Abstract
Introduction
Patients who are overweight or obese have an increased risk of developing type 2 diabetes mellitus (T2DM). Weight loss can have a positive effect on glycemic control.
Objective
We aimed to investigate glycemic control in patients with T2DM and overweight or obesity during a structured weight-loss program.
Methods
This was a prospective, interventional study. We recruited 36 patients (14 men and 22 women) with a median age of 58.5 years and median body mass index (BMI) of 34.1, to a 15-week structured weight-loss program with a low-calorie (800 kcal) formula diet for 6 weeks. The primary end point, HbA<sub>1c</sub> level, and secondary end points, anthropometric data, medication, and safety, were assessed weekly. Laboratory values and quality of life were assessed at baseline and after 15 weeks.
Results
HbA<sub>1c</sub> decreased from 7.3% at baseline to 6.5% at 15 weeks (p < 0.001), median body weight by 11.9 kg (p < 0.001), median BMI by 4.3 (p < 0.001) and median waist circumference by 11.0 cm (p < 0.001). Two participants discontinued insulin therapy, 4 could reduce their dosage of oral antidiabetic agents, and 6 completely discontinued their antidiabetic medication. Insulin dose decreased from 0.63 (0.38–0.89) to 0.39 (0.15–0.70) units/kg body weight (p < 0.001). No patient experienced hypoglycemic episodes or hospital emergency visits. Triglycerides and total cholesterol decreased as well as surrogate markers of liver function. However, the levels of high-density and low-density lipoprotein cholesterol (HDL-C and LDL-C) as well as uric acid remain unchanged. Regarding quality of life, the median physical health score increased from 44.5 (39.7–51.4) at baseline to 48.0 (43.1–55.3; p = 0.007), and the median mental health score decreased from 42.1 (36.1–46.7) to 37.4 (30.3–43.7; p = 0.004).
Conclusions
A structured weight-loss program is effective in the short term in reducing HbA<sub>1c</sub>, weight, and antidiabetic medication in patients with T2DM who are overweight or obese. Levels of HDL-C and LDL-C were not affected by short-term weight loss. The decline in mental health and the long-term effects of improved glycemic control require further trials.
Keywords: Obesity, Diabetes mellitus, Medical treatment, Formula diet, Quality of life
Introduction
Overweight and obesity are mainly caused by a combination of high-calorie intake, an unhealthy diet, little physical activity, and genetic predisposition, and are a crucial risk factor contributing to the global disease burden [1, 2]. In overweight and obese individuals, adipose tissue releases increased amounts of nonesterified fatty acids, hormones, and proinflammatory cytokines that are involved in the complex development of insulin resistance [3]. In addition, cytokines or proinflammatory signals from other organs may also play a substantial role in insulin resistance [4]. The risk of developing type 2 diabetes mellitus (T2DM) is 3 times greater in subjects with obesity [5]. According to the International Diabetes Federation, 463 million people worldwide suffer from diabetes, 90% of whom have T2DM [6].
Patients with T2DM who are overweight or obese often suffer from concomitant diseases like hypertension, dyslipidemia, and nonalcoholic fatty liver disease (NAFLD). Moreover, the percentage of comorbidities such as fatal stroke or cardiovascular disease are much higher in this group [5]. Therefore, a complex therapy is necessary to improve patients' outcomes. Improvement in glycemic control is essential to prevent microvascular complications [7]. Reducing high blood pressure and dyslipidemia are important to avoid macrovascular events [8, 9].
One option to achieve these treatment goals are lifestyle changes including weight loss by means of a formula diet as a total dietary replacement [10]. The DROPLET trial compared a weight-loss program using a total dietary replacement of 800 kcal for 8 weeks, followed by food reintroduction to weight-loss regimes according to guidelines. After 1 year, the average and adjusted weight loss between the intervention and control group was 7.2 kg [11]. Astbury et al. [12] conducted a systematic review and meta-analysis of the effectiveness of meal replacement for weight loss in 2018, and concluded that weight-loss programs incorporating meal replacements led to a greater weight loss after 1 year than comparator weight-loss programs, and that this is a valid option for the management of obesity and overweight.
However, it has been proposed that patients with T2DM may have less success in maintaining a sustained weight loss due to their medications including insulin [13, 14]. Recently, several studies investigating lifestyle interventions including a formula diet showed that patients with obesity and T2DM could reduce 13–23% of their body weight and achieved improved glycemic control [10, 15, 16, 17, 18, 19, 20, 21] This observation was underlined by a systematic review and meta-analysis showing that weight loss with formula diets was very similar in patients with and without diabetes [22]. In several research settings, patients with the need of insulin therapy were excluded from the study and, in some trials, a formula diet <800 kcal was used [15, 23].
The purpose of this study was to investigate glycemic control in patients with T2DM who were overweight or obese and on dietary therapy, oral antidiabetic medication, or insulin treatment during an established and evaluated 15-week structured weight-loss program that included multimodal therapy, a 6-week formula diet (i.e., a low-calorie diet, LCD), and an intensified monitoring of blood glucose levels.
Materials and Methods
This monocentric and prospective study is registered at ClinicalTrials.gov (ID. NCT02970838). The study was started in November 2012 and completed in May 2014.
Investigation of specific trial data regarding changes of the fecal microbiome [24] and changes in abdominal compartments composition by magnetic resonance imaging [25] were published previously.
Subjects
Advertisements in several local newspapers invited subjects interested in participating in a standardized weight-loss program to contact the investigators by calling a central telephone number. Inclusion criteria were: an age between 18 and 70 years, known T2DM, and a body mass index (BMI) of 27–45. Interested subjects were excluded in case of treatment with incretin mimetic drugs of <3 months; pregnancy; immobilization; allergy to the formula diet; severe heart, liver, or renal failure; dementia; eating disorders; and alcoholism. The participants had to bear the cost of the formula diet.
Inclusion criteria were verified by the trial physician at the initial examination. If necessary, blood values or further tests were conducted to verify the sustainability of the patients for the trial. In order to diagnose concomitant diseases, already-prescribed medical therapy or the following cut-off values were used: (i) hypertension: >130 mm Hg systolic blood pressure (BP) and >80 mm Hg diastolic BP; (ii) dyslipidemia: total triglycerides (TG) >1.7 mmol/L, total cholesterol (TC) >5.2 mmol/L or high-density lipoprotein cholesterol (HDL-C) <1.05 mmol/L for males and <1.25 mmol/L for females; and (iii) hyperuricemia: serum uric acid >387 µmol/L.
Standardized Weight-Loss Program
In the first 6 weeks of the standardized weight-loss program (OPTIFAST® II Short program, Nestlé Health Science, Germany), patients received a balanced-formula low-calorie diet (LCD). Daily consumption consisted of 5 sachets fully replacing normal food and corresponding to an energy content of 800 kcal. Five sachets contained an average of 96 g carbohydrates, 6.5 carbohydrate units (1.0–1.5 carbohydrate units per sachet), 70 g proteins, 15 g fat, and the recommended daily amounts of vitamins and minerals. Patients were advised to drink >2.5 L of water and other calorie-free beverages each day. This fasting phase was followed by a 4-week refeeding phase, during which regular food was reintroduced and the formula diet was gradually replaced until a daily total intake of 1,200 kcal was reached. During the last 5 weeks of the program, energy intake was gradually increased to an individual level of between 1,200 and 1,500 kcal that allowed subjects to keep their weight stable.
Once a week, participants visited the study center to have their health status monitored and take part in supervised exercises. The exercise course combined cardiovascular and strength training. It is part of the standardized weight-loss program. Training intensity was increased gradually, from 30% and 1–2 series with 15–25 repetitions to 70% and 1–3 series with 15–25 repetitions. The program was adjusted to an individual's fitness level and disease at the discretion of the trainer. A dietician supervised the group throughout the study and provided nutritional and behavioral counseling. To monitor the dosage of antidiabetics and other drugs as well as to minimize side effects, the participants met once a week with the study physician. Moreover, patients were able to contact the study team in the case of uncertainty about the management of their T2DM. The trainer, dietician, and study physician were trained before working with the weight-loss program to ensure standardized study implementation.
During the fasting phase, oral insulinotropic drugs and metformin were paused. In patients taking insulin, the prandial insulin was reduced to 2 insulin units per carbohydrate units, and the basal insulin rate was left unchanged unless the fasting blood glucose was <5.6 mmol/L. The patients were encouraged to measure their blood glucose at least 6 times daily and call the physician as soon as measured levels were <5.6 mmol/L or repeatedly >12 mmol/L. During the program, blood glucose levels were checked at least weekly by the study physician, and the insulin dosage was individually adjusted. During the refeeding phase, metformin was reintroduced when blood glucose levels were repeatedly >12 mmol/L, and other oral antidiabetic drugs were added when necessary. Due to possible negative aspects like hypoglycemia or weight gain, insulinotropic drugs were avoided whenever possible. Incretin mimetic drugs were not started until the end of the program.
Medications prescribed for dyslipidemia, hypertension, and hyperuricemia were left unchanged. We recommended that patients with repeatedly measured BP >130/80 mm Hg and low-density lipoprotein cholesterol (LDL-C) >1.8 mmol/L (>2.6 mmol/L in diabetics without further cardiovascular risk factors) consult their family physician to optimize their therapy and reduce their cardiovascular risk. Diuretic medication was paused until overt edema or dyspnea developed, or BP rose to >160 mm Hg.
Outcomes
We defined an improvement in HbA1c after 15 weeks of the standardized weight-loss program as the primary end point. Secondary end points were anthropometrical measurements, medication intake and safety end points, especially hypoglycemia, further blood value measurements, and quality of life. With subjects lightly clothed and not wearing shoes, weight was measured using a digital scale (Seca 635, Hamburg, Germany). Height was measured in a standing position (not wearing shoes) using a stadiometer (Seca 240, Hamburg, Germany). BMI was calculated. Waist circumference was measured midway between the superior iliac spine and the lower rib margin without exerting pressure on the body surface. Medication intake and safety issues were assessed at the weekly meeting with the trial physician. Blood value measurements were taken at baseline and after 15 weeks at the end of the weight-loss program. Blood samples were drawn from the cubital vein in the supine position in the morning after an overnight fasting period of >8 h and analyzed by the Institute of Clinical Chemistry and Laboratory Medicine, University Medicine, Greifswald, Germany. HbA1c concentrations were determined by high-performance liquid chromatography (HPLC; Bio-Rad Diamat, Munich, Germany). Fasting serum concentrations of glucose, TC, and uric acid, as well as the serum activity of alanine transaminase (ALT), aspartate transaminase (AST), and γ-glutamyl transferase (γ-GT) were measured photometrically (Dimension VISTA, Siemens Healthcare Diagnostics, Eschborn, Germany). HDL-C and LDL-C were measured using standard methods (Dimension VISTA, Siemens Healthcare Diagnostics). Plasma insulin levels were measured on a Centaur XP (Siemens Healthcare Diagnostics). All measurements complied with the regulations for internal and external quality controls according to the Guidelines of the German Medical Association on Quality Assurance in Medical Laboratory Examinations (Rili-BAEK). Homeostasis model assessment of insulin resistance (HOMAIR) values were calculated using fasting insulin and fasting glucose multiplied and then divided by 22.5 [26]. To investigate quality of life, the standardized questionnaire SF-12 was used. Quality of life was divided into physical and mental health, with higher SF-12 scores (on a scale of 0–100) corresponding to better health [27, 28].
Statistical Methods
Considering the study from Capstick et al. [16], we defined the effect size for the chosen intervention on our primary end point as 0.5. Using the Wilcoxon signed-rank test for matched pairs, 2-tailed normal distribution, an α-error of 0.05, and a power of 80%, the calculation of sample size resulted in n = 35.
All data were analyzed with STATA13 (Stata Corp., College Station, TX, USA). The Wilcoxon signed-rank test with the Bonferroni correction for paired samples was used to test for significant changes during the weight-loss program. All data are presented as median with 25th and 75th percentile due to the small sample size. Regression analyses were performed to evaluate associations between one dependent variable and multiple independent variables (age, sex, insulin therapy, and initial value of the dependent variable). We tested for heteroscedasticity using the Breusch-Pagan test. If the test detected heteroscedasticity (p < 0.05), we used robust standard errors. p < 0.05 was considered statistically significant.
Results
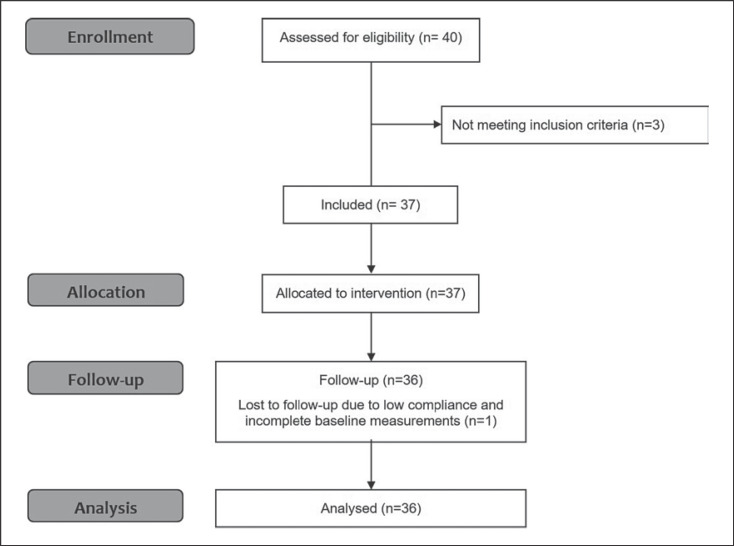
Overall, 36 subjects with T2DM, belonging to 4 consecutive cohorts, completed the standardized weight-loss program (Fig. 1). One patient withdrew from the study because of personal problems. Because of low attendance from the beginning, we excluded their data from the analysis.
Fig. 1.
Consort flow sheet.
The baseline characteristics of the patient population including diabetic therapy and concomitant diseases are shown in Table 1. Changes in the primary outcome parameter HbA1c are shown in Table 2. Median HbA1c decreased significantly from 7.3% at baseline to 6.5% at 15 weeks (p < 0.001). We searched for prognostic parameters regarding changes in HbA1c levels after 15 weeks. Regression analysis was performed with age, sex, insulin therapy, weight change, and baseline HbA1c as predictors (Table 3). In our model, age and weight loss were not significantly associated with changes in HbA1c. Patients on insulin treatment had higher initial HbA1c levels than patients without insulin treatment (0.47 percentage points), but insulin treatment was not significantly associated with a change in HbA1c. Gender analysis showed that women had 0.68 percentage points higher HbA1c levels than men. We found a relationship between HbA1c at the beginning of the study and a reduction at 15 weeks; if the HbA1c was one percentage point higher at the beginning, it decreased on average by 0.54 percentage points during the weight-loss program. In consequence, men without insulin treatment and a high initial HbA1c attained the greatest reduction in HbA1c whereas women taking insulin with a low initial HbA1c achieved the weakest change.
Table 1.
Baseline characteristics of 36 patients
| Female sex | 22 (61.1) |
| Male sex | 14 (38.9) |
| Age, years | 58.5 (53.0–64.0) |
| Weight, kg | 106.7 (93.8–117.2) |
| Height, m | 1.69 (1.65–1.76) |
| BMI | 34.1 (32.2–40.6) |
| Patients with overweighta | 32 (88.9) |
| Patients with obesityb | 4 (11.1) |
| Diabetes therapy | |
| Dietetic | 6 (16.7) |
| Oral agents | 13 (36.1) |
| Insulin | 6 (16.7) |
| Insulin + oral agents | 11 (30.6) |
| Concomitant diseases | |
| Hypertension | 31 (86.1) |
| On medication | 31 (100.0) |
| Dyslipidemia | 32 (88.9) |
| On medication | 20 (62.5) |
| Hyperuricemia | 12 (33.3) |
| On medication | 1 (8.3) |
Values are expressed as n (%) or median (1st–3rd quartile).
BMI 25.0–29.9
BMI >30.
Table 2.
Outcome parameters at baseline and after 15 weeks
| Parameters | Baseline | 15 weeks | Δ15 weeks | p value |
|---|---|---|---|---|
| Weight, kg | 106.7 (93.8–117.2) | 93.4 (81.8–105.2) | −11.9 (−16.6 to −7.4) | <0.001 |
| BMI | 34.1 (32.2–40.6) | 30.5 (28.1–35.2) | −4.1 (−5.7 to −2.7) | <0.001 |
| WC, cm | 114.5 (104.0–122.5) | 103.0 (93.0–114) | −11.0 (−15.0 to −7.0) | <0.001 |
| Systolic BP, mm Hg | 134.0 (124.0–152.0) | 133.5 (125.0–150.0) | −0.5 (−4.0 to 3.0) | 0.759 |
| Diastolic BP, mm Hg | 80.0 (72.0–83.5) | 78.0 (72.0–82.5) | −1.0 (−2.5 to 1.0) | 0.308 |
| HbA1c % | 7.3 (6.5–8.2) | 6.5 (6.1–7.3) | −0.5 (−1.2 to −0.3) | <0.001 |
| Glucose, mmol/L | 8.3 (6.4–9.5) | 6.7 (5.9–8.9) | −0.9 (−2.4 to 0.1) | 0.020 |
| Insulin, pmol/L | 153.9 (89.3–216.3) | 105.1 (68.1–149.5) | −32.0 (−102.0 to 14.1) | 0.024 |
| HOMAIR | 8.1 (4.1–11,5) | 4.8 (3.1–7.1) | −2.1 (−5.2 to 0.9) | 0.015 |
| Triglycerides, mmol/L | 2.13 (1.45–3.45) | 1.70 (1.10–2.51) | −0.33 (−1.40 to 0.07) | <0.001 |
| Cholesterol, mmol/L | 5.1 (4.3–5.9) | 4.6 (4.0–5.1) | −0.3 (−0.8 to 0.1) | 0.003 |
| LDL, mmol/L | 2.76 (2.33–3.55) | 2.77 (2.11–3.49) | −0.05 (−0.45 to 0.29) | 0.315 |
| HDL, mmol/L | 1.15 (0.86–1.44) | 1.12 (0.90–1.43) | 0.03 (−0.11 to 0.22) | 0.172 |
| ALT, µkat/L | 0.59 (0.47–0.79) | 0.42 (0.35–0.50) | −0.17 (−0.32 to −0.17) | <0.001 |
| AST, µkat/L | 0.33 (0.28–0.46) | 0.29 (0.25–0.36) | −0.05 (−0.13 to 0.02) | 0.001 |
| γ-GT, µkat/L | 0.67 (0.45–1.20) | 0.45 (0.37–0.92) | −0.13 (−0.34 to −0.02) | 0.002 |
| Uric acid, µmol/L | 323 (282–402) | 315 (267–366) | −15 (−47 to 25) | 0.242 |
Values are expressed as median (1st–3rd quartile). Bold type denotes statistical significance (Wilcoxon signed-rank test); p < 0.003 was statistically significant (Bonferroni correction). WC, waist circumference; BMI, body mass index; BP, blood pressure; HbA1c, glycated hemoglobin; HOMAIR, homeostatic model assessment; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ALT, alanine transaminase; AST, aspartate transaminase; γ-GT, γ-glutamyl transferase.
Table 3.
Results of linear regression analyses for 36 patients: change in HbA1c, weight, and HOMAIR after 15 weeks in relation to age, sex, insulin therapy, weight change, and initial value
| β | 95% CI | p value | ||
|---|---|---|---|---|
| Model 1: Change in HbA1c (R2 = 0.60) | ||||
| Age, years | <0.001 | −0.034 to 0.035 | 0.992 | |
| Female, yes/no | 0.675 | 0.153 to 1.196 | 0.013 | |
| Insulin, yes/no | 0.466 | −0.118 to 1.051 | 0.114 | |
| weight change, kg | 0.016 | −0.021 to 0.053 | 0.382 | |
| Initial HbA1c % | −0.543 | −0.781 to −0.304 | <0.001 | |
| Model 2: Change in weight (R2 = 0.32) | ||||
| Age, years | −0.630 | −0.278 to 0.152 | 0.554 | |
| Female, yes/no | 0.295 | −3.871 to 4.461 | 0.886 | |
| Insulin, yes/no | 2,400 | −1.463 to 6.262 | 0.215 | |
| Initial weight, kg | −0.156 | −0.261 to −0.051 | 0.005 | |
| Model 3: Change in HOMAIR (R2 = 0.96) | ||||
| Age, years | 0.062 | −0.182 to 0.307 | 0.607 | |
| Female, yes/no | 0.235 | −3.546, 4.016 | 0.900 | |
| Insulin, yes/no | −1,117 | −4.875 to 2.642 | 0.548 | |
| weight change, kg | 0.446 | 0.133 to 0.759 | 0.007 | |
| Initial HOMAIR | −0.990 | −1.067 to −0.912 | <0.001 | |
CI, confidence interval; HbA1c, glycated hemoglobin; HOMAIR, homeostasis model assessment of insulin resistance.
Median body weight decreased significantly by 11.9 kg (p < 0.001), median BMI by 4.3 (p < 0.001), and median waist circumference by 11.0 cm (p < 0.001) at 15 weeks (Table 2). To investigate associations with body weight reduction at 15 weeks, regression analyses were performed with age, sex, insulin therapy, and initial body weight as predictors of outcome (Table 3). Participants with greater weight at baseline lost significantly more body weight after 15 weeks in our weight-loss program (β = −0.156, p = 0.005). For each 10 kg higher initial weight, patients lost 1.6 kg more body weight. The factors age, sex, and insulin therapy were not associated significantly with weight loss at 15 weeks.
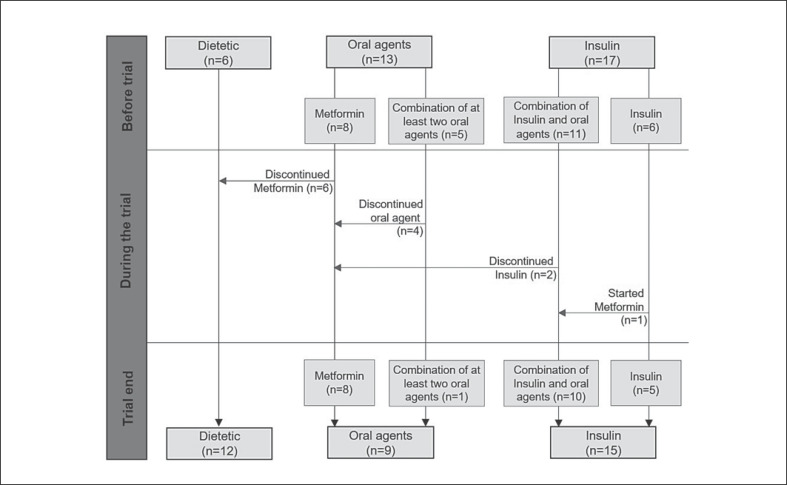
The course of antidiabetic drug dosage during the weight-loss program is shown in Figure 2. Two participants discontinued insulin therapy completely and required only 1 oral antidiabetic agent to control their blood glucose levels. Four patients were able to reduce their medication from ≥2 oral antidiabetic agents to 1 only, and 6 participants discontinued their oral antidiabetic agents completely. The median daily insulin usage decreased significantly (p < 0.001) from 0.63 (0.38–0.89) to 0.39 (0.15–0.70) units/kg of body weight after 15 weeks of the weight-loss program. Drugs for hypertension, hyperlipidemia, and hyperuricemia were not reduced or stopped during the weight-loss program.
Fig. 2.
Course of medication intake.
No serious adverse events related to the weight-loss program were reported during the intervention at all. Two patients had scheduled elective orthopedic interventions, but they could continue the weight-loss program with small adjustments in physical activity. Four patients made use of the offer to call outside of the scheduled appointment for treatment adjustments. The questions asked were mainly related to changes in blood glucose levels. Five patients reported constipation, which resolved after sufficient hydration and increasing their physical activity. No hospital emergency visits were recorded.
As shown in Table 2, TG decreased significantly by 32% (p = 0.003) and TC by 46% (p = 0.003) after the 15-week weight-loss program, but HDL-C and LDL-C remained unchanged. Surrogate markers of liver function, namely ALT, AST, and γ-GT, decreased significantly, but levels of uric acid did not change significantly during the study period.
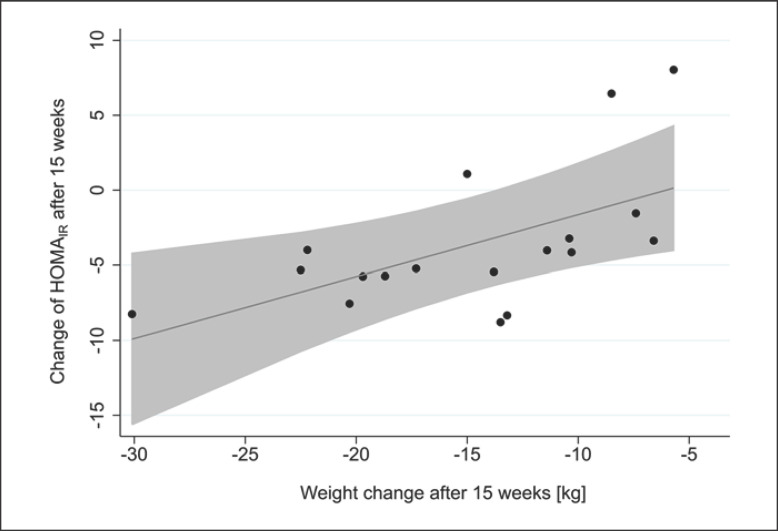
Changes in insulin and glucose levels as well as HOMAIR after the 15 weeks weight-loss program are shown in Table 2. Insulin and glucose levels and, consequently, HOMAIR, improved, but after the Bonferroni correction for multiple tests, the pvalues were not significant. To investigate associations with changes in HOMAIR after 15 weeks, regression analyses were performed with age, sex, insulin therapy, weight change after 15 weeks, and initial HOMAIR as predictors of outcome (Table 3). We found significant associations between changes in HOMAIR and initial HOMAIR (β = −0.990, p < 0.001) as well as weight change after 15 weeks (β = 0.446, p = 0.007), respectively. The association between changes in HOMAIR and weight change after 15 weeks is shown in Figure 3. For each 0.4 kg weight change, HOMAIR decreased by approximately 1.0 unit. The predictors age, sex, and insulin therapy were not associated significantly with a change in HOMAIR at 15 weeks.
Fig. 3.
Association between HOMAIR and weight change after 15 weeks.
Median physical health improved significantly (p = 0.007), from a score (on a scale of 0–100) of 44.5 (39.7–51.4) at baseline to 48.0 (43.1–55.3) after 15 weeks, while median mental health declined significantly (p = 0.004), from 42.1 (36.1–46.7) to 37.4 (30.3–43.7).
Discussion
We showed that, independent of their medical therapy, patients with T2DM who are overweight or obese were able to improve their glycemic control and reduce their body weight by participating in a standardized weight-loss program that included a LCD, with no adverse events, especially hypoglycemia. The primary end point, HbA1c value, decreased significantly from 7.3 to 6.5% during the weight-loss program. This was accompanied by a reduced intake or even discontinuation of antidiabetic medication. Previous programs achieved similar changes in HbA1c values [16, 18, 19], but 2 of the studies used a formula diet with an energy content <800 kcal [16, 18]. Rothberg et al. [19] investigated patients with T2DM and obesity on a 15-week outpatient LCD (800 kcal), and observed a decrease in HbA1c from 7.4 to 6.5%. These data are in line with our results, even though the duration of intake of the formula diet only in our study was shorter. In the DiRECT trial, patients with T2DM participated in a weight-loss program using total diet replacement for 3–5 months (800–900 kcal), and 2–8 weeks for food reintroduction [29]; 46% of the patients achieved diabetes remission in the intervention group and 4% in the control group. One year later, diabetes remission had been sustained in more than one-third of the trial population. The weight-loss program was therefore appropriate for overweight or obese patients with T2DM, independent of insulin therapy to improve glycemic control as a short-term treatment.
In our study, the improvement in HbA1c was significantly associated with gender and the initial HbA1c value. Men with a higher baseline level had the greatest improvement in HbA1c value. The gender-specific effect concerning changes in HbA1c was recently described [30] and further research is needed to optimize more specific treatments. Furthermore, Rothberg et al. [19] identified initial HbA1c value, changes in BMI, and insulin therapy as predictors of change in HbA1c. In our study, changes in BMI and insulin therapy were not associated significantly with changes in HbA1c. In contrast, HOMAIR and weight change were significantly linked in our trial. Further research is needed to identify predictors for changes in HbA1c levels and further diabetes data.
The participants in our study were able to reduce their body weight by 11.9 kg (12%). The amount of weight loss was significant and correlated positively with initial body weight, independent of their medication, especially insulin therapy. National guidelines for the therapy of overweight and obesity recommend a weight loss of >5% for patients with a BMI between 25 and 35, and a weight loss of >10% for patients with a BMI >35 [31]. In our study, 18 of 19 participants with a baseline BMI >27 but <35 reached the goal of >5% weight loss and therefore met this criterion. In addition, in 10 of the 17 participants (58.8%) with a BMI >35, the weight-loss program achieved the goal of >10% weight loss. The remaining 7 patients were able to reduce their body weight by 5–10%. In the DiRECT trial, the mean weight loss was 10 kg in the intervention group and 1 kg in the control group. [15]. Furthermore, Li et al. [32] conducted a retrospective trial investigating the efficacy of weight-loss programs using a formula diet in patients with obesity and T2DM or prediabetes. They showed that all of the patients lost weight. There was no significant difference between the weight loss of patients with and without diabetes. Their data support the hypothesis that medically supervised diets, including formula diets, should be more widely used in prevention and treatment. The meta-analysis by Leslie et al. [22] also supported this hypothesis; they found that weight losses with formula diets were very similar for patients with and without diabetes. They concluded that patients with severe obesity can potentially achieve a weight loss of >15–20%. The success rate of short-term weight loss in our trial, especially for patients with a BMI <35, is in line with several trials and meta-analyses; it shows that national recommendations can be reached with the standardized weight-loss program. Patients with a BMI >35 should consider taking part in either a follow-up program or a program with an extended period on a formula diet.
The change in weight had an influence on body composition. A decrease in waist circumference indicates a reduction in abdominal obesity, claimed to be responsible for metabolic imbalances [33, 34]. In addition, transaminase levels were significantly decreased, suggesting an effect on liver fat content. The pandemic fatty liver disease, NAFLD, is a concomitant disease of obesity, and may result in a nonalcoholic steatohepatitis (NASH). The latter is characterized by elevation of liver enzymes and overt fatty liver on ultrasound without a history of alcohol consumption. NASH is a disease which leads to fibrosis, cirrhosis, and finally hepatocellular carcinoma, similar to alcoholic steatohepatitis. So far, there is no specific medical treatment for NAFLD, and weight loss is the only option to reverse steatohepatitis [35, 36]. In this context, the improvement of transaminases in our study can be explained with a reduction in liver fat content. This result is especially relevant for patients with T2DM who are overweight or obese, because of the strong relationship between insulin resistance and NAFLD [37, 38].
Blood levels of TG and TC were significantly improved during the standardized weight-loss program, but levels of LDL-C and HDL-C did not change significantly. The influence of weight loss on HDL-C and LDL-C is still not clear, because of inconsistencies in the published data [16, 17, 20]. A possible explanation for the discrepancy in TC and HDL-C and LDL-C might be the improved metabolic capacity of the liver during the weight loss. NAFLD is known to increase the production and secretion of very (V)LDL particles, most likely due to impaired hepatic insulin sensitivity [39, 40, 41]. Hence, the improvement in liver function suggested by the reduction in hepatic fat content as well as diminished plasma transaminase activity during the study period may be paralleled by a diminished production of larger VLDL particles. The latter would explain the drop in TC without affecting LDL-C and HDL-C measurements. However, a comprehensive assessment of lipoprotein particles during similar interventions would be needed to confirm this hypothesis.
The standardized weight-loss program did not affect changes in BP or hypertensive medication. A standardized weight-loss program over 1 year showed that BP was significantly improved in patients who completed the entire program, but there was no significant improvement detected when patients who discontinued the program were included in the analysis [10]. This finding may pertain to our results, in so far as the 15-week duration of the program may have been too short to achieve significant improvement in hypertension.
To investigate the influence of body changes on quality of life, we included the SF-12 in our study. As expected, physical health improved during the weight-loss program, which confirms the findings of other studies that observed obese patients after weight loss [42, 43]. Nadalini et al. [42] investigated patients before and after bariatric surgery and identified physical function as a predictor for weight loss.
Next to physical health, we hypothesized that mental health would also improve during the weight-loss program. A strong relationship exists between obesity and mental disorders [44, 45], and so researchers assume that weight loss helps to improve mental health [46]. The decline in mental health observed in our study was thus unexpected. Researchers in other trials did not find a significant change in mental health [42, 43]. One possible explanation for our result could be that a patient's daily energy intake must remain at a lower level than before the weight-loss program to maintain a reduced body weight [47]. This long-term restriction may lead to dissatisfaction and stress for the patients, which outweigh the joy of the achieved weight loss and improved glycemic control. However, this finding remains limited, because no further psychological tests were carried out; nevertheless, the importance of psychological support during and after weight loss must be emphasized.
Our study has several limitations. One limitation was the heterogeneous study population regarding the diabetes therapy. Half of the participants received insulin therapy and the other half were on oral hypoglycemic agents or dietary therapy. In addition, we were not able to include further data such as diabetes duration, characterization of the study population (according to the novel subgroups in Ahlqvist et al. [48]), and did not perform an oral glucose tolerance test. Other limitations are that there was no control group without diabetes, and the long-term effects of weight loss were not investigated. However, our study has also strengths. The trial population was representative of the average clinical diabetes population and, despite its heterogenous nature, we observed significant results for several parameters like HbA1c and weight changes. In addition, only 1 patient dropped out despite the complex trial design. There were no hypoglycemic episodes, underlining the feasibility and safety of the program. Possible reasons for the compliance could be, on the one hand, the close monitoring, and on the other hand, the financial contribution the patients had to make themselves to purchasing the diet formula, which may have increased their motivation [49].
In conclusion, for patients with T2DM who were overweight or obese, a standardized weight-loss program, that included a formula LCD and regular physical activity, was found to be effective in the short term in reducing HbA1c, weight, antidiabetic medication including insulin, and improving liver function tests. It was also safe regarding potential side effects, such as hypoglycemia, when blood glucose levels and antidiabetic medication were monitored on a regular basis. However, BP, HDL-C, and LDL-C were not affected by weight loss at 15 weeks. The influence of the decline in mental health and the long-term effects of improved glycemic control on patients' mortality require further trials.
Statement of Ethics
The trial was conducted in accordance with the local Institutional Review Board of Greifswald University Hospital, Germany (No. BB062/12). All clinical investigations were conducted according to the principles expressed in the World Medical Association Declaration of Helsinki. Written informed consent was obtained before study inclusion.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Nestlé Health Science Germany supported the study by granting study participants a 15% discount for the formula diet. L.J.S. and J.R. received a Gerhard Domagk scholarship from University Medicine Greifswald made possible through an unrestricted educational grant from Baxter Deutschland GmbH (Unterschleissheim, Germany), Profusio GmbH (Greven, Germany), and Nutriticia GmbH (Erlangen, Germany).
Author Contributions
Conceptualization, project administration, and supervision were conducted by A.S., M.M.L., and M.K. L.J.S., S.G., and J.R. were in charge of data curation. P.J.M. conducted the statistical analysis. L.J.S. wrote the original draft. All authors reviewed and edited the manuscript and approved the final version.
Acknowledgements
We thank all patients for participating in our trial. We also thank Eckhart Weber and Kathrin Radü-Thurow for their support in the course of the study.
References
- 1.WHO Technical Report Series 894. Obesity: preventing and managing the global epidemic. World Health Organization. 2000 Geneva. [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005 Oct;366((9492)):1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006 Dec;444((7121)):840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017 Jan;14((1)):32–42. doi: 10.1038/nrgastro.2016.147. [DOI] [PubMed] [Google Scholar]
- 5.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009 Mar;9((1)):88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Diabetes Federation . Brussels, Belgium: International Diabetes Federation, 9th edn. 2019. IDF Diabetes Atlas. http://www.diabetesatlas.org. [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998 Sep;352((9131)):837–53. [PubMed] [Google Scholar]
- 8.Chalmers J, Arima H, Woodward M, Mancia G, Poulter N, Hirakawa Y, et al. Effects of combination of perindopril, indapamide, and calcium channel blockers in patients with type 2 diabetes mellitus: results from the Action In Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation (ADVANCE) trial. Hypertension. 2014 Feb;63((2)):259–64. doi: 10.1161/HYPERTENSIONAHA.113.02252. [DOI] [PubMed] [Google Scholar]
- 9.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008 Feb;358((6)):580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff SC, Damms-Machado A, Betz C, Herpertz S, Legenbauer T, Löw T, et al. Multicenter evaluation of an interdisciplinary 52-week weight loss program for obesity with regard to body weight, comorbidities and quality of life—a prospective study. Int J Obes. 2012 Apr;36((4)):614–24. doi: 10.1038/ijo.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astbury NM, Aveyard P, Nickless A, Hood K, Corfield K, Lowe R, et al. Doctor Referral of Overweight People to Low Energy total diet replacement Treatment (DROPLET): pragmatic randomised controlled trial. BMJ. 2018 Sep;362:k3760. doi: 10.1136/bmj.k3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astbury NM, Piernas C, Hartmann-Boyce J, Lapworth S, Aveyard P, Jebb SA. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes Rev. 2019 Apr;20((4)):569–87. doi: 10.1111/obr.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pi-Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care. 2005 Jun;28((6)):1526–7. doi: 10.2337/diacare.28.6.1526. [DOI] [PubMed] [Google Scholar]
- 14.Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2004 Sep;65(Suppl 1):S23–7. doi: 10.1016/j.diabres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018 Feb;391((10120)):541–51. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 16.Capstick F, Brooks BA, Burns CM, Zilkens RR, Steinbeck KS, Yue DK. Very low calorie diet (VLCD): a useful alternative in the treatment of the obese NIDDM patient. Diabetes Res Clin Pract. 1997 May;36((2)):105–11. doi: 10.1016/s0168-8227(97)00038-7. [DOI] [PubMed] [Google Scholar]
- 17.Snel M, Jonker JT, Hammer S, Kerpershoek G, Lamb HJ, Meinders AE, et al. Long-term beneficial effect of a 16-week very low calorie diet on pericardial fat in obese type 2 diabetes mellitus patients. Obesity (Silver Spring) 2012 Aug;20((8)):1572–6. doi: 10.1038/oby.2011.390. [DOI] [PubMed] [Google Scholar]
- 18.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011 Oct;54((10)):2506–14. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH. Very-low-energy diet for type 2 diabetes: an underutilized therapy? J Diabetes Complications. 2014 Jul-Aug;28((4)):506–10. doi: 10.1016/j.jdiacomp.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delahanty LM, Dalton KM, Porneala B, Chang Y, Goldman VM, Levy D, et al. Improving diabetes outcomes through lifestyle change—A randomized controlled trial. Obesity (Silver Spring) 2015 Sep;23((9)):1792–9. doi: 10.1002/oby.21172. [DOI] [PubMed] [Google Scholar]
- 21.Mottalib A, Sakr M, Shehabeldin M, Hamdy O. J Diabetes Res. 2015. Diabetes Remission after Nonsurgical Intensive Lifestyle Intervention in Obese Patients with Type 2 Diabetes. 2015: Artikel ID 468704, 4 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie WS, Taylor R, Harris L, Lean ME. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: systematic review and meta-analysis. Int J Obes. 2017 Jan;41((1)):96–101. doi: 10.1038/ijo.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalmers J, Arima H, Woodward M, Mancia G, Poulter N, Hirakawa Y, et al. Fat quantification with IDEAL gradient echo imaging: correction of bias from T1 and noise. Diabetes Care. 2014;51((3)):354–64. [Google Scholar]
- 24.Frost F, Storck LJ, Kacprowski T, Gärtner S, Rühlemann M, Bang C, et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: A pilot study. PLoS One. 2019 Jul;14((7)):e0219489. doi: 10.1371/journal.pone.0219489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogt LJ, Steveling A, Meffert PJ, Kromrey ML, Kessler R, Hosten N, et al. Magnetic Resonance Imaging of Changes in Abdominal Compartments in Obese Diabetics during a Low-Calorie Weight-Loss Program. PLoS One. 2016 Apr;11((4)):e0153595. doi: 10.1371/journal.pone.0153595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28((7)):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997 Jun;19((2)):179–86. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 28.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998 Nov;51((11)):1171–8. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 29.Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019 May;7((5)):344–55. doi: 10.1016/S2213-8587(19)30068-3. [DOI] [PubMed] [Google Scholar]
- 30.Schütt M, Zimmermann A, Hood R, Hummel M, Seufert J, Siegel E, et al. DPV initiative. German BMBF Competence Network Diabetes Mellitus Gender-specific effects of treatment with lifestyle, metformin or sulfonylurea on glycemic control and body weight: a german multicenter analysis on 9108 patients. Exp Clin Endocrinol Diabetes. 2015 Nov;123((10)):622–6. doi: 10.1055/s-0035-1559608. [DOI] [PubMed] [Google Scholar]
- 31.Adipositas-Gesellschaft D. (DAG), Deutsche Diabetes Gesellschaft (DDG), Deutsche Gesellschaft für Ernährung (DGE) e.V. Deutsche Gesellschaft für Ernährungsmedizin (DGEM) e.V. Interdisziplinäre Leitlinie der Qualität S3 zur “Prävention und Therapie der Adipositas”. 2014;2 Auflage. [Google Scholar]
- 32.Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes. 2014 Feb;4((2)):e105. doi: 10.1038/nutd.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord. 2004 Dec;28((4 Suppl 4)):S12–21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 34.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec;444((7121)):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 35.Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, et al. Fatty Liver Subgroup of the Look AHEAD Research Group Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010 Oct;33((10)):2156–63. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004 Jun;39((6)):1647–54. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 37.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab. 2012 May;15((5)):574–84. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sporea I, Mare R, Lupușoru R, Sima A, Sirli R, Popescu A, et al. Liver Stiffness Evaluation by Transient Elastography in Type 2 Diabetes Mellitus Patients with Ultrasound-proven Steatosis. J Gastrointestin Liver Dis. 2016 Jun;25((2)):167–74. doi: 10.15403/jgld.2014.1121.252.lsf. [DOI] [PubMed] [Google Scholar]
- 39.Adiels M, Taskinen MR, Borén J. Fatty liver, insulin resistance, and dyslipidemia. Curr Diab Rep. 2008 Feb;8((1)):60–4. doi: 10.1007/s11892-008-0011-4. [DOI] [PubMed] [Google Scholar]
- 40.Borén J, Taskinen MR, Olofsson SO, Levin M. Ectopic lipid storage and insulin resistance: a harmful relationship. J Intern Med. 2013 Jul;274((1)):25–40. doi: 10.1111/joim.12071. [DOI] [PubMed] [Google Scholar]
- 41.Poulsen MK, Nellemann B, Stødkilde-Jørgensen H, Pedersen SB, Grønbæk H, Nielsen S. Impaired Insulin Suppression of VLDL-Triglyceride Kinetics in Nonalcoholic Fatty Liver Disease. J Clin Endocrinol Metab. 2016 Apr;101((4)):1637–46. doi: 10.1210/jc.2015-3476. [DOI] [PubMed] [Google Scholar]
- 42.Nadalini L, Zenti MG, Masotto L, Indelicato L, Fainelli G, Bonora F, et al. Improved quality of life after bariatric surgery in morbidly obese patients. Interdisciplinary group of bariatric surgery of Verona (G.I.C.O.V.) G Chir. 2014 Jul-Aug;35((7-8)):161–4. [PMC free article] [PubMed] [Google Scholar]
- 43.van Gemert WA, van der Palen J, Monninkhof EM, Rozeboom A, Peters R, Wittink H, et al. Quality of Life after Diet or Exercise-Induced Weight Loss in Overweight to Obese Postmenopausal Women: The SHAPE-2 Randomised Controlled Trial. PLoS One. 2015 Jun;10((6)):e0127520. doi: 10.1371/journal.pone.0127520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumeister H, Härter M. Mental disorders in patients with obesity in comparison with healthy probands. Int J Obes. 2007 Jul;31((7)):1155–64. doi: 10.1038/sj.ijo.0803556. [DOI] [PubMed] [Google Scholar]
- 45.de Zwaan M, Petersen I, Kaerber M, Burgmer R, Nolting B, Legenbauer T, et al. Obesity and quality of life: a controlled study of normal-weight and obese individuals. Psychosomatics. 2009 Sep-Oct;50((5)):474–82. doi: 10.1176/appi.psy.50.5.474. [DOI] [PubMed] [Google Scholar]
- 46.Osama AJ, Shehab AK. Psychological wellbeing and biochemical modulation in response to weight loss in obese type 2 diabetes patients. Afr Health Sci. 2015 Jun;15((2)):503–12. doi: 10.4314/ahs.v15i2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995 Mar;332((10)):621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 48.Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018 May;6((5)):361–9. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 49.LaRose JG, Leahey TM, Lanoye A, Reading J, Wing RR. A Secondary Data Analysis Examining Young Adults' Performance in an Internet Weight Loss Program with Financial Incentives. Obesity (Silver Spring) 2020 Jun;28((6)):1062–7. doi: 10.1002/oby.22797. [DOI] [PMC free article] [PubMed] [Google Scholar]