Abstract
Introduction
Struma ovarii is a teratoma of the ovaries predominantly composed of thyroid tissue. Hyperthyroidism associated with struma ovarii is rare, occurring in approximately 8% of cases. Due to the rarity of struma ovarii, available data are limited to case reports and small case series.
Methods and results
We report on a 61-year-old female patient with known Hashimoto’s thyroiditis on levothyroxine replacement therapy for years with transition to clinical and biochemical hyperthyroidism despite antithyroid medication with carbimazole (10 mg/day), new diagnosis of urothelial carcinoma and an adnexal mass suspicious of ovarian cancer. The patient underwent resection of the adnexal mass and histopathology revealed a mature teratoma predominantly composed of thyroid tissue showing high levels of sodium iodide symporter protein expression. Following struma ovarii resection and disappearance of autonomous production of thyroid hormones, the patient developed hypothyroidism with severely decreased thyroid hormone levels fT4 and fT3 (fT4 0.4 ng/dL, reference interval 0.9–1.7 and fT3 < 1.0 pg/mL, reference interval 2.0–4.4). This has previously been masked by continued thyroid-stimulating hormone suppression due to long-term hyperthyroidism pre-surgery indicating secondary hypothyroidism, in addition to primary hypothyroidism based on the known co-existing chronic lymphocytic thyroiditis of the orthotopic thyroid gland. Levothyroxine administration was started immediately restoring euthyroidism.
Conclusion
This case illustrates possible diagnostic pitfalls in a patient with two concurrent causes of abnormal thyroid function.
Learning points
Struma ovarii is an ovarian tumor containing either entirely or predominantly thyroid tissue and accounts for approximately 5% of all ovarian teratomas.
In rare cases, both benign and malignant struma ovarii can secrete thyroid hormones, causing clinical and biochemical features of hyperthyroidism.
Biochemical features of patients with struma ovarii and hyperthyroidism are similar to those of patients with primary hyperthyroidism. In such cases, thyroid scintigraphy should reveal low or absent radioiodine uptake in the thyroid gland, but the presence of radioiodine uptake in the pelvis in a whole body radioiodine scintigraphy.
We give advice on possible diagnostic pitfalls in a case with two simultaneous causes of abnormal thyroid function due to the co-existence of struma ovarii.
Patient Demographics: Adult, Female, White, Germany
Clinical Overview: Thyroid, Thyroid
Diagnosis and Treatment: Levothyroxine
Related Disciplines: Gynaecology
Publication Details: Error in diagnosis/pitfalls and caveats, March, 2021
Background
Struma ovarii is an ovarian tumor containing either entirely or predominantly thyroid tissue (> 50%) and accounts for approximately 5% of all ovarian teratomas (1, 2, 3). Traditionally, struma ovarii does not secrete thyroid hormones, however, in 8% of cases clinical and biochemical features of hyperthyroidism have been described (4). Around 40% of cases are asymptomatic and are accidentally discovered by routine ultrasound (5). In symptomatic cases of struma ovarii, common symptoms include palpable abdominal mass, abdominal pain or vaginal bleeding as well as tachycardia and ascites (5). Biochemical features of patients with struma ovarii and hyperthyroidism are similar to those of patients with hyperthyroidism of the orthotopic thyroid gland, showing low thyroid-stimulating hormone (TSH) and elevated peripheral thyroid hormones. In such cases, thyroid scintigraphy should reveal low or absent radioiodine uptake in the orthotopic thyroid gland, but whole body 123I scintigraphy should confirm the presence of functional thyroid tissue within the ovarian mass.
The treatment of benign struma ovarii is unilateral surgical resection of the ovarian tumor. In case of malignant transformation, the decision on performing total thyroidectomy followed by radioiodine therapy should be made based upon the risk of recurrence and the presence of metastastic disease (6, 7). Furthermore, molecular genetic analysis of tumor tissue may assist in managing difficult cases (8, 9, 10).
We present an extraordinary case of a woman with two simultaneous causes of abnormal thyroid function, that transitioned from Hashimoto’s thyroiditis-induced hypothyroidism to struma ovarii-induced hyperthyroidism and, after unilateral adnexectomy, back to hypothyroidism with persistent TSH suppression.
Case presentation
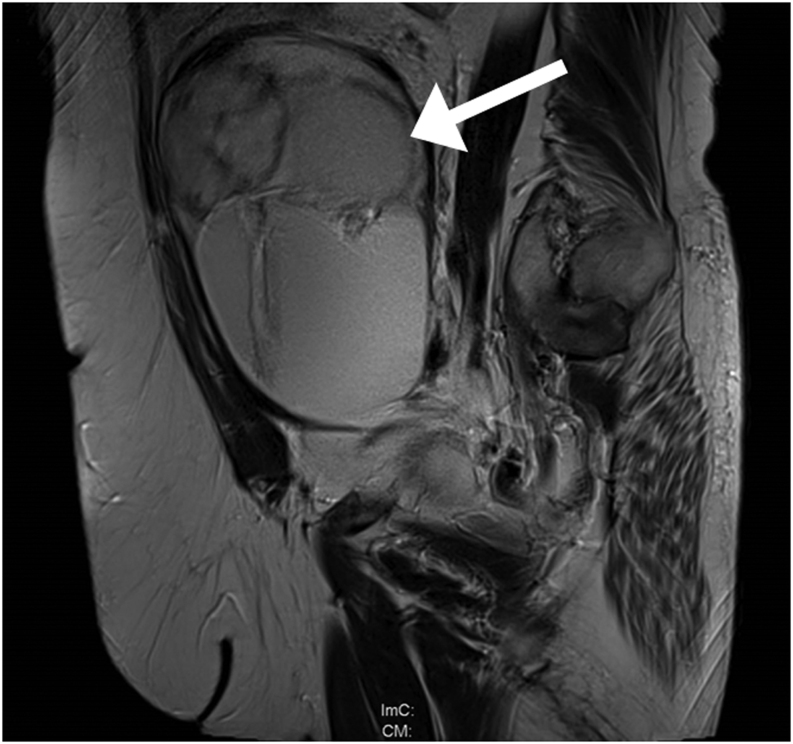
A 61-year-old female patient was admitted to the urology department because of urothelial carcinoma of the bladder. In the diagnostic work up an adnexal mass suspicious of ovarian cancer was detected by MRI (Fig. 1). Radical cystectomy and ileal neobladder reconstruction was performed as well as resection of the adnexal mass.
Figure 1.
Pelvic MRI. The sagittal T2-weighted image revealed a large high-intensity cystic mass of the right ovary with a diameter of 15 cm.
Medical history revealed Hashimoto’s thyroiditis for 3 years that was supplemented with levothyroxine (LT4) until the diagnosis of Graves' hyperthyroidism was made 1.5 years ago by her general practitioner based on her history of autoimmune disease despite the lack of TSH receptor antibodies. Antithyroid medication (carbimazole) was started 1.5 years ago and titrated to a dose of 10 mg/day at the time of admission.
Investigation
Preoperative thyroid function tests (TFTs) revealed a suppressed TSH of < 0.01 µU/mL (reference interval: 0.27–4.2), a free thyroxine (fT4) of 0.8 ng/dL (reference interval 0.9–1.7) and free triiodothyronine (fT3) of 3.3 pg/mL (reference interval: 2.0–4.4). Thyroid-specific autoantibody (TSH-receptor-, thyroid peroxidase- and thyroglobulin-antibodies) levels were within the reference range.
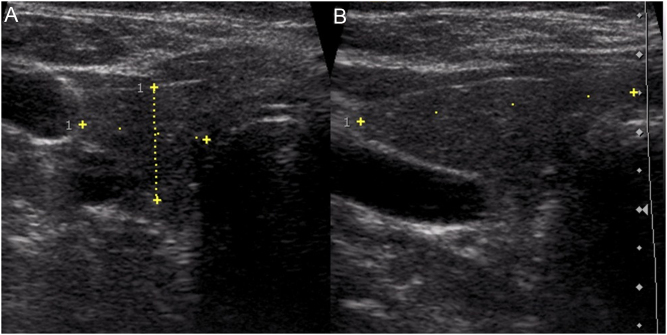
The patient was clinically euthyroid and thyroid ultrasound findings were highly suggestive of chronic lymphocytic thyroiditis (Fig. 2). Due to low fT4-levels, carbimazole was reduced to 5 mg/day.
Figure 2.
A sonogram of the right lobe of the thyroid ((A) transverse scan view, (B) longitudinal scan view) shows an inhomogeneous echotexture with hypoechoic areas compatible with chronic lymphocytic thyroiditis.
Treatment
Meanwhile, the patient underwent radical cystectomy and ileal neobladder reconstruction as well as unilateral adnexectomy. A giant cystic tumor of 15 × 14 × 7 cm in size was present in the right ovary, suspicious of a benign tumor. Histopathology of the adnexal mass revealed a mature teratoma predominantly composed of thyroid tissue, without signs of malignancy. Due to decreasing fT4-levels, carbimazole was stopped completely post-operatively.
Outcome and follow-up
Three days after withdrawal of carbimazole, TFTs were as follows: low TSH levels of 0.6 µU/mL in the presence of significantly decreased peripheral thyroid hormone levels of fT4 0.4 ng/dL and fT3 < 1.0 pg/mL. The failure to mount a TSH response post-operatively is consistent with secondary hypothyroidism after long-term thyrotropin-releasing hormone (TRH)/TSH suppression from extra-thyroidal thyroid hormone secretion. Biochemical assessment, including cortisol, corticotropin (ACTH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin as well as an ACTH stimulation test, revealed no additional pituitary hormone deficiencies. Due to post-surgical risk of developing myxedema coma, intravenous LT4 therapy was started immediately with 250 µg LT4/day for 3 days and switched to oral LT4 treatment with 100 µg/day. Follow-up of the patient showed TFTs within the normal range; TSH was 1.0 µU/mL, fT4 was 1.3 ng/mL and fT3 was 2.0 pg/mL.
Immunohistochemical analysis of sodium iodide symporter (NIS) expression of paraffin-embedded tissue sections of the teratoma was performed as described previously (11). Immunohistochemical staining revealed strong basolateral NIS staining in the thyroid follicular cells indicative of overactive thyroid tissue (Fig. 3).
Figure 3.
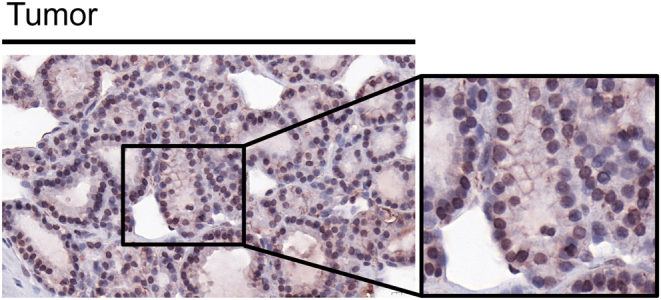
High NIS protein expression in teratoma. NIS-specific immunoreactivity (red) was detected in thyroid follicular cells in the teratoma tissue. The image is shown at ×40 magnification and ×60 magnification.
Discussion
Hyperthyroidism can be caused by a number of conditions, including Graves' disease, hyperfunctioning thyroid nodules and thyroiditis. Struma ovarii is one of the rare causes of hyperthyroidism and diagnosis can be even more difficult in the presence of co-existing thyroid disorders. In such cases, autonomous production of thyroid hormones by struma ovarii causes suppression of the thyroid gland. In 1390 cases of surgically removed ovarian tumors, Dunzendorfer et al. reported only five cases of struma ovarii and only one with co-existing hyperthyroidism (1). Another review of 1501 ovarian neoplasms did not detect a single case of hyperthyroidism due to struma ovarii (12). Approximately 8% of patients with struma ovarii develop hyperthyroidism, mostly originating from adenomas, rarely from differentiated thyroid carcinomas (1, 4).
Biochemical features of patients with struma ovarii and hyperthyroidism are similar to those of patients with primary hyperthyroidism. In such cases, thyroid scintigraphy should reveal low or absent radioiodine uptake in the thyroid gland, but the presence of radioiodine uptake in the pelvis in a whole body radioiodine scintigraphy. Histopathological findings of benign struma ovarii are identical to cervical thyroid tissue. Malignant struma ovarii is exceedingly rare and diagnostic criteria are similar to those for differentiated thyroid cancer.
Based on clinical and laboratory findings in our case, transition from hypothyroidism due to Hashimoto’s thyroiditis to overt hyperthyroidism 1.5 years before adnexectomy had been caused by autonomously functioning thyroid tissue in the teratoma that was controlled by antithyroid medication. After resection of the teratoma, the patient switched back to hypothyroidism due to known Hashimoto's thyroiditis of the orthotopic thyroid gland that was initially masked by continued TRH/TSH suppression as a result of long-term insufficiently controlled hyperthyroidism caused by the struma ovarii indicating additional secondary hypothyroidism. Based on the patient’s history and clinical and laboratory parameters, hypothyroidism was diagnosed and thyroid hormone replacement therapy was started immediately. After 3 weeks of LT4-replacement therapy, normalization of TFTs was achieved.
Comparing our case to other cases in the literature, Carvalho et al. described an almost identical case of a 62-year-old woman with symptoms of hypothyroidism after struma ovarii tumor resection most likely due to Hashimoto’s thyroiditis of the orthotopic thyroid gland (13). Amareen et al. reported a comparable case of clinical hypothyroidism following a resection of struma ovarii with positive thyroid peroxidase-antibodies in a 41-year-old woman (14). After initiation of LT4-replacement therapy, clinical symptoms and laboratory findings returned to normal. The authors assume that the ovarian tumor probably maintained normal thyroid function until surgical resection. The only difference to our case, which presented with preoperative hyperthyroidism, is that the patient was euthyroid before surgical resection of struma ovarii.
Our case, as one of the very few cases reported so far, illustrates two simultaneous causes of abnormal thyroid function due to the co-existence of struma ovarii causing hyperthyroidism in the presence of an orthotopic thyroid gland with chronic lymphocytic thyroiditis as well as possible diagnostic pitfalls.
Declaration of interest
Viktoria F Koehler has received honoraria for lectures and travel expenses from Novartis and Sanofi. Christine Spitzweg has received honoraria for advisory boards and lectures from Ipsen, Lilly, Bayer, Eisai, Genzyme. Patrick Keller, Elisa Waldmann, Nathalie Schwenk, Carolin Kitzberger, Kathrin A Schmohl, Thomas Knösel, Christian Georg Stief have declared no conflicts of interest.
Funding
This research was supported by the ‘Förderprogramm Forschung und Lehre (FöFoLe), Reg.-Nr. 1031’ of the medical faculty of the LMU Munich.
Patient consent
Written informed consent was obtained from the patient for publication of the submitted article and accompanying images.
References
- 1.Dunzendorfer T, deLas Morenas A, Kalir T, Levin RM.Struma ovarii and hyperthyroidism. Thyroid 1999. 9 499–502. ( 10.1089/thy.1999.9.499) [DOI] [PubMed] [Google Scholar]
- 2.Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS, Kim HS.Clinical characteristics of struma ovarii. Journal of Gynecologic Oncology 2008. 19 135–13. ( 10.3802/jgo.2008.19.2.135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondi-Pafiti A, Mavrigiannaki P, Grigoriadis Ch, Kontogianni-Katsarou K, Mellou A, Kleanthis CK, Liapis A.Monodermal teratomas (struma ovarii). Clinicopathological characteristics of 11 cases and literature review. European Journal of Gynaecological Oncology 2011. 32 657–65. [PubMed] [Google Scholar]
- 4.Dardik RB, Dardik M, Westra W, Montz FJ.Malignant struma ovarii: two case reports and a review of the literature. Gynecologic Oncology 1999. 73 447–4. ( 10.1006/gyno.1999.5355) [DOI] [PubMed] [Google Scholar]
- 5.Al Hassan MS, Saafan T, El Ansari W, Al Ansari AA, Zirie MA, Farghaly H, Abdelaal A.The largest reported papillary thyroid carcinoma arising in struma ovarii and metastasis to opposite ovary: case report and review of literature. Thyroid Research 2018. 11 10. ( 10.1186/s13044-018-0054-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anagnostou E, Polymeris A, Morphopoulos G, Travlos A, Sarantopoulou V, Papaspyrou I.An unusual case of malignant struma ovarii causing thyrotoxicosis. European Thyroid Journal 2016. 5 207–2. ( 10.1159/000448474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gild ML, Heath L, Paik JY, Clifton-Bligh RJ, Robinson BG.Malignant struma ovarii with a robust response to radioactive iodine. Endocrinology, Diabetes and Metabolism Case Reports 2020. 2020 19–0130. ( 10.1530/EDM-19-0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff EF, Hughes M, Merino MJ, Reynolds JC, Davis JL, Cochran CS, Celi FS.Expression of benign and malignant thyroid tissue in ovarian teratomas and the importance of multimodal management as illustrated by a BRAF-positive follicular variant of papillary thyroid cancer. Thyroid 2010. 20 981–98. ( 10.1089/thy.2009.0458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne C, Nikiforov YE.RAS mutation-positive follicular variant of papillary thyroid carcinoma arising in a struma ovarii. Endocrine Pathology 2010. 21 144–14. ( 10.1007/s12022-009-9097-8) [DOI] [PubMed] [Google Scholar]
- 10.Gobitti C, Sindoni A, Bampo C, Baresic T, Giorda G, Alessandrini L, Canzonieri V, Franchin G, Borsatti E.Malignant struma ovarii harboring a unique NRAS mutation: case report and review of the literature. Hormones 2017. 16 322–32. ( 10.14310/horm.2002.1750) [DOI] [PubMed] [Google Scholar]
- 11.Spitzweg C, Baker CH, Bergert ER, O'Connor MK, Morris JC.Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Human Gene Therapy 2007. 18 916–9. ( 10.1089/hum.2007.081) [DOI] [PubMed] [Google Scholar]
- 12.Ayhan A, Yanik F, Tuncer R, Tuncer ZS, Ruacan S.Struma ovarii. International Journal of Gynaecology and Obstetrics 1993. 42 143–14. ( 10.1016/0020-7292(9390628-a) [DOI] [PubMed] [Google Scholar]
- 13.Carvalho JP, Carvalho FM, Lima de Oliveira FF, Asato de Camargo RY.Hypothyroidism following struma ovarii tumor resection: a case report. Revista do Hospital das Clínicas 2002. 57 112–11. ( 10.1590/s0041-87812002000300006) [DOI] [PubMed] [Google Scholar]
- 14.Amareen VN, Haddad FH, Al-Kaisi NS.Hypothyroidism due to Hashimoto thyroiditis post struma ovarii excision. Saudi Medical Journal 2004. 25 948–9. [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a