Abstract
Due to the use of improvised explosive devices, blast exposure and mild traumatic brain injury (mTBI) have become hallmark injuries of the Iraq and Afghanistan wars. Although the mechanisms of the effects of blast on human neurobiology remain active areas of investigation, research suggests that the cerebrovasculature may be particularly vulnerable to blast via molecular processes that impact cerebral blood flow. Given that recent work suggests that blast exposure, even without a subsequent TBI, may have negative consequences on brain structure and function, the current study sought to further understand the effects of blast exposure on perfusion. One hundred and eighty military personnel underwent pseudo-continuous arterial spin labeling (pCASL) imaging and completed diagnostic and clinical interviews. Whole-brain analyses revealed that with an increasing number of total blast exposures, there was significantly increased perfusion in the right middle/superior frontal gyri, supramarginal gyrus, lateral occipital cortex, and posterior cingulate cortex as well as bilateral anterior cingulate cortex, insulae, middle/superior temporal gyri and occipital poles. Examination of other neurotrauma and clinical variables such as close-range blast exposures, mTBI, and PTSD yielded no significant effects. These results raise the possibility that perfusion may be an important neural marker of brain health in blast exposure.
Keywords: ASL, blast, cerebral blood flow, CBF, mTBI, mild traumatic brain injury, perfusion, veterans
Introduction
The use of improvised explosive devices and other weapons that emit explosive blasts in combat has made blast exposure a hallmark trauma of the Iraq and Afghanistan wars. It is estimated that 75% of traumatic brain injuries (TBI) that occurred during these wars were due to blast and 55% of Veterans of these wars were exposed to two or more blasts despite screening negative for TBI (i.e. losing consciousness, not remembering the injury, or being dazed, confused, or seeing stars) during their service.1 Blast exposure is commonly associated with physical injury such as hearing impairment, limb amputation, pulmonary complications, and vestibular dysfunction,2 but recent evidence suggests that brain structure and function are also impacted by blast even without a diagnosed TBI.3 For example, work by Robinson et al.4 showed that blast exposure within 10 m or less was associated with decreased functional connectivity of bilateral primary somatosensory and motor cortices. Other work has shown that blast exposure, independent of a TBI diagnosis, is associated with reduced white matter structural integrity,5–7 which may be further impacted by genetic risk for neurodegenerative disease.8 However, the underlying neurobiological mechanisms linking blast exposure to these neural correlates remain an active area of investigation.
One potential mechanism that may link blast exposure to the deleterious effects on brain health is its effects on cerebrovascular function.9,10 This link is well supported in the healthy and pathological aging literature as recent work has pointed to the importance of cerebrovascular health in the maintenance of neural integrity and function in healthy older adults and those with neurodegenerative disease.11–14 More specifically, research suggests that damage to the cerebrovasculature that occurs via alterations in cerebral blood flow (CBF; or perfusion) and the blood–brain barrier may lead to disruptions in neural and oxygen transport systems that are required for optimal brain function and maintenance.15 Consistent with this notion, Brickman et al.16 examined a group of healthy older adults and found that brain regions with reduced CBF were more likely to be classified as white matter hyperintensities. This finding is consistent with longitudinal work in cognitively intact older adults that showed that brain regions with low CBF at baseline were significantly more likely to have white matter hyperintensities 18 months later.17 Further, studies of pathological aging (e.g. Alzheimer’s disease) have shown a link between reduced CBF and faster rates of cognitive decline.18,19 Together, these studies suggest that overall blood supply to the brain is an important factor in brain health and point to the importance of examining CBF in conditions that have long-term negative consequences on brain structure and function.
To date, several studies have examined cerebrovascular health in mild TBI (mTBI) using arterial spin labeling (ASL), a non-invasive perfusion magnetic resonance imaging (MRI) technique that directly quantifies CBF with magnetically labeled arterial blood water. Grossman et al.20 found reduced CBF in the thalamus of mTBI patients one month and then again nine months after injury, suggesting residual cerebrovascular dysfunction in mTBI. Similarly, Ge et al.21 found reduced CBF in mTBI patients who were on average two years postinjury compared to controls. Recent work has extended these findings to blast-related TBI, with reduced global CBF in Veterans with mild and moderate-severe TBI who were on average three years postinjury compared to Veteran controls.22 Further, Clark et al.23 found that reduced CBF in the cingulate cortex was associated with reduced white matter integrity in the cingulum bundle in Veterans further removed from their injury, suggesting that disruptions in CBF may contribute to the degradation of the brain’s structural integrity in blast-related TBI over time. However, despite the body of work pointing to the role of cerebrovascular dysfunction in mTBI, no in-vivo human studies have examined cerebrovascular integrity in blast exposure per se, although rodent work has suggested that low-level blast exposure is associated with persistent structural and functional disruptions to the cerebrovasculature.24–26 This research suggests that blast exposure may impact cebrovasculature integrity via alterations to the neurovascular unit, including prominent effects on the CBF and vascular permeability, even without neuronal pathology. Together, this work points to the potential relevance of blast exposure on perfusion even in the absence of an accompanying mTBI.
In this study, we examined the association between cerebrovascular health and blast exposure in an expansive Operations Enduring Freedom/Iraqi Freedom/New Dawn (OEF/OIF/OND) military personnel cohort without moderate and severe TBI. We hypothesized that blast exposure would be associated with disruptions in CBF throughout the brain even in the absence of moderate and severe TBI and regardless of whether acute mTBI symptoms were present at the time of injury. To test this hypothesis, we used pseudo-continuous ASL (pCASL) imaging to examine whole-brain perfusion associations with the number of total blast exposures within 100 m. Additionally, given previous work suggesting that close-range blast exposures (CBEs; i.e. within 10 meters) may be particularly relevant to brain health in OEF/OIF/OND Veterans,4,8 we also examined perfusion in relation to the number of CBEs. Secondarily, to replicate previous work and to probe whether blast exposure was uniquely sensitive to cerebrovascular dysfunction, we explored whether other neurotrauma variables such as lifetime and military-related mTBI were associated with perfusion disruptions.
Materials and methods
Participants
Participants were 180 Veterans deployed to OEF/OIF/OND (n = 177) or active duty service members not yet deployed to OEF/OIF/OND serving in the Reserves or National Guard (n = 3) enrolled in the Translational Research Center for TBI and Stress Disorders (TRACTS)27 at the VA Boston Healthcare System, Jamaica Plain campus. Participants were excluded if they reported a history of seizures or neurological illness (unrelated to head injuries), serious mental illness such as bipolar disorder or other psychotic disorders (unrelated to posttraumatic stress disorder, PTSD), unstable psychological diagnosis that would interfere with accurate data collection (determined by consensus of at least three doctorate-level psychologists and includes acute distress, using/abusing alcohol or substances that morning or very recently (e.g. intoxication), or have a personal/psychiatric issue that does not allow for them to accurately complete research-based assessments for a full day), active suicidal or homicidal ideation, cognitive disorder due to a general medical condition, incompatibility with MRI due to ferromagnetic objects or pregnancy, or a history of moderate or severe TBI at any epoch (pre-deployment, blast/military, or post-deployment), although mild TBI (mTBI) was not excluded and approximately 70% of the sample reported a history of mTBI during the lifetime. Participants were further excluded if they had poor MRI data quality that resulted in unusable data or unavailable data on MRI, clinical assessments, or covariates used in analyses. Table 1 lists the participant characteristics and demographics.
Table 1.
Demographic and clinical participant characteristics (n = 180).
| Variable | M (SD) | n (%) |
|---|---|---|
| Demographic | ||
| Sex (male) | 167 (92.8) | |
| Age | 33.8 (8.8) | |
| BMI | 28.0 (3.9) | |
| Smoking status (smoker) | 32 (19.6) | |
| Branch of Service | ||
| Army | 99 (55.0) | |
| Navy | 6 (3.3) | |
| Marines | 45 (25.0) | |
| Airforce | 11 (6.1) | |
| Coast Guard | 1 (0.6) | |
| Unknown | 11 (6.1) | |
| Multiple branchesa | 7 (3.9) | |
| Clinical | ||
| CAPS total score | 48.7 (29.1) | |
| Lifetime mTBI diagnosis | 125 (69.4) | |
| Number of lifetime mTBIs | 1.8 (2.4) | |
| Military mTBI diagnosis | 90 (50.0) | |
| Number of military mTBIs | 1.1 (2.1) | |
| Number of total blast exposures | 41.1 (140.7) | |
| Number of CBEs | 4.9 (33.7) |
Note: The demographic characteristics for the full sample are shown before removal of individuals with extreme values on blast exposure measures (n = 4 for number of total blast exposures; n = 2 for number of CBEs). BMI data were unavailable for two participants (n = 178) and smoking status (cigarettes) was unavailable for 17 participants (n = 163).
aSix individuals served in the army as well as the marines (n = 2), air force (n = 2), and navy (n = 2) and one individual served in the navy as well as the air force.
BMI: body mass index; CBE: close-range blast exposure; mTBI: mild traumatic brain injury; PTSD: posttraumatic stress disorder.
All participants provided written and informed consent. The study was approved by the VA Boston Healthcare System Institutional Review Board and Research and Development and was conducted in accordance with the World Medical Association Declaration of Helsinki.
Clinical assessments and self-report questionnaires
The Boston Assessment of TBI-Lifetime (BAT-L)28 was used to assess history of blast exposure and TBI and was administered by doctoral-level psychologists. The BAT-L is a semi-structured interview based on Department of Veterans Affairs and Department of Defense TBI diagnostic criteria, in which blast and TBI exposures from pre, during, and post-military experiences are queried in detail using a forensic approach. The BAT-L also includes classification of blast exposure events into distance ranges (0–10 m, 11–25 m, 26–100 m) as a proxy for blast severity. Total number of blast exposures across all ranges as well as total number of blast exposure events in the 0–10 m range were summed for each individual to obtain a continuous measure of total and close-range blast exposures. Approximately 19% (n = 35) individuals were not exposed to blast in this study but were included in analyses. MTBI was defined as the presence of altered mental status less than 24 h, posttraumatic amnesia less than 24 h, and/or loss of consciousness less than 30 min pre, during, or post-military experience (lifetime; yes/no and number) or during military experience related to blast or other deployment mechanisms (military; yes/no and number). Individuals who had a military mTBI (n = 90) are counted as having a lifetime mTBI, but it is possible that someone with a lifetime mTBI (n = 125) did not have a military mTBI (n = 35). Moderate and severe TBI were exclusionary criteria and therefore no participants had altered mental status or posttraumatic amnesia greater than 24 h or a loss of consciousness greater than 30 min. Table 2 lists the mTBI injury characteristics and metrics.
Table 2.
MTBI characteristics.
| Variable | M (SD) | n (%) |
|---|---|---|
| Lifetime mTBI | ||
| Time since most recent (years) | 9.6 (8.4) | |
| Altered mental status (minutes/individuals presenting) | 110.3 (257.2) | 125 (100) |
| Posttraumatic amnesia (minutes/individuals presenting) | 42.7 (160.5) | 103 (82.4) |
| Loss of consciousness (minutes/individuals presenting) | 2.1 (5.2) | 74 (59.2) |
| Military mTBI | ||
| Time since most recent (years) | 6.4 (3.6) | |
| Altered mental status (minutes/individuals presenting) | 84.2 (231.4) | 90 (100) |
| Posttraumatic amnesia (minutes/individuals presenting) | 13.6 (62.5) | 66 (73.3) |
| Loss of consciousness (minutes/individuals presenting) | 1.8 (5.3) | 46 (51.1) |
Note: The mTBI characteristics for the full sample are shown before removal of individuals with extreme values on blast exposure measures (n = 4 for number of total blast exposures; n = 2 for number of CBEs). One hundred and twenty-five individuals reported a lifetime mTBI (pre-, during, or post-deployment) and 90 individuals reported a military mTBI (during deployment and related to military operations). Altered mental status, posttraumatic amnesia, loss of consciousness are presented in average minutes (listed in M(SD) column) as well as how many individuals presented with these characteristics (listed in n (%) column).
mTBI: mild traumatic brain injury; PTSD: posttraumatic stress disorder.
PTSD was assessed with the Clinician-Administered PTSD Scale (CAPS) for DSM-IV29 and was administered by doctoral level psychologists. The CAPS is currently the gold standard for assessment of PTSD and captures the presence of PTSD within the past 30 days. Total CAPS score was used in analyses as a measure of current PTSD symptom severity.
The Fagerstrom Test for Nicotine Dependence (FTND)30 was used to assess smoking status. The FTND is a self-report questionnaire in which individuals answer various questions relating to nicotine dependence. Endorsement of cigarette smoking was used in analyses as a measure of smoking status (yes/no).
All diagnoses are reviewed by a diagnostic team consensus of at least three Ph.D. or M.D.s for confirmation of TBI and DSM-IV diagnoses.
Image acquisition and processing
Acquisition
One hundred and twenty-seven participants’ neuroimaging data were collected with a 32-channel head coil (12-channel for structural scans) on a Siemens 3-Tesla TIM Trio whole-body scanner at VA Boston Healthcare System. Two Magnetization Prepared Rapid Gradient Echo (MP-RAGE) T1-weighted structural scans were acquired with the following parameters: TR = 2530 ms, TE = 3.32 ms flip angle = 7°, FOV = 256, Matrix = 256 × 256, voxel size = 1 mm3. The pCASL sequence obtained for this research study was previously reported in Wu et al.31 and successfully tested on humans. PCASL scans were acquired with the following parameters: TR = 4000 ms, TE = 11 ms, post-labeling delay = 1.5 s, labeling duration = 1.5 s, mean Gz × 10 = 6 mT/m, 30 tag-control pairs, Hanning window‐shaped RF pulse with duration = 500 μs, RF gap = 360 μs, flip angle = 25°, slice‐selective gradient = 6 mT/m, FOV = 220 mm, matrix = 64 × 64, 20 5 mm slices with 1 mm gap acquired sequentially inferior to superior, label placed at 9 cm below center of slices, no background suppression used, and a gradient echo planar imaging (EPI) sequence was used for image readout. Following an upgrade to the scanner, 53 participants had structural (collected with a 20-channel head coil) and pCASL (collected with a 64-channel head coil) scans on a Siemens Prisma Fit whole-body scanner at VA Boston Healthcare System. Every effort was made to keep sequence specifications the same after the upgrade, resulting in identical parameters for both structural and perfusion imaging except that the TE for the pCASL scan was changed from 11 ms to 12 ms. To account for potential scanner software differences, a scanner flag was included in analyses as a covariate. Individuals scanned with the Prisma were significantly older than those scanned with the Trio (Prisma, M (SD) = 36.5 (9.0); Trio, M(SD) = 32.6 (8.5); t(178) = −2.71, p = 0.007). No other demographic variables were significantly different between scanners. MP-RAGE scans were averaged to create a single high contrast-to-noise image. A second MP-RAGE was unavailable for three individuals and structural registration for those individuals was completed with a single MP-RAGE.
Processing
Data were processed with Bayesian Inference for Arterial Spin Labelling (BASIL), a collection of tools in FMRIB’s Software Library (FSL, version 6.0.1, http://www.fmrib.ox.ac.uk/fsl/) that creates quantitative CBF images from ASL data. First, structural images were pre-processed with fsl_anat, which is a general anatomical pipeline script that calls FMRIB tools such as brain extraction (BET), bias-field correction (FAST), tissue-type and subcortical structure segmentation (FAST and FIRST), orientation to standard MNI space (fslreorient2std), and linear and non-linear registration (FLIRT and FNIRT) for a comprehensive integrated structural analysis. Next, Oxford ASL (oxford_asl), a command line utility within BASIL, was used to process pCASL data to produce a calibrated map of resting state perfusion.32 PCASL data were motion corrected with MCFLIRT33 and partial volume corrected using the appropriate flags within the oxford_asl tool.34 Adaptive spatial smoothing on perfusion was also applied.35 The oxford_asl tool performed a tag-control subtraction to remove static tissue contribution and the resulting time series was used to calculate relative CBF by using a Bayesian model inversion technique,32 an arterial transit time of 1.3 s, and a T1 relaxation time of 1.65 s for arterial blood and 1.3 s for tissue. Relative CBF in scanner units was then converted to absolute physiological units (ml/100 g/min) with a calibration image, which was created using the mean of the unlabeled (control) images from the data. Specifically, asl_calib was called to model the M0 of cerebrospinal fluid (CSF) assuming 3 T default T1 CSF, T2 CSF, and T2 blood values (4.30 ms, 750 ms, and 150 ms, respectively) for calibration. The calibration process computed and applied the calibration factor by estimating equilibrium magnetization in the CSF in the ventricles, which relied on the structural image provided via fsl_anat to the oxford_asl command. This automatically generated a ventricle mask that was then transformed into ASL space and applied to the calibration image for conversion of relative CBF maps to physiological units. Resulting calibrated perfusion maps were visually inspected for data quality.
Whole-brain group-level analyses were conducted with flameo in FSL. First, the partial volume corrected, calibrated perfusion maps in standard space for each subject were combined into a single 4D image for further group-level processing. Next, to determine voxel-wise perfusion correlates with variables of interest, group-level analyses were conducted using mixed effects model FLAME stage 1.36–38 Age, scanner, sex, CAPS score, and blast exposure (either the number of total blast exposures or the number of CBEs) were entered into the model as regressors. Analyses were repeated including mTBI as an additional covariate in separate models for lifetime (yes/no or number) and military (yes/no or number) as well as including body mass index (BMI) and smoking status as additional covariates in separate models (along with age, scanner, sex, CAPS score, and blast exposure). For analyses examining mTBI group differences, age, scanner, sex, and CAPS score were entered in the model as regressors and group-level activation maps were generated for group contrasts (mTBI vs. no-mTBI), which were computed in separate models for lifetime and military TBI. Analyses examining the main effect of the number of mTBIs followed the same model as blast exposure but replaced the number of blast exposures with the number of lifetime or military mTBIs. Smoothness estimates were generated from the residuals using FSL’s smoothest command line utility. Z statistic images were thresholded using clusters determined by Z < 3.1 (p < 0.001) with a corrected cluster significance threshold of p = 0.05 using FSL’s cluster tool.
Results
Blast exposure
Several individuals had extreme values on the number of total blast exposures (n = 4, number of total blast exposures > 500) and CBEs (n = 2, number of CBEs > 50). These individuals were removed from the blast exposure analyses to ensure that they did not drive effects, leaving the final sample n = 176 for the number of total blast exposures: M(SD) = 22.5 (57.5) and n = 178 for the number of CBEs: M (SD) = 1.6 (3.3).
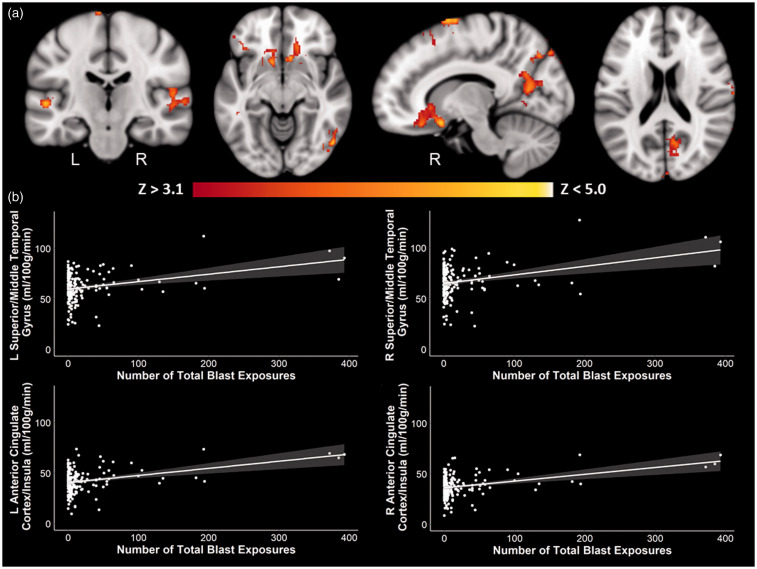
Whole-brain results revealed an effect of total blast exposure on perfusion after controlling for age, scanner, sex, and current PTSD symptom severity such that with an increasing number of total blast exposures, there was increased perfusion in right middle/superior frontal gyri, supramarginal gyrus, lateral occipital cortex, and posterior cingulate cortex as well as bilateral anterior cingulate cortex, insulae, middle/superior temporal gyri and occipital poles (Table 3, Figure 1). There were no significant negative associations between perfusion and the number of total blast exposures. Additionally, there were no significant effects that survived multiple comparison correction when the number of CBEs was examined.
Table 3.
Significant whole-brain positive associations between perfusion and the number of total blast exposures with and without TBI, BMI, and smoking status as covariates.
| Contrast | Cluster size | Z Max | X | Y | Z | Brain region |
|---|---|---|---|---|---|---|
| Positive blast exposure effect, controlling for age, scanner, sex, and PTSD symptom severity | 1793 | 5.92 | 42 | 8 | 60 | Right middle frontal gyrus/superior frontal gyrus |
| 815 | 5.29 | −10 | 14 | −12 | Left anterior cingulate cortex/frontal orbital cortex/insula | |
| 727 | 5.01 | 10 | 14 | −16 | Right anterior cingulate cortex/frontal orbital cortex/insula | |
| 594 | 5.02 | 52 | −68 | −6 | Right lateral occipital cortex | |
| 389 | 4.59 | 62 | −48 | 36 | Right angular gyrus/supramarginal gyrus | |
| 346 | 4.25 | 14 | −60 | 28 | Right precuneus/posterior cingulate cortex | |
| 308 | 4.42 | 2 | −88 | 34 | Bilateral occipital pole | |
| 296 | 4.43 | 56 | −22 | 10 | Right superior temporal gyrus/middle temporal gyrus | |
| 188 | 4.75 | −52 | −22 | 0 | Left superior temporal gyrus/middle temporal gyrus | |
| Positive blast exposure effect, controlling for age, scanner, sex, PTSD symptom severity, and lifetime mTBI (yes/no) | 1361 | 5.68 | 42 | 8 | 60 | Right middle frontal gyrus/superior frontal gyrus |
| 735 | 5.44 | −10 | 14 | −12 | Left anterior cingulate cortex/frontal orbital cortex/insula | |
| 591 | 4.88 | 10 | 14 | −16 | Right anterior cingulate cortex/frontal orbital cortex/insula | |
| 487 | 4.99 | 52 | −68 | −6 | Right lateral occipital cortex | |
| 262 | 4.28 | 68 | −14 | 14 | Right superior temporal gyrus/postcentral gyrus | |
| 250 | 4.27 | 2 | −86 | 34 | Bilateral occipital pole | |
| 233 | 4.12 | 14 | −60 | 28 | Right precuneus/posterior cingulate cortex | |
| 227 | 4.37 | 62 | −48 | 36 | Right angular gyrus/supramarginal gyrus | |
| Positive blast exposure effect, controlling for age, scanner, sex, PTSD symptom severity, and lifetime mTBI (number) | 1832 | 5.95 | 42 | 8 | 60 | Right middle frontal gyrus/superior frontal gyrus |
| 816 | 5.30 | −10 | 14 | −12 | Left anterior cingulate cortex/frontal orbital cortex/insula | |
| 741 | 5.00 | 10 | 14 | −16 | Right anterior cingulate cortex/frontal orbital cortex/insula | |
| 580 | 4.99 | 52 | −68 | −6 | Right lateral occipital cortex | |
| 379 | 4.56 | 62 | −48 | 36 | Right angular gyrus/supramarginal gyrus | |
| 331 | 4.22 | 14 | −60 | 28 | Right precuneus/posterior cingulate cortex | |
| 300 | 4.40 | 56 | −22 | 10 | Right planum temporale/superior temporal gyrus | |
| 298 | 4.41 | 2 | −88 | 34 | Bilateral occipital pole | |
| 197 | 4.83 | −52 | −22 | 0 | Left planum temporale/superior temporal gyrus | |
| Positive blast exposure effect, controlling for age, scanner, sex, PTSD symptom severity, and military mTBI (yes/no) | 1542 | 5.75 | 42 | 8 | 60 | Right middle frontal gyrus/superior frontal gyrus |
| 735 | 5.37 | −10 | 14 | −12 | Left anterior cingulate cortex/frontal orbital cortex/insula | |
| 628 | 4.94 | 10 | 14 | −16 | Right anterior cingulate cortex/frontal orbital cortex/insula | |
| 507 | 5.00 | 52 | −68 | −6 | Right lateral occipital cortex | |
| 420 | 4.41 | 54 | −22 | 8 | Right superior temporal gyrus/middle temporal gyrus | |
| 326 | 4.39 | 2 | −88 | 34 | Bilateral occipital pole | |
| 304 | 4.18 | 14 | −60 | 28 | Right precuneus/posterior cingulate cortex | |
| 282 | 4.50 | 62 | −48 | 36 | Right angular gyrus/supramarginal gyrus | |
| 229 | 4.86 | −52 | −22 | 0 | Left superior temporal gyrus/middle temporal gyrus | |
| Positive blast exposure effect, controlling for age, scanner, sex, PTSD symptom severity, and military mTBI (number) | 1816 | 5.97 | 42 | 8 | 60 | Right middle frontal gyrus/superior frontal gyrus |
| 807 | 5.29 | −10 | 14 | −12 | Left anterior cingulate cortex/frontal orbital cortex/insula | |
| 730 | 5.01 | 10 | 14 | −16 | Right anterior cingulate cortex/frontal orbital cortex/insula | |
| 530 | 4.98 | 52 | −68 | −6 | Right lateral occipital cortex | |
| 349 | 4.21 | 14 | −64 | 12 | Right intracalcarine cortex/precuneus | |
| 344 | 4.39 | 68 | −14 | 14 | Right postcentral gyrus/superior temporal gyrus | |
| 299 | 4.56 | 62 | −48 | 36 | Right angular gyrus/supramarginal gyrus | |
| 290 | 4.42 | 2 | −88 | 34 | Bilateral occipital pole | |
| 213 | 4.90 | −52 | −22 | 0 | Left superior temporal gyrus/middle temporal gyrus | |
| Positive blast exposure effect controlling for age, scanner, sex, PTSD symptom severity, and BMIa | 2021 | 5.89 | 42 | 8 | 60 | Right middle frontal gyrus/superior frontal gyrus |
| 1489 | 5.10 | −10 | 14 | −12 | Left anterior cingulate cortex/frontal orbital cortex/insula | |
| 721 | 5.33 | 52 | −68 | −6 | Right lateral occipital cortex | |
| 555 | 4.81 | 62 | −48 | 36 | Right angular gyrus/supramarginal gyrus | |
| 484 | 4.57 | 2 | −88 | 34 | Bilateral occipital pole | |
| 471 | 4.18 | 32 | −38 | −6 | Right parahippocampus/hippocampus | |
| 422 | 4.35 | 14 | −60 | 28 | Right precuneus/posterior cingulate cortex | |
| 392 | 4.16 | −8 | −18 | 18 | Bilateral thalami | |
| 326 | 4.48 | 56 | −22 | 10 | Right superior temporal gyrus/middle temporal gyrus | |
| Positive blast exposure effect controlling for age, scanner, sex, PTSD symptom severity, and smoking statusb | 1551 | 5.63 | 42 | 8 | 60 | Right middle frontal gyrus/superior frontal gyrus |
| 415 | 4.92 | −10 | 14 | −12 | Left anterior cingulate cortex/frontal orbital cortex/insula | |
| 402 | 4.91 | 30 | 24 | −24 | Right frontal orbital cortex/temporal pole/anterior cingulate cortex | |
| 386 | 4.77 | 44 | −74 | −18 | Right lateral occipital cortex | |
| 302 | 5.03 | 20 | −88 | 44 | Right occipital pole/lateral occipital cortex | |
| 247 | 4.44 | 52 | −70 | 32 | Right lateral occipital cortex |
Note: Coordinates are in Montreal Neurological Institute (MNI) space. Cluster size is number of voxels in standard space (2 mm3 voxel size). Only peak coordinates of clusters are listed. Subclusters are not reported. There were no significant negative associations between perfusion and the number of total blast exposures.
aBMI data were unavailable for two participants (n = 174).
bSmoking status was unavailable for 17 participants (n = 159).
BMI: body mass index; PTSD: posttraumatic stress disorder; mTBI: mild traumatic brain injury.
Figure 1.
Positive whole-brain associations between blast exposure and perfusion. There was a significant positive association between the number of total blast exposures and perfusion in regions such as the middle/superior temporal gyri, superior/middle frontal gyri, anterior cingulate cortex, insulae, and occipital cortex. (a) Coronal, axial, and sagittal slices of perfusion in association with the number of total blast exposures are shown in MNI space, controlling for age, scanner, sex, and current PTSD symptom severity. Color scale indicates Z-score threshold. (b) Corresponding scatter plots of selected regions, with 95% confidence intervals shaded in gray, show the positive association between the number of total blast exposures and perfusion. Other brain regions showed similar positive associations as those represented in the scatter plots and are not shown here. L: left; R: right.
To determine whether the removal of blast exposure outliers impacted the results, we re-ran the whole-brain analyses examining perfusion associations with the number of total blast exposures and the number of CBEs including the whole sample (n = 180) without the removal of the n = 4 outliers on the number of total blast exposures or n = 2 outliers on the number of CBEs. Results revealed a similar pattern with the exception that after inclusion of outliers, there was increased perfusion in several brain regions with a greater number of CBEs. These results are reported in the Supplemental Materials (see Supplemental Materials Figure S1).
Additional whole-brain analyses examining blast exposure groups (yes/no and no/low/moderate/high groups) rather than a continuous measure of blast exposure revealed increased perfusion in high blast exposed individuals (15–500 blast exposures) compared to unexposed individuals (0 blast exposures) in the left anterior cingulate cortex extending into the frontal orbital cortex and insula. Moreover, high blast exposed individuals had increased perfusion in the left inferior frontal gyrus/frontal orbital cortex and the left angular gyrus/supramarginal gyrus compared to low (1–4 blast exposures) and moderate (5–14 blast exposures) blast exposed groups. There were no significant whole-brain differences in perfusion between no blast exposure and low and moderate groups or between dichotomous yes/no blast exposure groups that survived multiple comparison correction. Further details regarding the methods and results of these blast exposure group analyses are reported in the Supplemental Materials.
To confirm that the effect was associated with blast exposure and not with underlying TBI effects, we repeated analyses including lifetime TBI (yes/no or number) or military TBI (yes/no or number) separately as additional covariates in the model. The pattern of results did not change when mTBI variables were additionally included in the analyses such that all regions listed remained significantly associated with blast (Table 3). Similarly, because weight and cigarette use can affect perfusion, we also ran follow-up analyses controlling for BMI (n = 174) and self-reported cigarette usage (yes/no, n = 159), separately. Again, the pattern of results did not change when these variables were included in the analyses such that all regions listed remained significant (Table 3).
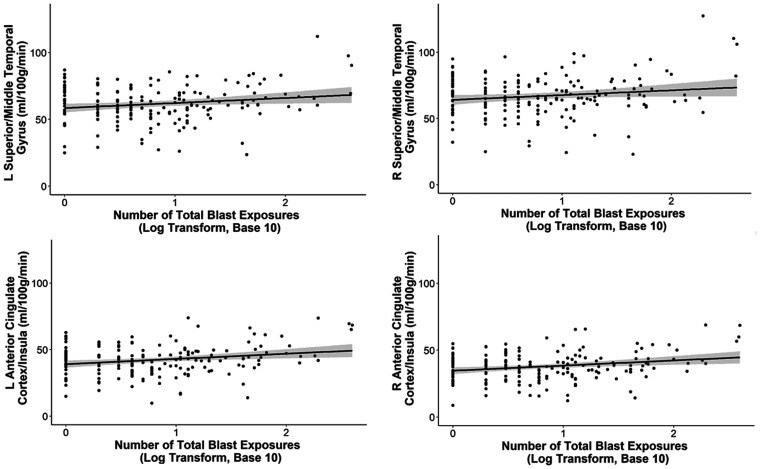
Lastly, to account for the potential effects of the skewed distribution of the number of total blast exposures, we ran follow-up regression models that included the log transform (with base 10) of the number of total blast exposures as the independent variable. Separate hierarchal regression models were performed for each significant cluster that survived multiple comparison correction in the primary whole-brain analysis with the non-log transformed data. Perfusion values of significant clusters were extracted from each participant’s partial volume corrected, calibrated perfusion image in standard space. Age, scanner, sex, and current PTSD symptom severity were included in the first step of the model and the log transform of the number of total blast exposures was added in the second step. The pattern of results did not change with a significant change in the model when the log transform of the number of total blast exposures was added, with a positive effect on all clusters examined with the exception of the right precuneus/posterior cingulate cortex, which just missed the standard threshold significance (p = 0.061): right middle frontal gyrus/superior frontal gyrus: ΔR2 = 0.031, ΔF(1, 170) = 6.363, β = 0.184, unstandardized B = 4.589, p = 0.013; left anterior cingulate cortex/frontal orbital cortex/insula: ΔR2 = 0.05, ΔF(1, 170) = 9.957, β = 0.235, unstandardized B = 4.158, p = 0.002; right anterior cingulate cortex/frontal orbital cortex/insula: ΔR2 = 0.049, ΔF(1, 170) = 9.658, β = 0.232, unstandardized B = 3.108, p = 0.002; right lateral occipital cortex: ΔR2 = 0.04, ΔF(1, 170) = 8.527, β = 0.211, unstandardized B = 4.402, p = 0.004; right angular gyrus/supramarginal gyrus: ΔR2 = 0.036, ΔF(1, 170) = 6.892, β = 0.198, unstandardized B = 5.11, p = 0.009; right precuneus/posterior cingulate cortex: ΔR2 = 0.019, ΔF(1, 170) = 3.558, β = 0.144, unstandardized B = 3.084, p = 0.061; bilateral occipital pole: ΔR2 = 0.025, ΔF(1, 170) = 4.807, β = 0.166, unstandardized B = 4.035, p = 0.03; right superior temporal gyrus/middle temporal gyrus cluster: ΔR2 = 0.025, ΔF(1, 170) = 5.009, β = 0.167, unstandardized B = 3.986, p = 0.027; left superior temporal gyrus/middle temporal gyrus cluster: ΔR2 = 0.036, ΔF(1, 170) = 6.478, β = 0.198, unstandardized B = 4.22, p = 0.012. Figure 2 shows the scatter plots of the association between the log transformation of the number of total blast exposures and perfusion in selected brain regions.
Figure 2.
Positive associations between perfusion and the log transformation of the number of total blast exposures. The significant positive associations between the number of total blast exposures and perfusion in regions such as the middle/superior temporal gyri, superior/middle frontal gyri, anterior cingulate cortex, insulae, and occipital cortex remained significant when the number of total blast exposures was log transformed (base 10) to account for the potential skew of the data. The 95% confidence intervals are shaded in gray. Other brain regions showed similar positive associations as those represented in the scatter plots and are not shown here. L: left; R: right.
mTBI
To examine the main effects of mTBI on perfusion, we performed whole-brain analyses examining (1) lifetime mTBI (yes/no or number) and (2) military mTBI (yes/no or number) in place of blast exposure. Results revealed no significant mTBI group differences in perfusion after correction for multiple comparison. However, there was a significant overlapping positive linear association between the number of lifetime and military mTBIs and perfusion in the left cerebellum (number of lifetime mTBIs: number of voxels = 277, Z-Max = 6.25, peak MNI coordinates = −10 −66 −56; number of military mTBIs: number of voxels = 315, Z-Max = 7.9, peak MNI coordinates = −32 −48 −58).
The interaction between mTBI and blast exposure
To investigate whether mTBI had impact on perfusion within the context of a blast, we performed follow-up hierarchal regression analyses in which we examined the interaction between the number of total blast exposures and (1) the number of mTBIs (lifetime or military mTBIs in separate models) as well as (2) the history of mTBI (yes/no, lifetime or military mTBI in separate models) on each significant cluster that survived multiple comparison correction in the whole-brain blast exposure analysis. Age, scanner, sex, and current PTSD symptom severity were included in the first step of the model, the main effects of the number of total blast exposures and (1) the number of mTBIs (lifetime or military) or (2) history of mTBI (yes/no, lifetime or military) were added to the second step, and the interaction between the number of total blast exposures and (1) the number of mTBIs (lifetime or military) or (2) the history of mTBI (yes/no, lifetime or military) was included in the final step.
After Bonferroni multiple comparison correction (correcting across nine significant clusters for each set of mTBI models), there was only one significant interaction between the number of total blast exposures and the number of military mTBIs that survived correction on the right angular gyrus/supramarginal gyrus cluster (overall model: R2 = 0.217, F(7,175) = 6.632, p < 0.001; change in model: Δ R2 = 0.038, ΔF(1,168) = 8.217, β = −0.273, unstandardized B = −0.029, p = 0.005). Inspection of this interaction revealed that individuals with a greater number of military mTBIs and low blast exposure had decreased perfusion in this region. There were no other significant interactions between the number of total blast exposures and the number of lifetime or military mTBIs that survived multiple comparison correction on any of the remaining perfusion clusters (number of lifetime mTBIs interactions: ΔR2 ranged from 0.000 to 0.031, ΔF(1,168) ranged from 0.002 to 6.689, unstandardized B ranged from −0.027 to 0.002, β ranged from −0.282 to 0.037, p’s > 0.011 (ranged from 0.011 to 0.968); number of military mTBIs interactions: ΔR2 ranged from 0.000 to 0.031, ΔF(1,168) ranged from 0.011 to 6.431, unstandardized B ranged from −0.022 to 0.001, β ranged from −0.246 to 0.014, p’s > 0.012 (ranged from 0.012 to 0.918)). Similarly, there were no significant interactions between the number of total blast exposures and the history of lifetime or military mTBI (yes/no) that survived multiple comparison correction on any perfusion cluster (history of lifetime mTBI interactions: ΔR2 ranged from 0.002 to 0.024, ΔF(1,168) ranged from 0.400 to 2.978, unstandardized B ranged from 0.042 to 0.225, β ranged from 0.224 to 0.580, p’s > 0.02 (ranged from 0.02 to 0.528); history of military mTBI interactions: ΔR2 ranged from 0.000 to 0.018, ΔF(1,168) ranged from 0.005 to 3.697, unstandardized B ranged from −0.075 to 0.021, β ranged from −0.262 to 0.097, p’s > 0.056 (ranged from 0.056 to 0.946)).
It is important to note that individuals with a lifetime mTBI in this sample had a significantly greater number of total blast exposures than those without (No lifetime mTBI, M(SD) = 8.51(21.13); Yes lifetime mTBI, M(SD) = 28.50 (66.61); t(164.6) = −2.996, p = 0.003). However, the total number of lifetime mTBIs was not significantly correlated with the number of total blast exposures (r = 0.073, p = 0.337). These results were similar for military mTBI, with a significant group difference in the number of total blast exposures (No military mTBI, M(SD) = 13.17(43.88); Yes military mTBI, M(SD) = 31.78(67.51); t(149.38) = −2.169, p = 0.032), but no significant correlation between the number of military mTBIs and total blast exposures (r = 0.088, p = 0.244).
Other factors related to perfusion
Examination of the main effects of covariates revealed significant negative associations between age and perfusion such that with increasing age, there was decreased perfusion in bilateral occipital poles and frontal poles as well as the left temporal pole, inferior temporal gyrus, and insula (Supplemental Materials Table S1). Additionally, there was a significant effect of scanner on perfusion such that individuals scanned with the Prisma scanner (compared to those with the Trio) had decreased perfusion in the right cerebellum (Supplemental Materials Table S2). There was a main effect of sex on perfusion such that females (n = 13) had increased perfusion in bilateral cerebellum, occipital cortices, and frontal poles compared to males (Supplemental Materials Table S3). There were no significant main effects of current PTSD symptom severity as measured by the CAPS on perfusion. Examination of the main effects of BMI and smoking status revealed a significant negative main effect of BMI on perfusion such that with increasing BMI, there was reduced perfusion within the right occipital cortex and bilateral cerebellum (Supplemental Materials Table S4) but there were no significant main effects of smoking status on perfusion.
Discussion
To our knowledge, this is the first study to investigate the consequences of blast exposure and perfusion in a large human cohort of young Iraq and Afghanistan war Veterans and military personnel. Using pCASL imaging, we found that the number of total blast exposures (irrespective of close-range distance) was associated with increased perfusion in the middle/superior frontal gyri, anterior cingulate cortex, middle/superior temporal gyri, supramarginal gyrus, and insula. This relationship was not apparent in other neurotrauma and clinical variables such as the presence or number of lifetime or military mTBIs or current PTSD symptom severity. Additionally, although individuals with more lifetime and military mTBIs had higher levels of blast exposure, there were no significant mTBI by blast exposure interactions except for the right angular gyrus/supramarginal gyrus. Interestingly, when blast exposure was examined by group, we found that individuals exposed to a high amount of blast exposure (15–500 blasts) had increased perfusion in the left anterior cingulate cortex compared to unexposed individuals (0 blasts) as well as in the left inferior frontal gyrus/frontal orbital cortex and the left angular gyrus/supramarginal gyrus compared to individuals exposed to low (1–4 blasts) and moderate (5–14 blasts) amounts of blast. Together, these results add to the growing literature surrounding the importance of blast exposure in brain health and integrity.4–8
Our finding that the number of blast exposures, even without accompanying symptoms of an mTBI, is associated with increased perfusion suggests that the mechanistic properties of multiple blast exposures (i.e. how blasts interact with the body and brain) may be important in cerebrovascular health. Although previous work has shown that the vasculature is especially vulnerable to the effects of blast via molecular processes that may impact CBF,25,39–41 our results extend this work by suggesting a potential dose-effect of blast exposure on cerebral perfusion in diffuse brain regions. Moreover, our work suggests that perfusion in the left anterior cingulate cortex, left inferior frontal gyrus/frontal orbital cortex, and left angular gyrus/supramarginal gyrus may require a critical exposure threshold for perfusion disruptions. Specifically, we found that only individuals in a high blast exposure group (15–500 blast exposures) had increased perfusion in these regions compared to no, low, and moderate blast exposed individuals, suggesting that perfusion within the left anterior cingulate cortex, inferior frontal gyrus/frontal orbital cortex, and angular gyrus/supramarginal gyrus may not be dose-responsive but may require some critical threshold of blast. However, further work is needed to confirm these results.
Our finding points to the importance of examining blast exposures even in the absence of symptoms of acute mTBI at the time of the injury, as a blast exposure metric may account for sub-concussive injuries not measured by mTBI diagnosis. Recent work has shown that sub-concussive blows to the head without acute symptoms are important for brain health and neurodegenerative processes.4,5,8,42–44 Therefore, a metric that accounts for sub-concussive hits (such as the number of blast exposures) may be a particularly sensitive marker for sub-clinical but persistent effects on the brain. Interestingly, this finding of increased perfusion was irrespective of close-range blast exposure distance. This is contrary to previous work by our group, in which close-range blast exposure within 10 m has been associated with diffuse white matter abnormalities and resting state functional connectivity disruptions.4,8 Taken together, the results suggest that gray matter perfusion may be more sensitive to diffuse disruptions via blast at greater distances than other brain metrics such as functional connectivity and white matter integrity, which may require increased proximity for discernable impacts on the brain.
Contrary to our hypotheses, we did not find decreased perfusion in association with blast exposure and were unable to replicate previous work that reported an association between mTBI and perfusion.20–22 However, it is important to note that our study included a large, predominately combat cohort, which was different from past research in civilians that reported TBI associations with perfusion. Nonetheless, we did include a metric of mTBI that accounted for any history of mTBI (pre-, during, and post- deployment) including civilian-related etiologies as well as a military-specific mTBI measure that examined mTBI that occurred only during deployment (not accounting for pre or post deployment histories of mTBI), potentially isolating blast effects within the context of deployment. In the one study that did report an association between TBI and cerebrovascular dysfunction in a Veteran cohort,22 moderate and severe TBI were included in the sample, which were exclusionary in our study. Instead, we showed that whole-brain perfusion increases were related to blast exposure irrespective of the presence of a TBI. Specifically, we report increased perfusion in diffuse brain regions including middle/superior frontal gyri, anterior cingulate cortex, middle/superior temporal gyri, supramarginal gyrus, and insula. Consistent with these findings, studies that have examined the white matter of blast exposed samples have also reported diffuse patterns of disruptions,6–8,45,46 potentially suggesting that diffuse alterations to the brain may be a marker of blast injury. Moreover, studies of mild cognitive impairment patients who are in the prodromal phase of Alzheimer’s disease have also reported increased perfusion in frontal, hippocampal, and temporal regions, raising the possibility that increased perfusion in these brain regions may be a marker of metabolic stress on the system as a direct result of pathology or may reflect a compensatory response for cognitive decline.47–50 Work within the Alzheimer’s disease framework further suggests that increases in brain activity and metabolism may be the first signs of neurodegeneration, representing excitotoxic reactions and failures to properly inhibit activity, which may thereby result in further synaptic and tissue dysfunction and degradation.51–54
Nonetheless, this finding of increased perfusion contrasts with animal work of cerebral perfusion in blast,55 although acute (i.e. within 72 h) rather than chronic blast effects were examined. Many aspects of vascular health exhibit temporal progressions25 and this study examined Veterans years after their blast exposures. Thus, it is possible that the increased perfusion noted here indicates long-lasting impairments such as disruptions in autoregulation and vasoconstriction or dilation as well as hypercapnia and inflammation.25,56–59 Another possibility is that our results may reflect vascular remodeling in which increased perfusion compensates for dead-end vessels that resulted from the blast.25,60,61 Additionally, increased perfusion may reflect consequences of cavitation, a phenomenon in which blast-induced hyper-pressurized bubbles form within the body and upon collapse can generate intense shock waves and impact deep neural tissues and vessels.62,63 However, these hypotheses notwithstanding, more work is needed to confirm our results.
There are several limitations of the current study. First, this study is cross-sectional, and we cannot make causal inferences. Second, although the present study performed several follow-up analyses to determine whether the perfusion effects observed were blast-related rather than mTBI- or PTSD-related, there remain many other untested variables that could potentially account for the main effects of blast exposure observed in this study. Future work should aim to replicate these blast effects and examine other potential confounds. Third, mTBI and blast exposure estimates were based on retrospective self-report and may be subject to reporting bias. However, the BAT-L uses a forensic semi-structured interview approach, which helps account for some limitations associated with retrospective reports.28 Nonetheless, the consistency of retrospective recall of TBI is poor over time64 and thus, assessment of blast exposure may be subject to similar issues. Fourth, use of outgoing weapons and munitions, such as the shoulder-launched multipurpose assault weapon were not included in our blast measure, which may underestimate overall reporting of low-level blast exposure. Fifth, we were unable to quantify the spatially heterogenous nature of blast exposure injury in the context of perfusion. Recent work has pointed to the importance of considering spatial heterogeneity with respect to blast in other brain tissue such as white matter.7,45,65 It will be important for future work to examine blast exposure in perfusion within a spatially heterogenous context. Sixth, we were unable to study the functional consequences of the reported alterations of perfusion in blast exposure. Future work is needed to determine whether increases in perfusion in blast exposure have any bearing on functional outcome. Finally, our sample was primarily combat Veterans who were predominately male, limiting our ability to generalize our results to women or cohorts consisting of non-military groups.
In summary, we provide novel evidence that blast exposure (irrespective of close-range distance) is associated with cerebrovascular dysfunction, even after accounting for mTBI and PTSD. Specifically, our results point to a potential dose-effect of blast exposure such that with an increasing number of blast exposures, there is increased perfusion in diffuse brain regions. Given that blast exposure and sub-concussive impacts to the head are emerging as potential risk factors for neurodegenerative disease,8,66,67 the current findings place emphasis on the importance of investigating the neural correlates of blast exposure and provide preliminary insights into perfusion as a potential mechanism that warrants further study.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20935190 for Cerebral perfusion is associated with blast exposure in military personnel without moderate or severe TBI by Danielle R Sullivan, Mark W Miller, Erika J Wolf, Mark W Logue, Meghan E Robinson, Catherine B Fortier, Jennifer R Fonda, Danny JJ Wang, William P Milberg, Regina E McGlinchey and David H Salat in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors thank the team of investigators at TRACTS for their assistance with data collection and management. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Authors’ contributions: DRS conceptualized the manuscript, processed all of the neuroimaging data, conducted the data analysis, and wrote the manuscript. MER, CBF, and JRF performed some of the data collection. DJJW provided the pCASL sequence. MWM, EJW, MER, and DHS, assisted with interpretation of the results. DRS, MWM, EJW, MWL, MER, CBF, JRF, DJJW, WPM, REM, & DHS provided critical feedback and intellectual content and helped shape the research, analysis, and final manuscript. All authors approved the final version.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Career Development Award (grant number: 1 IK2 CX001772-01) from the United States Department of Veterans Affairs, Clinical Science Research and Development Service awarded to D.R.S.; and the Translational Research Center for TBI and Stress Disorders (TRACTS), a VA Rehabilitation Research and Development National Network Research Center (B9254-C). This work was further supported with resources and the use of facilities at the National Center for PTSD, the Neuroimaging Research for Veterans Center, VA Boston Healthcare System, and the Core for Advanced MRI at Baylor College of Medicine.
ORCID iD: Danielle R Sullivan https://orcid.org/0000-0002-0141-5887
Supplemental material: Supplemental material for this article is available online.
References
- 1.Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 2008; 358: 453–463. [DOI] [PubMed] [Google Scholar]
- 2.Cernak I, Noble-Haeusslein LJ.Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab 2010; 30: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salat DH, Robinson ME, Miller DR, et al. Neuroimaging of deployment-associated traumatic brain injury (TBI) with a focus on mild TBI (mTBI) since 2009. Brain Injury 2017; 31: 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson ME, Lindemer ER, Fonda JR, et al. Close-range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. Hum Brain Mapp 2015; 36: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trotter BB, Robinson ME, Milberg WP, et al. Military blast exposure, ageing and white matter integrity. Brain 2015; 138: 2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazarian JJ, Donnelly K, Peterson DR, et al. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during operations enduring freedom and Iraqi freedom. J Head Trauma Rehabil 2012; 28: 1–12. [DOI] [PubMed] [Google Scholar]
- 7.Taber KH, Hurley RA, Haswell CC, et al. White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil 2015; 30: E15–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan DR, Logue M, Wolf EJ, et al. Close-range blast exposure is associated with altered white matter integrity in APOE ε4 carriers. J Neurotrauma 2019; 36: 3264–3273. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan DR.A cerebrovascular hypothesis of neurodegeneration in mTBI. J Head Trauma Rehabil 2019; 34: E18–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson M, Clark D, Milberg W, et al. Characterization of differences in functional connectivity associated with close-range blast exposure. J Neurotrauma 2017; 34: S53–S61. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Das SR, Xie SX, et al. Arterial spin labeled MRI in prodromal Alzheimer’s disease: a multi-site study. Neuroimage 2013; 2: 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernbaum M, Menon BK, Fick G, et al. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J Cereb Blood Flow Metab 2015; 35: 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Thrippleton MJ, Makin SD, et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2016; 36: 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xekardaki A, Rodriguez C, Montandon M-L, et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 2014; 274: 490–499. [DOI] [PubMed] [Google Scholar]
- 15.Zlokovic BV.Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 2005; 28: 202–208. [DOI] [PubMed] [Google Scholar]
- 16.Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatr Res 2009; 172: 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Promjunyakul N, Lahna D, Kaye JA, et al. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. Neuroimage 2015; 8: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedictus MR, Leeuwis AE, Binnewijzend MAA, et al. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur Radiol 2017; 27: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao LL, Buckley ST, Kornak J, et al. ASL Perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord 2010; 24: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossman EJ, Jensen JH, Babb JS, et al. Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. Am J Neuroradiol 2013; 34: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge Y, Patel MB, Chen Q, et al. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labeling MR imaging at 3T. Brain Injury 2009; 23: 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponto LLB, Brashers-Krug TM, Pierson RK, et al. Preliminary investigation of cerebral blood flow and amyloid burden in Veterans with and without combat-related traumatic brain injury. J Neuropsychiatr Clin Neurosci 2015; 28: 89–96. [DOI] [PubMed] [Google Scholar]
- 23.Clark AL, Bangen KJ, Sorg SF, et al. Dynamic association between perfusion and white matter integrity across time since injury in Veterans with history of TBI. Neuroimage 2017; 14: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenney K, Amyot F, Haber M, et al. Cerebral vascular injury in traumatic brain injury. Exp Neurol 2016; 275: 353–366. [DOI] [PubMed] [Google Scholar]
- 25.Elder GA, Gama Sosa MA, De Gasperi R, et al. Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front Neurol 2015; 6: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gama Sosa MA, De Gasperi R, Perez Garcia GS, et al. Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain. Acta Neuropathol Commun 2019; 7: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGlinchey RE, Milberg WP, Fonda JR, et al. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudinal prospective cohort study. Int J Meth Psychiatr Res 2017; 26: e1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortier CB, Amick MM, Grande L, et al. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. J Head Trauma Rehabil 2014; 29: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress 1995; 8: 75–90. [DOI] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991; 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 31.Wu WC, Fernández‐Seara M, Detre JA, et al. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magnet Reson Med 2007; 58: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 32.Chappell MA, Groves AR, Whitcher B, et al. Variational Bayesian inference for a nonlinear forward model. IEEE Transac Signal Process 2008; 57: 223–236. [Google Scholar]
- 33.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17: 825–841. [DOI] [PubMed] [Google Scholar]
- 34.Chappell MA, Groves AR, MacIntosh BJ, et al. Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magnet Reson Med 2011; 65: 1173–1183. [DOI] [PubMed] [Google Scholar]
- 35.Groves AR, Chappell MA, Woolrich MW.Combined spatial and non-spatial prior for inference on MRI time-series. Neuroimage 2009; 45: 795–809. [DOI] [PubMed] [Google Scholar]
- 36.Beckmann CF, Jenkinson M, Smith SM.General multilevel linear modeling for group analysis in FMRI. Neuroimage 2003; 20: 1052–1063. [DOI] [PubMed] [Google Scholar]
- 37.Woolrich M.Robust group analysis using outlier inference. Neuroimage 2008; 41: 286–301. [DOI] [PubMed] [Google Scholar]
- 38.Woolrich MW, Behrens TE, Beckmann CF, et al. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 2004; 21: 1732–1747. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed F, Plantman S, Cernak I, et al. The temporal pattern of changes in serum biomarker levels reveals complex and dynamically changing pathologies after exposure to a single low-intensity blast in mice. Front Neurol 2015; 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling G, Bandak F, Armonda R, et al. Explosive blast neurotrauma. J Neurotrauma 2009; 26: 815–825. [DOI] [PubMed] [Google Scholar]
- 41.Simard JM, Pampori A, Keledjian K, et al. Exposure of the thorax to a sublethal blast wave causes a hydrodynamic pulse that leads to perivenular inflammation in the brain. J Neurotrauma 2014; 31: 1292–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012; 4: 134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee AC, Stein TD, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baugh CM, Stamm JM, Riley DO, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imag Behav 2012; 6: 244–254. [DOI] [PubMed] [Google Scholar]
- 45.Miller DR, Hayes JP, Lafleche G, et al. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum Brain Mapp 2016; 37: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller DR, Hayes JP, Lafleche G, et al. White matter abnormalities are associated with overall cognitive status in blast-related mTBI. Brain Imag Behav 2017; 11: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luckhaus C, Flüß MO, Wittsack H-J, et al. Detection of changed regional cerebral blood flow in mild cognitive impairment and early Alzheimer’s dementia by perfusion-weighted magnetic resonance imaging. Neuroimage 2008; 40: 495–503. [DOI] [PubMed] [Google Scholar]
- 48.Johnson K, Moran E, Becker J, et al. Single photon emission computed tomography perfusion differences in mild cognitive impairment. J Neurol Neurosurg Psychiatr 2007; 78: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Wahlund L-O, Almkvist O, et al. Voxel-and VOI-based analysis of SPECT CBF in relation to clinical and psychological heterogeneity of mild cognitive impairment. Neuroimage 2003; 19: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 50.Alsop DC, Casement M, de Bazelaire C, et al. Hippocampal hyperperfusion in Alzheimer’s disease. Neuroimage 2008; 42: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beason-Held LL, Goh JO, An Y, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci 2013; 33: 18008–18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012; 74: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sojkova J, Beason-Held L, Zhou Y, et al. Longitudinal cerebral blood flow and amyloid deposition: an emerging pattern? J Nucl Med 2008; 49: 1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapogiannis D, Mattson MP.Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 2011; 10: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bir C, VandeVord P, Shen Y, et al. Effects of variable blast pressures on blood flow and oxygen saturation in rat brain as evidenced using MRI. Magnet Reson Imag 2012; 30: 527–534. [DOI] [PubMed] [Google Scholar]
- 56.Ito H, Kanno I, Ibaraki M, et al. Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab 2003; 23: 665–670. [DOI] [PubMed] [Google Scholar]
- 57.Deibler A, Pollock J, Kraft R, et al. Arterial spin-labeling in routine clinical practice, part 2: hypoperfusion patterns. Am J Neuroradiol 2008; 29: 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kovesdi E, Gyorgy AB, Kwon S-KC, et al. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front Neurosci 2011; 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svetlov SI, Prima V, Glushakova O, et al. Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to “composite” blast. Front Neurol 2012; 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Onetti Y, Dantas AP, Pérez B, et al. Middle cerebral artery remodeling following transient brain ischemia is linked to early postischemic hyperemia: a target of uric acid treatment. Am J Physiol 2015; 308: H862–H874. [DOI] [PubMed] [Google Scholar]
- 61.Gama Sosa MA, De Gasperi R, Janssen PL, et al. Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury. Acta Neuropathol Commun 2014; 2: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panzer MB, Myers BS, Capehart BP, et al. Development of a finite element model for blast brain injury and the effects of CSF cavitation. Ann Biomed Eng 2012; 40: 1530–1544. [DOI] [PubMed] [Google Scholar]
- 63.Goeller J, Wardlaw A, Treichler D, et al. Investigation of cavitation as a possible damage mechanism in blast-induced traumatic brain injury. J Neurotrauma 2012; 29: 1970–1981. [DOI] [PubMed] [Google Scholar]
- 64.Alosco ML, Aslan M, Du M, et al. Consistency of recall for deployment-related traumatic brain injury. J Head Trauma Rehab 2016; 31: 360–368. [DOI] [PubMed] [Google Scholar]
- 65.Jorge RE, Acion L, White T, et al. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry 2012; 169: 1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009; 68: 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKee AC, Robinson ME.Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement 2014; 10: S242–S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20935190 for Cerebral perfusion is associated with blast exposure in military personnel without moderate or severe TBI by Danielle R Sullivan, Mark W Miller, Erika J Wolf, Mark W Logue, Meghan E Robinson, Catherine B Fortier, Jennifer R Fonda, Danny JJ Wang, William P Milberg, Regina E McGlinchey and David H Salat in Journal of Cerebral Blood Flow & Metabolism