab) The percent of Ki67-positive cells was calculated per region of interest (ROI). Data represent means ± SD of experiments conducted in triplicate (N = 3). **P<0.01 vs. control. (Ba) Representative images of viable (green) and dead (red) cells after ischemia/re-oxygenation were visualized using live/dead cell assay. Representative micrograph of neuronal cells exposed to OGD/R with or without Gal3 pretreatment is shown. (Bb) Gal3 pretreatment significantly decreased the number of dead cells as indicated by decreased number of propidium iodide (PI) stained red cells. Data represent means ± SD of experiments conducted in triplicate (N = 3). **P<0.01 vs. control. (c) Mitochondrial reductase activity, a measure of cell viability was calculated using MTT assay and relative cell viability was expressed as arbitrary value of optical density at 570 nm. Data represent means ± SD of experiments conducted in triplicate (N = 3). *P < 0.05 vs. control group.
Gal3 regulates pro-survival and pro-apoptotic pathway specific protein levels
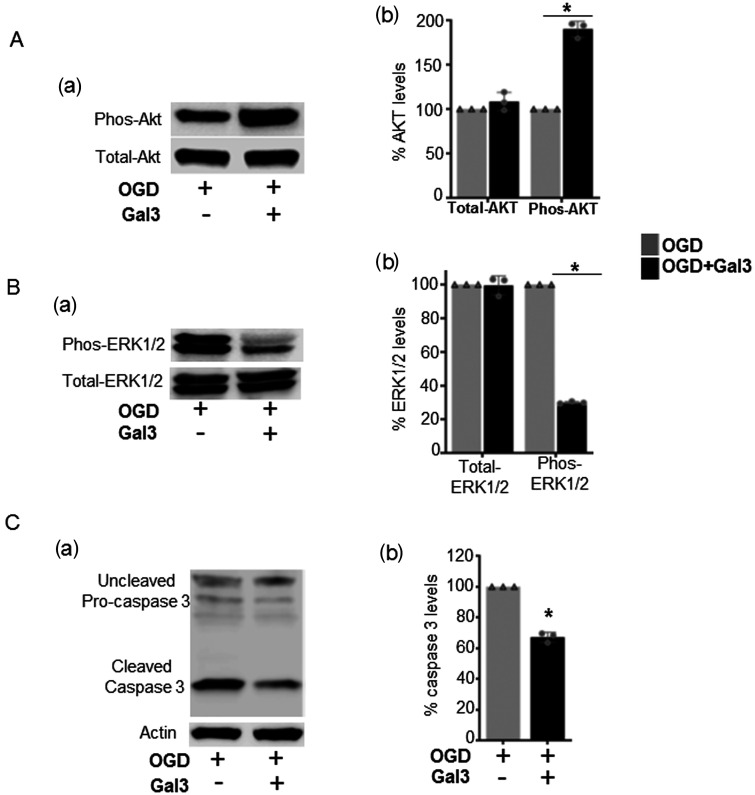
Activation of the phosphoinositide 3‐kinase/Akt is linked to cell survival and angiogenesis. Also, activated mitogen‐activated protein kinase/ERK and caspase pathways promote neuronal apoptosis. Thus, we investigated whether Gal3 regulates these pathways. Western blot analysis revealed that Gal3 pre-treatment of neuronal cells at the time of OGD exposure, significantly increases the levels of phosphorylated AKT (Figure 6(Aa) and (Ab)), while decreasing the levels of phosphorylated ERK1/2 (Figure 6(Ba) and (Bb)), and also cleaved caspase-3, a key inducer of apoptosis (Figure 6(Ca) and (Cb)). Thus, Gal3 is associated with increased protein levels of pro-survival molecules and decreased levels of pro-apoptotic molecules in neuronal cells exposed to in vitro ischemia.
Figure 6.
Gal3 increases phos-AKT and decreased the levels of phos-ERK1/2, and caspase-3 in neuronal cells exposed to in vitro ischemia/oxygen glucose deprivation. (Aa) The expressions of phos-Akt were increased in Gal3-treated cells compared to untreated control cells. Total-Akt remained unchanged in both groups. (Ab) Quantitation of total and phos-Akt levels. *P<0.05. (Ba) The expressions of phos-ERK1/2 were decreased in Gal3-treated cells compared to untreated control cells. Total-ERK1/2 remained unchanged in both groups. (Bb) Quantitation of total and phos-ERK1/2 levels. *P < 0.05. (Ca) Gal3 significantly decreased the levels of cleaved caspase-3 in Gal3-treated cells compared to untreated control cells. (Cb) Quantitation of cleaved caspase-3 levels. *P < 0.05. Representative Western blot and analysis are shown for untreated and Gal3-treated neuronal cells (N=3).The results were normalized to internal control protein actin.
Gal3 is associated with the alterations of mRNA levels of genes involved in angiogenesis and cell survival
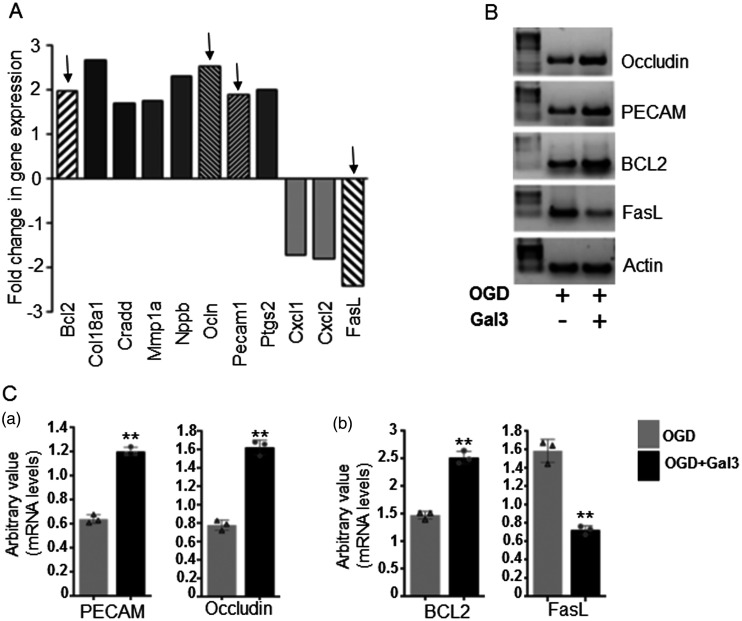
Pathway specific PCR array screening identified the expression of genes involved in angiogenesis and apoptosis pathway. Results showed that, as compared to untreated Neuro-2a cells, Gal3 pre-treatment correlated with more than two-fold upregulation of mRNA levels of pro-angiogenic molecules including occludin and PECAM1, and pro-survival molecule BCL2. Gal3 treatment was also associated with decreased mRNA levels of pro-apoptotic Fas ligand in neuronal cells exposed to OGD/R (Figure 7(a)). Semi-quantitative PCR, in agreement with array data, further validated the significant (P < 0.01) alterations in the expression levels of occludin, PECAM1, BCL2, and Fas ligand. Representative images of PCR products of selected genes are shown in Figure 7(b). Quantitation of band density is shown in graphs for PECAM1 and occludin (Figure 7(Ca)) and for BCL2 and FasL (Figure 7(Cb)) in Gal3-treated cells as compared to control cells under in vitro ischemia.
Figure 7.
Gal3 is associated with the alterations of the mRNA levels of pro-angiogenic and apoptotic genes in neuronal cells under in vitro ischemic conditions.
(a) Determination of angiogenesis and apoptosis-related gene expression changes by quantitative real-time polymerase chain reaction using endothelial biology pathway specific PCR array. (b) Validation of changes in mRNA levels of selected genes by semi quantitative PCR analysis. Pretreatment with Gal3 increases mRNA levels of pro-angiogenic factors occludin and Platelet endothelial cell adhesion molecule (PECAM1)/CD31, and pro-survival molecule BCL2. It decreases mRNA levels of pro-apoptotic Fas ligand in neuronal cells exposed to oxygen glucose deprivation (in vitro ischemia). Levels of beta-actin mRNA were used as internal control. (c) Quantitative analysis showing reduced/increased expression of occludin, PECAM1/CD31 (Ca), BCL2, and Fas ligand (Cb). Bars represent the gene expression normalized to beta-actin. **P<0.01 vs. control (N = 3).
Discussion
In the present study, we have demonstrated that Gal3 not only reduces brain damage and but also ameliorates neurological deficits in a rat stroke model through neuro-vascular unit restoration. At the molecular level, Gal3 treatment upregulated phospho-Akt, and downregulated phospho-ERK and caspase-3 that are involved in cell survival and apoptosis. Furthermore, Gal3 treatment was associated with a decrease in mRNA levels of pro-apoptotic and inflammatory genes, and an increase in pro-survival and pro-angiogenic genes. These results demonstrate that Gal3 potentially provides neuro-vascular protection and improves post-stroke functional recovery through modulation of key signaling pathways.
Intense research has found that reperfusion following the ischemic stroke can aggravate brain damage through progressive loss of cortical neurons, and the main injury mechanism is related to disruption of vascular unit and apoptotic neuronal cell death.36–38 Thus, therapeutic approaches to reduce post-stroke brain damage involves protecting the neurons in an area surrounding the brain infarction, which requires restoration of the vascular unit. Interestingly, after ischemic injury, there is a pronounced increase in expression of pro-survival and angiogenic cytokines and growth factors as an attempt to self-repair the damaged brain.11–14 In support of this, we have previously shown that Gal3 is upregulated in rat brains following focal ischemic stroke induced by middle cerebral artery occlusion.50 It is likely that overexpression of Gal3 is an adaptive program and an essential part of self-defense mechanism to improve cell survival and regeneration under these stressing conditions. However, this self-defense mechanism may be insufficient for successful post-stroke recovery. There is an emerging body of evidence that Gal3 is upregulated under a hypoxic micro-environment due to vaso-occlusion or defective angiogenesis, particularly in solid tumors to enhance their survival, growth, and invasion.51–63 Gal3 is also associated with cognitive impairment and aging.64 Despite these compelling data, the specific role of Gal3 in post-stroke brain has not been systematically elucidated.
Gal3 is a member of the lectin family with β-galactoside carbohydrate recognition domains, and it plays an important role in cell–cell adhesion, cell–matrix interactions, macrophage activation, angiogenesis, metastasis, and apoptosis.16–20 The modulation of Gal3 expression under low oxygen microenvironment, and its involvement in multiple functions evoke deep interest in better understanding of the functions and signals regulated by this protein, and in particular, elucidating its role as a molecular target for promoting ischemic brain protection/repair and functional recovery.
Cerebral ischemia/stroke, a major degenerating disease, enhances destruction of neurons by activating a cascade of events such as oxidative stress, apoptosis, and inflammation.2–6 The brain is particularly susceptible to these events owing to its high metabolic rate, high content of polyunsaturated fatty acids, and post-mitotic characteristics of neurons. Studies are establishing that neuro-modulation therapies can noticeably decrease the post-stroke brain damage and functional deficits.3–10,71 Apoptosis is a major contributor to neuronal cell death following ischemia/reperfusion.36–38 However, the underlying mechanisms are still not fully understood. The choice of neuroprotective agents is primarily based on the abilities of specific drugs to reduce apoptosis and neuronal degeneration, thereby increasing cell proliferation and survival. Such neuroprotective effects have been generally mediated through activation of PI3K/Akt and caspase pathways. In this study, we demonstrate that Gal3 administration significantly reduces the number of degenerating and apoptotic neurons. We further verified whether a decrease in neuronal death by Gal3 treatment was the result of reduced caspase-3-positive cells, as caspase-3 has been identified as a key mediator of apoptosis in neuronal cells.40–45 Interestingly, as compared to saline-treated post-stroke brains, Gal3-treated brains showed decreased levels of caspase-3 protein in NeuN-positive neuronal cells in the peri-infarct region of ischemic brain. Since in the ischemic rat brain, multiple confounding factors are involved, we investigated the protective effects of Gal3 specifically on neuronal cells in an oxygen–glucose deprivation/reoxygenation (OGD/R)-induced neuronal injury model that mimics in vivo ischemic conditions. In agreement with our in vivo observation, Gal3 increased proliferation, and decreased cell death of neuronal cells under OGD conditions. This neuroprotective effect was associated with significantly increased levels of active phosphorylated Akt. However, the levels of active phosphorylated ERK1/2 were decreased by pre-treatment with Gal3, indicating that Akt and ERK1/2 signaling pathways play essentially independent roles after ischemic insults. Interestingly, ERK1/2 is involved in both cell survival and cell death events through suppressing PI3/Akt signaling. Indeed, studies have demonstrated that spontaneous activation of ERK1/2 that occurs via MEK1/2 induces the default pathway of apoptosis and may contribute to neuronal cell death.28–35 Notably, inhibition of ERK1/2 activity in kidney proximal tubular cells up-regulates Akt and prevents apoptosis.31 Thus, the positive effect of Gal3 on up-regulation of the activity of the kinase Akt appears to be mediated, at least in part, through ERK1/2 suppression. The final execution of cell death relies on the caspases, a family of intracellular proteases that are activated in response to apoptotic stimuli.39–44 Particularly, activated/cleaved caspase-3 induces cell death by promoting DNase-mediated nuclear DNA fragmentation, and this phenomenon is associated with the development of ischemic brain infarction.45,46 Of note, our study demonstrated that Gal3 indeed significantly decreases the number of apoptotic cells that are stained intensively by the TUNEL reaction that specifically labels fragmented DNA in apoptotic cells. Also cleaved caspase-3 levels were decreased in neuronal cells exposed to ischemic insults both in vivo and in vitro models. Interestingly, caspase-3 was shown to be upregulated after stroke in ischemic human brain, and both genetic disruption and pharmacological inhibition of caspases provide neuroprotective effects. Thus, targeting the apoptotic pathway may be a key approach to reduce cerebral ischemia/reperfusion injury. Our data further strengthen the role of Gal3 in promoting neuronal survival by inhibiting pro-apoptotic signals.
Neuronal restoration from endogenous precursors in the adult brain after stroke requires healthy restoration of blood vessels. Stroke induces transient and low levels of angiogenesis in sub-ventricular zone and adjacent striatum which is not sufficient for complete repair as the vasculature remodeling plays a key role for long-term neurogenesis after stroke. The enhancement of new blood vessel formation or angiogenesis is clearly identified as a promising therapeutic strategy for ischemic stroke, as restoration of the vascular unit through angiogenesis improves neuroprotection and functional recovery. In our previous study, we demonstrated that blocking Gal3 by neutralizing antibody suppresses the stroke-induced angiogenesis in rats subjected to middle cerebral artery occlusion.50 Further, we also have demonstrated that Gal3 enhances pro-angiogenic structure formation abilities of human vein endothelial cells.18 In this study, we showed that Gal3 administration at the time of reperfusion, leads to increased vascular density in the ischemic brain. In support of this, we observed significant increase in the levels of well-known angiogenic factors VEGF and KDR/VEGFR2 in Gal3-treated post-stroke rat brains. These findings thus highlight the importance of Gal3 as a key molecule in regulating angiogenic events after MCAO. Presumably, Gal3-induced vessel formation stimulates the survival signaling pathways that provide neuroprotection. In agreement with this putative protective role, Gal3 deficient diabetic mice show increased clotting tendencies as well as endothelial dysfunction.62 We next investigated the molecular mechanism of Gal3-regulated vasculo-neuroprotection, by employing pathway specific PCR arrays. This approach identified the possible angiogenic and apoptotic genes affected by Gal3. Interestingly, Gal3 pre-treatment resulted in more than two-fold upregulation of mRNA levels of pro-angiogenic molecules including occludin and PECAM1/CD31, and pro-survival molecule BCL2. An increase in BCL2 expression has been proven to be cyto-protective by preventing apoptotic pathway. There are evidence that Gal3 and Akt indirectly activate NF-κB and thus resulting in transcription of pro-survival genes and trophic factors.23–26,73 Up regulation of occludin, a tight junction protein protects blood–brain barrier by supporting vascular integrity, while PECAM1 promotes endothelial cell function.74–76 It is established that upregulation of the Fas ligand/Fas death pathway is involved in neuronal apoptosis.77–79 Interestingly, Gal3 treatment was also associated with decreased mRNA levels of pro-apoptotic Fas ligand in neuronal cells exposed to OGD/R. Overall, our results demonstrate that activation of intracellular signal transduction pathways occurs in cells upon Gal3 treatment, which play an important role in effectively preventing infarct expansion through promotion of angiogenesis and neuronal regeneration under ischemic conditions. These findings provide insight into the potential mechanistic basis for Gal3 function. Further investigations however may unravel the specific modes of the molecular mechanisms and signaling pathways regulated by Gal3 in post-stroke ischemic brain. Taken together, our study demonstrates that Gal3 is a potential therapeutic target in the treatment of stroke.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20931137 for Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation by Umadevi V Wesley, Ian C Sutton, Katelin Cunningham, Jacob W Jaeger, Allan Q Phan, James F Hatcher and Robert J Dempsey in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X20931137 for Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation by Umadevi V Wesley, Ian C Sutton, Katelin Cunningham, Jacob W Jaeger, Allan Q Phan, James F Hatcher and Robert J Dempsey in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the American Heart Association (AHA-17GRNT33700105) for UVW, and by the Department of Neurosurgery, School of Medicine and Public Health, The University of Wisconsin Madison, WI 53792.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: UVW developed the study concept, designed and conducted the experiments, and wrote the manuscript, RJD participated in the development of the study concept, experimental design, and manuscript review, KC, JFH, AP, IS, JJ provided technical assistance in conducting some experiments, data analysis and edited the manuscript. All authors read and approved the final manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018; 137: 467–492. [DOI] [PubMed] [Google Scholar]
- 2.Jayaraj RJ, Azimullah S, Beiram R, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation 2019; 16: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahota P, Savitz SI.Investigational therapies for ischemic stroke: neuroprotection and neurorecovery. Neurotherapeutics 2011; 8: 434–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharp FR, Jickling GC, Stamova B, et al. Molecular markers and mechanisms of stroke: RNA studies of blood in animals and humans. J Cereb Blood Flow Metab 2011; 31: 1513–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X, Michael De Silva T, Chen J, et al. Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res 2017; 120: 449–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006; 26: 13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thored P, Wood J, Arvidsson A, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007; 38: 3032–3039. [DOI] [PubMed] [Google Scholar]
- 8.Posada-Duque RA, Barreto GE, Cardona-Gomez GP.Protection after stroke: cellular effectors of neurovascular unit integrity. Front Cell Neurosci 2014; 8: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkat P, Chopp M, Chen J.Blood–brain barrier disruption, vascular impairment, and ischemia/reperfusion damage in diabetic stroke. J Am Heart Assoc 2017; 6: e005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doll DN, Barr TL, Simpkins JW.Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis 2014; 5: 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesley UV, Hatcher JF, Ayvaci ER, et al. Regulation of dipeptidyl peptidase IV in the post-stroke rat brain and In vitro ischemia: implications for chemokine mediated neural progenitor cell migration and angiogenesis. Mol Neurobiol 2017; 54: 4973–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambertsen KL, Biber K, Finsen B.Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 2012; 32: 1677–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalluri HS, Dempsey RJ.Growth factors, stem cells, and stroke. Neurosurg Focus 2008; 24: 3–4. [DOI] [PubMed] [Google Scholar]
- 14.Stonesifer C, Corey S, Ghanekar S, et al. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 2017; 158: 94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jablonska A, Lukomska B.Stroke induced brain changes: implications for stem cell transplantation. Acta Neurobiol Exp 2011; 71: 74–85. [DOI] [PubMed] [Google Scholar]
- 16.Abel WF, Funk CR, Blenda AV.Galectins in the pathogenesis of cerebrovascular accidents: an overview. J Exp Neurosci 2019; 13: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newlaczyl AU, Yu LG.Galectin-3-a jack-of-all-trades in cancer. Cancer Lett 2011; 313: 123–128. [DOI] [PubMed] [Google Scholar]
- 18.Wesley UV, Vemuganti R, Ayvaci R, et al. Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res 2013; 1496: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suthahar N, Meijers WC, Silljé HHW, et al. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: an update Theranostics 2018; 8: 593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon SB, Yoon HJ, Chang CY, et al. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol 2010; 185: 7037–7046. [DOI] [PubMed] [Google Scholar]
- 21.Turner MD, Nedjai B, Hurst T, et al. Cytokines and chemokines: at the crossroads of cell signaling and inflammatory disease. Biochim Biophys Acta 2014; 1843: 2563–2582. [DOI] [PubMed] [Google Scholar]
- 22.Melo FH, Butera D, Junqueira Mde S, et al. The promigratory activity of the matricellular protein galectin-3 depends on the activation of PI-3 kinase. PLoS One 2011; 6: e29313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata M, Yamawaki T, Sasaki T, et al. Upregulation of Akt phosphorylation at the early stage of middle cerebral artery occlusion in mice. Brain Res 2002; 942: 1–10. [DOI] [PubMed] [Google Scholar]
- 24.Jeon SJ, Han SH, Yang SI, et al. Positive feedback regulation of Akt-FMRP pathway protects neurons from cell death. J. Neurochem 2012; 123: 226–238. [DOI] [PubMed] [Google Scholar]
- 25.Schabbauer G, Tencati M, Pedersen B, et al. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol 2004; 24: 1963–1969. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Liu G, Guo J, et al. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 2018; 14: 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fruman DA, Chiu H, Hopkins BD, et al. The PI3K pathway in human disease. Cell 2017; 170: 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue L Murray JH, andTolkovsky AM.. The Ras/phosphatidylinositol 3-kinase and Ras/ERK pathways function as independent survival modules each of which inhibits a distinct apoptotic signaling pathway in sympathetic neurons. J Biol Chem 2010; 275: 8817–8824. [DOI] [PubMed] [Google Scholar]
- 29.Dong J, Ramachandiran S, Tikoo K, et al. EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol Renal Physiol 2004; 287: F1049–F1058. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Omori N, Jin G, et al. Cooperative expression of survival p-ERK and p-Akt signals in rat brain neurons after transient MCAO. Brain Res 2003; 962: 21–26. [DOI] [PubMed] [Google Scholar]
- 31.Sinha D, Bannergee S, Schwartz JH, et al. Inhibition of ligand-independent ERK1/2 activity in kidney proximal tubular cells deprived of soluble survival factors up-regulates Akt and prevents apoptosis. J Biol Chem 2004; 279: 10962–10972. [DOI] [PubMed] [Google Scholar]
- 32.Irving EA, Barone FC, Reith AD, et al. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res Mol Brain Res 2000; 77: 65–75. [DOI] [PubMed] [Google Scholar]
- 33.Kim YK, Kim HJ, Kwon CH, et al. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol 2005; 25; 374–382. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang S, Schnellmann RG.A death-promoting role for extracellular signal-regulated kinase. J Pharmacol 2006; 319: 991–997. [DOI] [PubMed] [Google Scholar]
- 35.Huang CY, Liou YF, Chung SY, et al. Role of ERK signaling in the neuroprotective efficacy of magnesium sulfate treatment during focal cerebral ischemia in the gerbil cortex. Chin J Physiol 2010; 53: 299–309. [DOI] [PubMed] [Google Scholar]
- 36.Radak D, Katsiki N, Resanovic I, et al. Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol 2017; 15: 115–122. [DOI] [PubMed] [Google Scholar]
- 37.Haviv R, Lindenboim L, Li H, et al. Need for caspases in apoptosis of trophic factor-deprived PC12 cells. J Neurosci Res 1997; 50: 69–80. [DOI] [PubMed] [Google Scholar]
- 38.Graham SH, Chen J.Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 2001; 21: 99–109. [DOI] [PubMed] [Google Scholar]
- 39.Zhou C, Yamaguchi M, Kusaka G, et al. Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 2004; 24: 419–431. [DOI] [PubMed] [Google Scholar]
- 40.Namura S, Zhu J, Fink K, et al. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 1998; 18: 3659–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endres M, Namura S, Shimizu-Sasamata M, et al. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J Cereb Blood Flow Metab 1998; 18: 238–247. [DOI] [PubMed] [Google Scholar]
- 42.Le DA, Wu Y, Huang Z, et al. Caspase activation and neuroprotection in caspase-3-deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A 2002; 99: 15188–15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma J, Endres M, Moskowitz MA.Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br J Pharmacol 1998; 124: 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arscott WT, LaBauve AE, May V, et al. Suppression of neuroblastoma growth by dipeptidyl peptidase IV: relevance of chemokine regulation and caspase activation. Oncogene 2009; 28: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rami A, Sims J, Botez G, et al. Spatial resolution of phospholipid scramblase 1 (PLSCR1), caspase-3 activation and DNA-fragmentation in the human hippocampus after cerebral ischemia. Neurochem Int 2003; 43: 79–87. [DOI] [PubMed] [Google Scholar]
- 46.McIlroy D, Sakahira H, Talanian RV, et al. Involvement of caspase-3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli. Oncogene 1999; 18: 4401–4408. [DOI] [PubMed] [Google Scholar]
- 47.Krześlak A, Lipińska A.Galectin-3 as a multifunctional protein. Cell Mol Biol Lett 2004; 9: 305–328. [PubMed] [Google Scholar]
- 48.Chou FC, Chen HY, Kuo CC, et al. Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci 2018; 19: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cay T.Immunhistochemical expression of galectin-3 in cancer: a review of the literature. Turk Patoloji Derg 2012; 28: 1–10. [DOI] [PubMed] [Google Scholar]
- 50.Yan YP, Lang BT, Vemuganti R, et al. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res 2009; 1288: 116–124. [DOI] [PubMed] [Google Scholar]
- 51.Kataoka Y, Ohshio Y, Teramoto K, et al. Hypoxia-induced galectin-3 enhances RhoA function to activate the motility of tumor cells in non-small cell lung cancer. Oncol Rep 2019; 41: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikemori RY, Machado CM, Furuzawa KM, et al. Galectin-3 up-regulation in hypoxic and nutrient deprived microenvironments promotes cell survival. PLoS One 2014; 9: e111592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandes Bertocchi AP, Campanhole G, Wang PH, et al. A Role for galectin-3 in renal tissue damage triggered by ischemia and reperfusion injury. Transpl Int 2008; 21: 999–1007. [DOI] [PubMed] [Google Scholar]
- 54.Ishibashi S, Kuroiwa T, Sakaguchi M, et al. Galectin-1 regulates neurogenesis in the subventricular zone and promotes functional recovery after stroke. Exp Neurol 2007; 207: 302–313. [DOI] [PubMed] [Google Scholar]
- 55.Matarrese P, Fusco O, Tinari N, et al. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer 2000; 85: 545–554. [PubMed] [Google Scholar]
- 56.Markowska AI, Jefferies KC, Panjwani N.Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem 2011; 286: 29913–29921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nangia-Makker P, Honjo Y, Sarvis R, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol 2000; 156: 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qu WS, Wang YH, Wang JP, et al. Galectin-1 enhances astrocytic BDNF production and improves functional outcome in rats following ischemia. Neurochem Res 2010; 35: 1716–1724. [DOI] [PubMed] [Google Scholar]
- 59.Satoh K, Niwa M, Binh NH, et al. Increase of galectin-3 expression in microglia by hyperthermia in delayed neuronal death of hippocampal CA1 following transient forebrain ischemia. Neurosci Lett 2011; 504: 199–203. [DOI] [PubMed] [Google Scholar]
- 60.Walther M, Kuklinski S, Pesheva P, et al. Galectin-3 is upregulated in microglial cells in response to ischemic brain lesions, but not to facial nerve axotomy. J Neurosci Res 2000; 61: 430–435. [DOI] [PubMed] [Google Scholar]
- 61.Lalancette-Hébert M, Swarup V, Beaulieu JM, et al. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci 2012; 32: 10383–10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darrow AL, Shohet RV, Maresh JG.Transcriptional analysis of the endothelial response to diabetes reveals a role for galectin-3. Physiol Genom 2011; 43: 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao W, Ajani JA, Sushovan G, et al. Galectin-3 mediates tumor cell-stroma interactions by activating pancreatic stellate cells to produce cytokines via integrin signaling. Gastroenterology 2018; 154: 1524–1537. [DOI] [PubMed] [Google Scholar]
- 64.Eli S, Michael PB, Larissa IE, et al. Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. Geroscience 2017; 39: 199–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cipolla MJ, Liebeskind DS, Chan SL.The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab 2018; 38: 2129–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu F, Schafer DP, McCullough LM.TTC, fluoro-jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods 2009; 179: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng F, Zhong X, Lu Y, et al. Refined qingkailing protects MCAO mice from endoplasmic reticulum stress-induced apoptosis with a broad time window. Evid Based Complement Alternat Med 2012; 2012: 567872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaar KL, Brenneman MM, Savitz SI.Functional assessments in the rodent stroke model. Exp Transl Stroke Med 2010; 2: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L, Schallert T, Zhang ZG, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods 2002; 117: 207–214. [DOI] [PubMed] [Google Scholar]
- 70.Bouet V, Boulouard M, Toutain J, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009; 4: 1560–1564. [DOI] [PubMed] [Google Scholar]
- 71.Zhao H, Nepomuceno R, Gao X, et al. Deletion of the WNK3-SPAK kinase complex in mice improves radiographic and clinical outcomes in malignant cerebral edema after ischemic stroke. J Cereb Blood Flow Metab 2017; 37: 550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wesley UV, Bhute VJ, Hatcher JF, et al. Local and systemic metabolic alterations in brain, plasma, and liver of rats in response to aging and ischemic stroke, as detected by nuclear magnetic resonance (NMR) spectroscopy. Neurochem Int 2019; 127: 113–124. [DOI] [PubMed] [Google Scholar]
- 73.Qian D, Lu Z, Xu Q, et al. Galectin-1 induces secretion of stromal cell-derived factor-1(SDF-1) in PSCs by activating NF-κB signaling. Cancer Lett 2018; 154: 1524–1537. [Google Scholar]
- 74.Kanayasu-Toyoda T, Ishii-Watabe A, Kikuchi Y, et al. Occludin as a functional marker of vascular endothelial cells on tube-forming activity. J Cell Physiol 2018; 233: 1700–1711. [DOI] [PubMed] [Google Scholar]
- 75.DeLisser HM, Christofidou-Solomidou M, Strieter RM, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 1997; 151: 671–677. [PMC free article] [PubMed] [Google Scholar]
- 76.Cao G, O’Brien CD, Zhou Z, et al. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol 2002; 282: 1181–1190. [DOI] [PubMed] [Google Scholar]
- 77.Jayanthi S, Deng X, Ladenheim B, et al. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA 2005; 102: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clayton E, Kinley-Cooper SK, Weber RA, et al. Brain stimulation: neuromodulation as a potential treatment for motor recovery following traumatic brain injury. Brain Res 2016; 1640: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uchino Y, Woodward AM, Mauris J, et al. Galectin-3 is an amplifier of the interleukin-1β-mediated inflammatory response in corneal keratinocytes. Immunology 2018; 154: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20931137 for Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation by Umadevi V Wesley, Ian C Sutton, Katelin Cunningham, Jacob W Jaeger, Allan Q Phan, James F Hatcher and Robert J Dempsey in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X20931137 for Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation by Umadevi V Wesley, Ian C Sutton, Katelin Cunningham, Jacob W Jaeger, Allan Q Phan, James F Hatcher and Robert J Dempsey in Journal of Cerebral Blood Flow & Metabolism