Abstract
Severe acute respiratory syndrome coronavirus 2 rapid antigen detection (RAD) test kits are widely used as primary screening test in Japan because rapid diagnosis of coronavirus disease 2019 (COVID-19) is critical for infection control. We report cases with RAD test false-positive results in a ward for patients with disabilities. RAD tests potentially evoke hospital operational risk. It is desirable that performing PCR test appropriately when patients admitted to a medical treatment ward with COVID-19 symptoms instead of RAD test.
Keywords: Rapid antigen detection test, SARS-CoV-2, COVID-19
1. Introduction
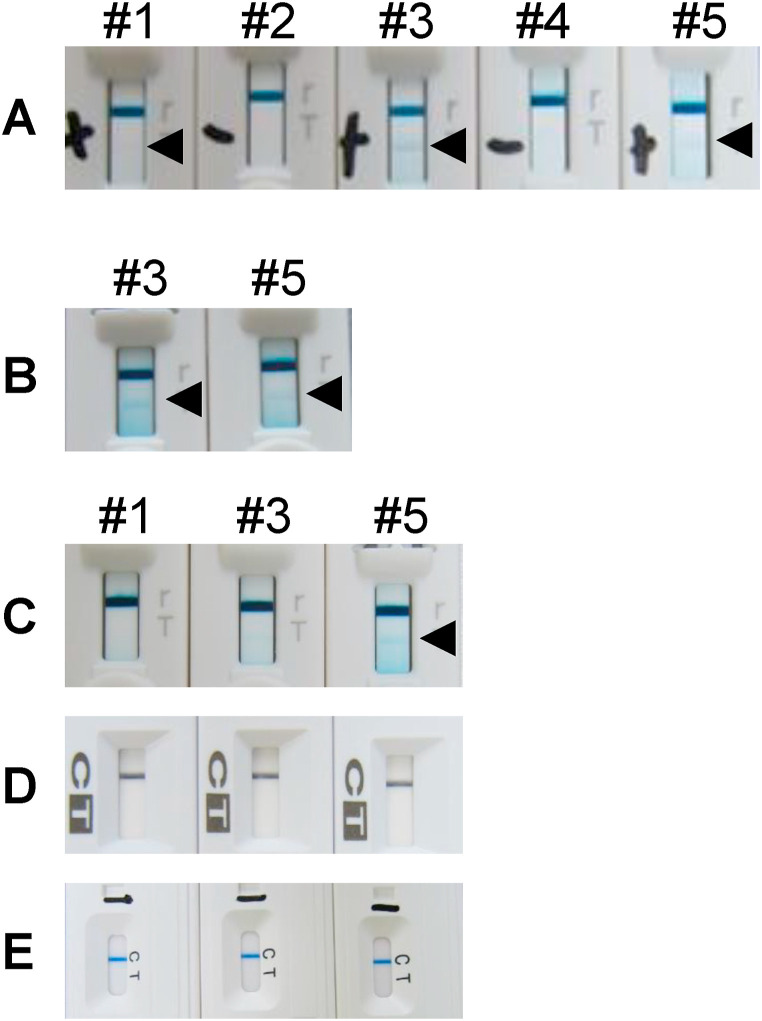
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus that causes coronavirus disease 2019 (COVID-19) infection, has recently emerged and caused a pandemic. COVID-19 outbreaks in hospitals or welfare facilities have become a significant social problem, such as the aggravation of inpatients and residents at risk of underlying diseases and old age and the difficulty of securing human resources because of staff infection [[1], [2], [3]]. In Japan, rapid antigen detection (RAD) test kits based on the immunochromatography method are widely used as primary screening test because early detection of COVID-19 is important for institutional infection control. We very recently experienced false-positive cases detected by RAD tests used for febrile patients hospitalized in the ward of persons with disabilities (see Fig. 1 ).
Fig. 1.
Detection of SARS-CoV-2 antigen using the ESPLINE SARS-CoV-2 kit (Lot no. K4B00811) on days 1, 2, and 4. The photograph taken on day 1 was taken 12 h after the test was read. Photographs taken on days 2 and 4 were taken immediately after the test was read. #1–#5 indicate patient number. Patients #1–#5 were tested on day 1 (A), and patients #3 and #5 were tested on day 2 (B). Patients #1, #3, and #5 were tested on day 4 using all three SARS-CoV-2 antigen rapid test kits currently available in Japan: ESPLINE SARS-CoV-2 kit (Lot no. K4B00815) (C), ImunoAce SARS-CoV-2 (D), and QuickNavi COVID-19 Ag kit (E). Triangles indicate positive bands.
2. Case report
Five patients hospitalized in the ward of disabled persons exhibited a fever for 2 days or more. These patients had been in reverse isolation for the last 7 months since the first wave of COVID-19 in Japan (Table 1 ). After the onset of fever, tazobactam/piperacillin were empirically administered to all patients, and azithromycin hydrate and favipiravir were additionally administered to the three patients with SARS-CoV-2 RAD test positive. Favipiravir was discontinued after confirming SARS-CoV-2 PCR test negative. Four patients showed defervescence on day 3, but one patient had a persistent fever.
Table 1.
Patient characteristics.
| Patient no. | Age | Gender | Underlying disease | WBCa (/μL) | CRPa (mg/dL) | Feverb |
||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 4 | ||||||
| 1 | <10 | M | Down syndrome | 4000 | 2.78 | + | +/− | – |
| 2 | 10 | M | Spastic quadriplegia | 6900 | 2.14 | + | ||
| 3 | 10 | M | Cerebral palsy | 18,600 | 0.82 | + | + | + |
| 4 | 10 | M | Cerebral palsy | 4300 | 4.75 | + | ||
| 5 | 20 | M | Lennox-Gastaut syndrome | 4700 | 14.26 | + | +/− | – |
CRP, C-reactive protein; M, male; WBC, white blood cell.
Inspection day; Day 1.
Fever; - (<37.0 °C), +/− (37.0–38.0 °C), + (>38.0 °C).
Specimens were collected from the innermost part of the nasal cavity according to the guidelines for the utilization of SARS-CoV-2 antigen detection kits and were prepared for assays according to the respective manufacturer’s manual [4].
Three SARS-CoV-2 RAD test kits based on the immunochromatography method are currently available on the Japanese market: ESPLINE® SARS-CoV-2 kit (Lot nos. K4B00811 and K4B00815; Fujirebio Inc., Tokyo, Japan); ImunoAce SARS-CoV-2 kit™ (Lot no. S201003; Tauns Laboratories Inc., Shizuoka, Japan); and QuickNavi™ COVID-19 Ag kit (Lot no. 140081; Denka Co., Tokyo, Japan). Each rapid test kit was used according to the respective manufacturer’s manual. At least two laboratory technicians conducted the tests. The SARS-CoV-2 virus genetic test was performed as follows according to the Pathogen Detection Manual [5].
At initial examination (day 1), ESPLINE kit (Lot no. K4B00811) results indicated 3 of 5 patients were positive for SARS-CoV-2. PCR testing was subsequently performed using nasopharyngeal swabs from 57 inpatients in the ward and 12 staff members (e.g., nurses), including the three patients with antigen-positive results.
On day 2, the three patients with antigen-positive results underwent PCR testing using nasopharyngeal swab samples. The RAD test was performed on only two of the three patients because two ESPLINE kits (Lot no. K4B00811) were available. Similar to the first test results, PCR-negative and antigen-positive results were obtained.
On day 4, antigen tests were performed on the three SARS-CoV-2 antigen-positive patients using ESPLINE (Lot no. K4B00815), ImunoAce, and QuickNavi kits. Of the three false-positive patients detected by the ESPLINE kit (Lot no. K4B00811), one patient with persistent fever was positive by the ESPLINE kit (Lot no. K4B00815). ImunoAce and QuickNavi kits showed negative results. The remaining two patients who had improved fever symptoms were antigen-negative as determined by all three test kits.
We have received a report that rhinoviruses were detected by direct sequencing in the remaining specimens from the PCR test by administrative testing. The rhinoviruses have been confirmed in four patients other than patient no. 4.
3. Discussion
The RAD test result using the ESPLINE kit was judged to give false-positive results because patients were PCR-negative, and the occurrence of COVID-19 was clinically denied. We cannot say that there are no true COVID-19 cases in which the antigen test is positive, and the PCR test is negative. However, we estimated that this case was with false positive, taking into account the reported specificity of both the antigen and PCR tests, the fact that there were no PCR test positive cases among the 69 patients and staff involved, and the prevalence situation in the area. In fact, rhinovirus infection was later reported by administrative testing using direct sequencing. Furthermore, because different lots of the ESPLINE kit yielded similar results, and the other two antigen test kits also gave negative results, we speculate that this was an issue specific to the ESPLINE kit.
The RAD test based on the immunochromatography method is widely used in Japan, and if the test is positive, the patient is diagnosed as COVID-19. Although several reports have noted low sensitivity with RAD tests, and false positives have already been reported for quantitative tests such as Lumipulse®, to our knowledge, in qualitative tests no reports have described false-positive results [[6], [7], [8], [9]]. However, more recently, false-positive results from the RAD test have become a problem. The Japanese Association for Infectious Diseases reported that the ESPLINE kit (Lot no. K4B00811) might give false positives, which was the kit used at initial screening [10]. False positives most often occur when a highly viscous sample is used [11]. Although the reported degree of viscosity cannot be accurately defined, the samples were not considered to have high viscosity in this case, and the two antigen-positive cases demonstrated reproducibility as both yielded positive results on the first and second tests. Therefore, these false positives likely did not result from problems with the sample or procedure. There are three types of RAD tests used this time, all of which are based on immunochromatography, but they differ in the coloration principle of the judgment line. ESPLINE uses alkaline phosphatase labeling for the color reaction, QuickNavi uses colored latex particles for the color reaction, and ImmunoAce uses platinum-gold colloid for the color reaction. We cannot deny the possibility that these different color reactions may have affected the results.
SARS-CoV-2 causes worse outcomes and a higher mortality rate in disabled or older people; thus, the RAD test may play an essential role as primary screening at facilities for the elderly or disabled persons. However, if the RAD test shows positive, the facility and staff will be considerably burdened. In our case, 43 staff members, including nurses, doctors, laboratory technicians, pharmacists, child instructors, and clerks, collectively worked overtime for 147 hours a day. Additionally, our institution experienced some reputational damage. Thus, the use of RAD tests potentially evokes hospital operational risk. It is important to remember that the patient is the one who bears the greatest burden when a false positive occurs. If a patient with false positive result admit to a room for COVID-19, it may increase the risk of nosocomial infection.
We consider that it is desirable to perform PCR testing promptly when patients admitted to the medical treatment ward have symptoms without using a RAD test.
Author contribution statement
KI: Data curation, Formal analysis, Investigation, Conceptualization, Methodology, Writing – original draft, review and editing.
TK: Data curation, Investigation, Writing – review and editing.
YO: Data curation, Investigation, Writing – review and editing.
KS: Data curation, Investigation, Writing – review and editing.
YS: Data curation, Investigation, Writing – review and editing.
CK: Data curation, Investigation, Writing – review and editing.
YM: Data curation, Investigation, Writing – review and editing.
RH: Data curation, Investigation, Writing – review and editing.
HI: Data curation, Investigation, Writing – review and editing.
MS: Data curation, Investigation, Writing – review and editing.
YM: Data curation, Investigation, Writing – review and editing.
KK: Data curation, Investigation, Writing – review and editing.
HM: Data curation, Investigation, Writing – review and editing.
HT: Data curation, Formal analysis, Investigation, Conceptualization, Methodology, Supervision, Writing – original draft, review and editing.
Ethics approval
This study was performed based on the Declaration of Helsinki and its amendments and the Ethical Guidelines for Clinical Research by the Ministry of Health, Labor and Welfare, Japan. Publication of this study was approved by the National Hospital Organization Awara Hospital Clinical Research Review Board (#2024). Since three patients did not have sufficient consent ability, the test was conducted with the patient’s family members’ consent, who were the substitutes. If it was possible to communicate easily with the person, the person consented to the inspection.
Funding
None.
Declaration of competing interest
The authors have read the journal’s policy on the disclosure of potential conflicts of interest and the journal’s authorship agreement. The authors declare that they have neither conflicts of interest nor competing interests.
References
- 1.Andrew M., Searle S.D., McElhaney J.E., McNeil S.A., Clarke B., Rockwood K., et al. COVID-19, frailty and long-term care: implications for policy and practice. J Infect Dev Ctries. 2020;14:428–432. doi: 10.3855/jidc.13003. [DOI] [PubMed] [Google Scholar]
- 2.D’Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J Am Geriatr Soc. 2020;68:912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- 3.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., et al. Epidemiology of covid-19 in a long-term care facility in king county, Washington. N Engl J Med. 2020;382 doi: 10.1056/NEJMoa2005412. 2005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Ministry of Health, Labor and Welfare . Japanese Ministry of Health, Labor and Welfare; Tokyo, Japan: 2020. The guidelines for the utilization of the SARS-CoV-2 antigen detection kit.https://www.mhlw.go.jp/content/000640554.pdf [Google Scholar]
- 5.National Institute of Infectious Diseases . National Institute of Infectious Diseases; Tokyo, Japan: 2020. The pathogen detection manual 2019-nCoV Ver.2.9.1.https://www.niid.go.jp/niid/images/lab-manual/2019-nCoV20200319.pdf [Google Scholar]
- 6.Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C.S., Lam E.T., et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129:104500. doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagura-Ikeda M., Imai K., Tabata S., Miyoshi K., Murahara N., Mizuno T., et al. Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), direct RT-qPCR, reverse transcription-loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scohy A., Anantharajah A., Bodéus M., Kabamba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol: The Official Publication of the Pan American Society for Clinical Virology. 2020;129 doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa T., Fukumori T., Nishihara Y., Sekine T., Okuda N., Nishimura T., et al. Another false-positive problem for a SARS-CoV-2 antigen test in Japan. J Clin Virol. 2020;131:104612. doi: 10.1016/j.jcv.2020.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Japanese Association for Infectious Diseases . The Japanese Association for Infectious Diseases; Tokyo, Japan: 2020. COVID-19 questionnaire on false positives of the simplified antigen qualitative test (October 27, 2020)http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_survey_201027.pdf [Google Scholar]
- 11.Fujirebio Inc Precautions for "ESPLINE SARS-CoV-2" operation and judgment method. 2020. https://www.fujirebio.co.jp/products/espline/pdf/EL_SARS-CoV-2_ASSAY_PROCEDUREFAQ.pdf