Abstract
Health systems in sub-Saharan Africa are facing an ongoing HIV epidemic and increasing burden of noncommunicable disease. With the focus shifting to the development of comprehensive primary health care and chronic disease treatment, multidisease modeling is integral to estimating future health care needs. We extended an established agent-based model of HIV transmission to include hypertension in two rural settings: KwaZulu-Natal, South Africa, and western Kenya. We estimated that from 2018 to 2028 hypertension prevalence would increase from 40 percent to 46 percent in KwaZulu-Natal and from 29 percent to 35 percent in western Kenya, while HIV prevalence is stabilizing and predicted to decrease. As the health system burden in sub-Saharan Africa is changing, innovative chronic disease treatment and the broadening of successful programs, such as integrated HIV and noncommunicable disease care, are necessary to reach universal health care coverage.
Health systems in sub-Saharan Africa are facing the challenges of aging populations, the persistent HIV epidemic, and rising prevalence of noncommunicable diseases.1 Globally, these diseases cause over forty million deaths annually, and low- and middle-income countries bear the largest burden related to the diseases, with mortality increasing most rapidly in sub-Saharan Africa.2 Hypertension is the leading risk factor for deaths due to cardiovascular disease, chronic kidney disease, and diabetes, which led the World Health Organization (WHO) to set the goal of a 25 percent reduction in hypertension prevalence worldwide by 2025.3,4
Concurrently, the scale-up of HIV treatment over the past fifteen years has greatly increased life expectancy in sub-Saharan Africa.5 However, the aging of people living with HIV has also resulted in an increasing number of patients who need care for comorbid noncommunicable diseases.6 Adding hypertension treatment to HIV care platforms is being piloted across sub-Saharan Africa, providing valuable information on the costs and effects of joint service delivery and showing improvements in blood pressure control.7 In addition to benefiting people with comorbid HIV and hypertension, integrated care may provide spillover benefits to those with only one chronic condition through health system synergies such as leveraging health system informatics or linkage strategies and increased testing availability.8
The extensive and lifelong care required to treat the HIV and noncommunicable disease epidemics has significant implications for health care systems as the focus shifts toward universal health coverage, one of the UN Sustainable Development Goals.9 A key prerequisite is having efficient and equitable health systems to provide access to essential health services, including chronic disease care.1 However, estimates are lacking of the co-occurrence of the disease burdens and the resulting health care needs, which policy makers must have to translate findings into workable health system implementations of integrated care. Mathematical modeling can help inform current and future epidemiology, as well as quantifying the population and health system burden.10,11 The informed development and expansion of integrated care requires models that can simulate the joint burden. Our multidisease model is the first to our knowledge that is fit to population screening for noncommunicable diseases and HIV and that quantifies the direct and indirect benefits of integrated care.
We estimated health system burden, morbidity, and mortality of HIV and hypertension in two regions in sub-Saharan Africa: KwaZulu-Natal, South Africa, and western Kenya. The health system burden is the proportion of people who should be engaged in care, as defined by national guidelines, to meet treatment coverage goals. For HIV, this refers to the 90-90-90 targets of the Joint United Nations Programme on HIV/AIDS, in which 90 percent of people with HIV know their status, 90 percent of those who know their status are enrolled in care, and 90 percent of those enrolled in care have viral suppression.12 The health system burden for hypertension is operationalized as having 50 percent of the hypertensive population engaged in care for counseling, drug therapy, or monitoring, derived from the WHO’s target in its action plan on non-communicable diseases to prevent heart attack and stroke.13 We also quantified all potential beneficiaries of integrated care, divided into intended beneficiaries (who have comorbid HIV and hypertension) and spillover beneficiaries (who have either HIV or hypertension).
We selected KwaZulu-Natal and western Kenya because both have the highest historical HIV burden in their respective countries: By 2012 HIV prevalence had reached 29 percent among adults ages 15–49 in KwaZulu-Natal and 10 percent among adults over age 18 in western Kenya.14,15 These regions are of key interest for the potential scale-up of integrated chronic disease care since they have been the sites of comprehensive responses to the HIV epidemic and are predominantly rural areas—which have lower preparedness for chronic noncommunicable disease care, compared to more urban areas.15-17 We used STDSIM, an established agent-based microsimulation model of the spread and control of HIV and other sexually transmitted infections (discussed in greater detail in the next section),5 and we expanded it to incorporate the natural history and epidemiology of hypertension alongside HIV. Our model has agents that can develop HIV, hypertension, or both, and it projects the morbidity, mortality, and health system implications of the dual burden. The model includes changing hypertension risk as a feature of both the aging population and increases in hypertension across age groups over time. Thus, the projected hypertension burden increased more rapidly than in other modeling studies, which resulted in a greater expected burden for health systems.
Study Data And Methods
MODEL OVERVIEW
STDSIM is an established agent-based model that creates a representative virtual population of individuals, or agents, that interact with each other to form a dynamic network of sexual contacts. Similar to real people, each agent has a set of demographic, clinical, and behavioral traits; based on these traits, the agents interact with each other to form networks. Each agent’s traits are drawn from distributions that are calibrated to make this virtual population similar to the population it represents. The model contains four input modules: demographic characteristics, sexual behavior, the natural history of the diseases, and interventions (including treatments). A detailed description of the model can be found elsewhere.18
MODELING HIV
We modeled HIV as having four consecutive states—early infection, asymptomatic infection, symptomatic infection, and AIDS—which were timed to replicate the progression of HIV from infection. Antiretroviral therapy treatment was operationalized with two submodels: the agents’ demand for the therapy as a function of disease status, and the health system’s capacity to meet that demand.18 The online appendix provides further details.19
MODELING HYPERTENSION
Blood pressure was modeled as either normotensive or hypertensive, with the latter defined as having blood pressure of at least 140/90 mmHg, as Kenya and South Africa use the cutoff recommended by the WHO instead of the more aggressive definition of at least 130/80 mmHg adopted by the American Heart Association and the American College of Cardiology in 2017.20,21 All agents are normotensive at birth and have an individual risk of developing hypertension during their lives. The timing of the hypertension development is drawn at birth and models the risk of hypertension associated with aging. Since men and women have differing risks of developing hypertension, they have separate distributions. The women’s distribution implicitly models the changing risk of developing hypertension beginning around menopause, which is consistent with evidence showing a cardiovascular-protective effect of estradiol in premenopausal women and a sharp rise in hypertension among postmenopausal women.22
In addition to this baseline risk of hypertension, which increases with age, changes in risk factors in sub-Saharan Africa attributed to economic development—including increased inactivity, unhealthy diet, and changes in stressors—have resulted in temporal growth of age-adjusted hypertension prevalence.23 We modeled this growth as a competing risk of becoming hypertensive if the agent is still normotensive at that time.
SETTINGS AND DATA
To calibrate the model, we used population surveillance data of hypertension and HIV from KwaZulu-Natal and western Kenya. The model inputs replicated disease development using a life-course approach, with multiple parameters to approximate the combination of age, cohort, and period effects. The model used these inputs to produce a simulated longitudinal cohort as output, which we used to estimate annual prevalence, incidence, and morbidity that we could project into the future. A robust calibration process ensured that our life-course inputs reproduced the observed data.
Data for KwaZulu-Natal were derived from the Africa Centre Demographic Information System population HIV screening surveillance surveys, which began in 2003–04 and have been used to calibrate the HIV component of STDSIM in prior studies.18 The 2003–04 and 2010 surveys also included hypertension screening, and we used this surveillance prevalence to calibrate the prevalences of hypertension, HIV, and both hypertension and HIV as comorbidities.24 The current levels of hypertension prevalence at the national and provincial levels and of awareness, treatment, and control of hypertension in South Africa came from the South African National Health and Nutrition Examination Survey.25
Data on hypertension prevalence, awareness, treatment, and control in Kenya were from the 2015 Kenya STEPwise Survey for Non Communicable Diseases Risk Factors.26 This nationally representative sample screened for noncommunicable diseases. Hypertension prevalences in counties in western Kenya were consistent with the prevalence in the nation as a whole, but there were limited respondents for some age groups for males in western Kenya, so the national prevalence of hypertension was used for calibration because of the larger sample size. Calibration of HIV modeling in western Kenya used reports from the 2016 Kenya HIV County Profiles and the 2007 and 2012 Kenya AIDS Indicator Surveys.27-29
PROJECTIONS AND OUTCOMES
We modeled total annual mortality, incidence, and prevalence, as well as the health system burden of hypertension, HIV, and comorbid HIV and hypertension, for the period 2018–28. We present the mean projections and 95% uncertainty intervals from all parameterizations of the model that fit the data (for the parameterizations, see the appendix).19 The health system burden for these conditions was quantified as the number of people per 100,000 population enrolled in HIV treatment to reach the first two 90-90-90 targets of the Joint United Nations Programme on HIV/AIDS; the number enrolled in noncommunicable disease care to reach the target of 50 percent of hypertensive people in care for monitoring, counseling, or receiving drug therapy; the number with both HIV and hypertension as comorbidities (people who could benefit directly from integrated treatment programs); and the number with either HIV or hypertension (who could benefit from integrated care through spillover even though they have only one of the chronic conditions).
In the base model we assumed that current levels of hypertension care would remain constant in 2018–28, incorporating the current levels of diagnosis, treatment use, and hypertension control. Since treatment does not influence transmission and increases healthy life-years while not providing a cure, an increase in hypertension treatment would result in more people living longer while still needing hypertension care. If treatment increased, our future projections would provide a lower estimate of morbidity and health system burden for hypertension. We also ran scenarios that held screening and treatment to current levels and maximized screening and treatment use, to explore the implication of improving these components of hypertension care on treatment targets.
Each model run simulated approximately 35,000 individuals. To account for the stochasticity in the model, we used the average result of 1,000 runs. To allow for easier comparison across the populations, projections of incidence and prevalence are presented as percentages of the population, and health system burden projections are quantified as per 100,000 population. Simulations were run on supercomputing clusters through Brown University’s Center for Computation and Visualization and the Population Studies and Training Center.
LIMITATIONS
This study had several limitations. The main one was the lack of complete surveillance data over multiple years in both populations. Panel surveys that include blood pressure screening and longitudinal assessments of population hypertension prevalence are rare, and low levels of knowledge of hypertensive status and low health care use for hypertension minimize the utility of self-report or health records for these estimates.23 Using the age- and sex-specific prevalence rates from the serial cross-sectional surveys in KwaZulu-Natal for hypertension and for hypertension and HIV as comorbidities allowed model calibration, but the fact that there were few years of surveillance for hypertension and for comorbid noncommunicable diseases and HIV was a limitation. The increased attention to hypertension and other noncommunicable diseases in sub-Saharan Africa is leading to more studies on the population prevalence of these conditions, so more panel data may become available.
Another limitation is that mathematical modeling is an abstraction of reality. We made simplifying assumptions for some components that could influence the development or control of hypertension or HIV, and as with all mathematical models, there was uncertainty in the parameter estimates. To address these issues, we conducted a sensitivity analysis of key parameters and assumptions used in the construction of the model, and we varied the parameters within the calibration process. For details, see the appendix.19
Study Results
DISEASE PREVALENCE
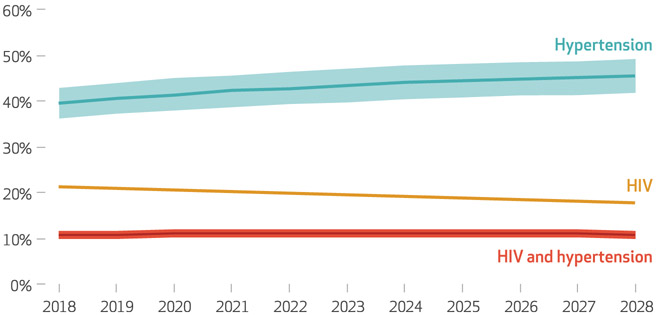
In both KwaZulu-Natal and western Kenya, the prevalence of hypertension is projected to increase from 2018 to 2028. In KwaZulu-Natal the prevalence increases from 40 percent to 46 percent (exhibit 1). In western Kenya it increases from 29 percent to 35 percent (exhibit 2). During the same period the prevalence of HIV is projected to decrease for both populations—from 21 percent to 18 percent in KwaZulu-Natal and from 5 percent to 3 percent in western Kenya. In KwaZulu-Natal the prevalence of comorbid HIV and hypertension increases modestly from 10 percent to 11 percent, while it decreases less than 1 percent in western Kenya from 2.3 percent to 1.8 percent. The changes in prevalence are driven by increasing incidence of hypertension and decreasing incidence in HIV (appendix exhibit A6).19 Mortality remains stable throughout this time period, since mortality due to HIV decreases as a result of increased treatment, while the aging of the population increases hypertension prevalence and other-cause mortality (appendix exhibit A7).19
EXHIBIT 1. Projected percent of people in KwaZulu-Natal, South Africa, with hypertension, HIV, or both, 2018–28.
SOURCE Authors’ analysis of data from an STDSIM simulation. NOTE The shaded areas represent 95% uncertainty intervals.
EXHIBIT 2. Projected percent of people in western Kenya with hypertension, HIV, or both, 2018–28.
SOURCE Authors’ analysis of data from an STDSIM simulation. NOTE The shaded areas represent 95% uncertainty intervals.
HEALTH SYSTEM BURDEN
Under the WHO guidelines to initiate antiretroviral therapy regardless of CD4 count, we projected a decrease in both Kwa-Zulu-Natal and western Kenya from 2018 to 2028 in the therapy needed to reach the target of 81 percent of HIV-infected people enrolled in care, driven by the decrease in HIV incidence (exhibit 3). In KwaZulu-Natal the number of people needing care for hypertension overtakes the number needing antiretroviral therapy by 2020, even with the relatively modest goal of treating 50 percent of people with hypertension (data not shown). In western Kenya the number of people in hypertension treatment needed to reach that target is 2.4 times larger than the population needing antiretroviral therapy to reach the HIV target in 2018, a difference that doubles by 2028. With the decreasing HIV prevalence and increasing hypertension prevalence, the population with both conditions who would directly benefit from integrated care remains stable in KwaZulu-Natal and even decreases slightly in western Kenya from 2018 to 2028. However, the number of people with either HIV or hypertension who could receive spillover benefits increases during this period.
EXHIBIT 3.
Estimated health system burdens of hypertension and HIV per 100,000 population in KwaZulu-Natal, South Africa, and western Kenya, 2018 and 2028
| 2018 |
2028 |
|||
|---|---|---|---|---|
| Number | 95% UI | Number | 95% UI | |
| KWAZULU-NATAL | ||||
| ART coverage targeta | 20,558 | (18,886, 20,609) | 17,172 | (17,139, 18,017) |
| Hypertension treatment targetb | 19,831 | (18,195, 21,466) | 23,787 | (20,908, 24,665) |
| People with HIV and hypertension | 10,711 | (9,827, 11,596) | 10,962 | (10,058, 11,866) |
| People with either HIV or hypertension | 54,330 | (49,878, 56,779) | 55,811 | (52,917, 59,707) |
| WESTERN KENYA | ||||
| ART coverage targeta | 5,934 | (5,645, 6,023) | 3,419 | (3,307, 3,532) |
| Hypertension treatment targetb | 14,452 | (13,528, 15,375) | 16,838 | (15,762, 17,919) |
| People with HIV and hypertension | 2,280 | (2,116, 2,445) | 1,746 | (1,621, 1,870) |
| People with either HIV or hypertension | 33,826 | (31,903, 35,741) | 36,151 | (33,986, 38,328) |
SOURCE Authors’ analysis of data from an STDSIM simulation. NOTE UI is uncertainty interval. ART is antiretroviral therapy.
Number of people with HIV who would have to be enrolled in care to reach the goals of having 90 percent of people with HIV aware of their status and 90 percent of those people enrolled in HIV care.
Number of people with hypertension who would have to be enrolled in care to reach the target of 50 percent of those people receiving blood pressure monitoring, counseling, or drug therapy.
HYPERTENSION TREATMENT SCENARIOS
For a population of 100,000 in 2028 the target is to treat 23,787 people with hypertension in Kwa-Zulu-Natal and 16,838 people with hypertension in western Kenya. In the base-case scenario with current screening and treatment, the population treatment coverage in 2028 is 8,059 (66 percent below the target) in KwaZulu-Natal and 2,267 (84 percent below the target) in western Kenya (exhibit 4). Under the alternative scenario that maximized diagnosis to a theoretical 100 percent while holding the proportion diagnosed in treatment at the current level, the population in treatment in KwaZulu-Natal is 36,631 (54 percent above the target). However, under the second alternative scenario, which maximized the proportion in treatment if diagnosed without changing diagnosis from the base case, the population in treatment was 10,466 (56 percent below the target). In western Kenya neither alternative scenario would achieve the target treatment, at 8,082 (52 percent below the target) and 11,113 (34 percent below the target), respectively, because of the low levels of both diagnosis and treatment if diagnosed.
EXHIBIT 4. Hypertension treatment scenarios for KwaZulu-Natal, South Africa, and western Kenya in 2028.
SOURCE Authors’ analysis of data from an STDSIM simulation; Shisana O, et al. The South African National Health and Nutrition Examination Survey, 2012 (note 37 in text); and Kenya Ministry of Health. Kenya STEPwise Survey for Non Communicable Diseases Risk Factors 2015 report (note 26 in text). NOTE The treatment target is for 50 percent of people with hypertension to receive care for it.
SENSITIVITY ANALYSIS
We evaluated the effect on trends and point estimates of varying key assumptions. Evaluating the assumption of changing age-specific risk resulted in lower hypertension prevalence than our main findings projected. Although the increase in hypertension was smaller, it did not change the overall pattern of our results. Varying the assumptions used in the quantification of hypertension risk change timing and growth in western Kenya similarly did not materially affect the pattern in our findings. We also tested the implication of the association between HIV and hypertension by increasing blood pressure for people on antiretroviral therapy. This made no significant changes in the population-level estimates, as it affected only the hypertensive classification of those still at risk and close to the threshold. For specifications and results, see the appendix.19
Discussion
We developed a multidisease model for HIV and hypertension and estimated current and future burden and health system needs for both diseases in parts of Kenya and South Africa. We projected an increase in hypertension prevalence and incidence in both settings, a decrease in HIV incidence and prevalence, stable rates of comorbid HIV and hypertension, and increased direct and spillover benefits for integrated health care.
The observed trend in hypertension prevalence is driven by the aging population, changing profiles of communicable diseases, and increases in hypertension risk across sex- and age-specific groups.5,23 By operationalizing the changing hypertension risk as the aggregate effect of conditions related to modernization and economic development for each individual, we could model multifactorial trends in hypertension with limited assumptions. This provided us with the flexibility to model temporal change without requiring data on changes in specific risk factors or the effects of changing nutritional exposures, adiposity, and psychosocial stress. As a result, our modeled hypertension prevalence increased more rapidly than other projections that rely on cross-sectional estimates of risk profiles by age and model all change through the aging population.30,31 The trends predicted from our model are consistent with a longitudinal cohort study in South Africa and trends in age-standardized and hypertension-attributed mortality in Kenya, so our projections of health systems’ need to reach treatment targets provide a more robust and greater expectation of burden for planning purposes than many other modeling studies have done.3,32 Our results can be considered more conservative than they would have been if we had used the stringent hypertension criteria adopted by the American Heart Association and American College of Cardiology.20
Our model purposefully did not include any interactions between hypertension and HIV for these populations in sub-Saharan Africa, except in the sensitivity analysis. In high-income countries the syndemic of HIV and hypertension is supported by a large body of evidence that shows increased hypertension and other noncommunicable diseases among people living with HIV.33 However, studies in sub-Saharan Africa have found conflicting evidence and lower rates of hypertension among these people.34 This may be due to a number of factors, including pretreatment wasting that reduced body mass index; lifestyle change advice for these people such as the avoidance of alcohol or dietary changes to improve hypertension risk profiles; and HIV care involvement that alerted patients to, and treated patients for, their high blood pressure.35 In sub-Saharan Africa, HIV infection’s influence on cardiovascular risk factors can be protective or lead to increased risk because of the many different pathways, at both the individual and population levels.36 Therefore, because of the lack of clear evidence regarding the relationship between HIV and hypertension in sub-Saharan Africa, we also modeled the diseases separately. The prevalence of both conditions as comorbidities was compared with KwaZulu-Natal data, as that was the only site with both conditions screened in the same survey, and modeling without any interaction fit these data.
While our models were limited to two specific regions, the relevance of this research is broader. We modeled the subnational regions with the highest prevalence of HIV because the HIV epidemic dynamics vary greatly by location. Focusing on specific regions instead of attempting to make a national estimate produced more realistic networks for infectious disease transmission in our model and robust estimates of the dual burden in priority areas. Because we did not model an interaction between HIV and hypertension, and the prevalence of hypertension was equivalent in both HIV-positive and -negative populations, modeling a high HIV burden did not increase the overall hypertension estimates in the model, compared to areas with lower HIV prevalence. The prevalence of hypertension within our modeled regions closely approximated the national prevalence in each country: In the 2012 South African National Health and Nutrition Examination Survey, the prevalence of hypertension in KwaZulu-Natal was 32.7 percent, compared to the national estimate for South Africa of 31.8 percent (95% confidence interval: 29.9, 33.7),37 while the prevalence in western Kenya was 23.1 percent per the 2015 Kenya STEPwise Survey for Non Communicable Diseases Risk Factors, and the Kenyan prevalence nationally was 24.5 percent (95% CI: 22.6, 26.6).26
Also, these two regions are predominantly rural, which provided the opportunity for us to model the burden of disease among this large (75 percent of Kenyans and 35 percent of South Africans) and understudied population. Rural health systems are significantly less prepared for treatment of noncommunicable diseases than are systems in urban areas in sub-Saharan Africa, and our research identifies a large and growing need to provide chronic disease care in these underprepared areas.16 As health systems are transforming, chronic disease care availability in rural areas must become a substantial component of the health services offered under universal health coverage in sub-Saharan Africa, because over half of the population in KwaZulu-Natal and a third of the population in western Kenya are projected to suffer from HIV, hypertension, or both by 2028.
Policy Implications
The similar trends in KwaZulu-Natal and western Kenya, despite the regions’ many underlying differences, can be used to shape health system priorities, while the variations in care coverage and disease prevalence identify more specific policy recommendations.
First, the population prevalence of hypertension in both Kenya and South Africa is increasing, despite differences in prevalence and changing risks. Policies that target changing risk, while important, are unlikely to contain the entire increasing need for care alone, and treatment scale-up is necessary to reach targets of controlling blood pressure to reduce hypertension-related mortality.38
Second, integrated HIV and noncommunicable disease screening programs to increase diagnosis are considered a low-cost way to address this priority.31 In addition to increasing the diagnosis of hypertension, as the prevalence of HIV is projected to decrease, such integrated screening programs may keep down the cost of screening per new diagnosis. However, as illustrated in the treatment scenarios, the benefit of screening programs in achieving treatment goals is dependent on care use. In South Africa, where 77 percent of people diagnosed with hypertension are in treatment, the return on investment of a screening program would be higher than in Kenya, where only 24 percent of people who are aware of their hypertension are in treatment. Screening initiatives in areas with lower treatment use require strategies to improve linkage and adherence to hypertension care, leveraging lessons learned in the HIV care cascade.
Finally, health systems need to increase the capacity for hypertension treatment. By 2028 we project at least a 20 percent increase in the demand for hypertension care in KwaZulu-Natal and a 17 percent increase in western Kenya, even before accounting for additional demand from increased diagnosis rates and linkage to care. Integrated HIV and noncommunicable disease care has many direct benefits, including increased screening and treatment use for people living with HIV. But scaling up the chronic care system can also include leveraging the spillover effects, such as those on health informatics systems, long-term care management, and differentiated care for different levels of disease control.
Conclusion
Disease risk for hypertension and HIV is changing, and health system planning in sub-Saharan Africa should account for this significant source of morbidity and mortality. Controlling both HIV and hypertension are major public health priorities, but they require credible projections of burden for health system planning.1 This research represents a first attempt at modeling the changing risks across all age groups and predicts growing population burdens of hypertension and of HIV and hypertension as comorbidities. Understanding multiple disease dynamics is integral to health system strengthening efforts for universal health coverage planning. This multidisease modeling underscores the importance of prioritizing the looming challenge of chronic disease care in sub-Saharan Africa.
Supplementary Material
Acknowledgments
This work was funded by the Fogarty International Center of the National Institutes of Health (NIH), the President’s Emergency Plan for AIDS Relief, and CRDF Global (Grant No. OISE-17-62967-1). The authors are grateful to the Population Studies and Training Center at Brown University, which receives funding from the NIH (Grant No. P2C-HD-041020), for general support. This work was also facilitated by the Providence/Boston Center for AIDS Research (Grant No. P30-AI-042853). The views expressed in this article are those of the authors and do not reflect the views of the National Institutes of Health.
Contributor Information
Brianna Osetinsky, Department of Health Services, Policy, and Practice, Brown University School of Public Health, in Providence, Rhode Island..
Jan A. C. Hontelez, Department of Public Health at Erasmus MC, Erasmus University Rotterdam, in the Netherlands, and at the Heidelberg Institute of Global Health, Heidelberg University, in Germany..
Mark N. Lurie, Department of Epidemiology, Brown University School of Public Health..
Stephen T. McGarvey, Department of Epidemiology, both at the Brown University School of Public Health..
Gerald S. Bloomfield, Department of Medicine and Global Health, Duke University School of Medicine, in Durham, North Carolina..
Sonak D. Pastakia, Purdue Kenya Partnership, Purdue University College of Pharmacy, in Eldoret, Kenya..
Richard Wamai, Department of Cultures, Societies, and Global Studies, Northeastern University, in Boston, Massachusetts..
Till Bärnighausen, Heidelberg Institute of Global Health, Heidelberg University. Africa Health Research Institute, in Somkhele, South Africa; global health at the Harvard T. H. Chan School of Public Health, in Boston..
Sake J. de Vlas, Department of Public Health at Erasmus MC, Erasmus University Rotterdam..
Omar Galárraga, Department of Health Services, Policy, and Practice, Brown University School of Public Health..
NOTES
- 1.Agyepong IA, Sewankambo N, Binagwaho A, Coll-Seck AM, Corrah T, Ezeh A, et al. The path to longer and healthier lives for all Africans by 2030: the Lancet Commission on the future of health in sub-Saharan Africa. Lancet. 2018;390(10114): 2803–59. [DOI] [PubMed] [Google Scholar]
- 2.Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou B, Bentham J, Di Cesare M, Bixby H, Danaei G, Cowan MJ, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389(10064): 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global status report on noncommunicable diseases: 2014 [Internet]. Geneva: WHO; c 2014. [cited 2019 May 3]. p. 67. Available from: https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf [Google Scholar]
- 5.Hontelez JA, de Vlas SJ, Baltussen R, Newell ML, Bakker R, Tanser F, et al. The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS. 2012;26(Suppl 1):S19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomfield GS, Khazanie P, Morris A, Rabadán-Diehl C, Benjamin LA, Murdoch D, et al. HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr. 2014; 67(Suppl 1):S40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Njuguna B, Vorkoper S, Patel P, Reid MJA, Vedanthan R, Pfaff C, et al. Models of integration of HIV and noncommunicable disease care in sub-Saharan Africa: lessons learned and evidence gaps. AIDS. 2018; 32(Suppl 1):S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tollman SM, Kahn K, Sartorius B, Collinson MA, Clark SJ, Garenne ML. Implications of mortality transition for primary health care in rural South Africa: a population-based surveillance study. Lancet. 2008; 372(9642):893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Nations. Sustainable Development Goals: 17 goals to transform our world [Internet]. New York (NY): UN; [cited 2019 May 3]. Available from: https://www.un.org/sustainabledevelopment/ [Google Scholar]
- 10.Olney JJ, Braitstein P, Eaton JW, Sang E, Nyambura M, Kimaiyo S, et al. Evaluating strategies to improve HIV care outcomes in Kenya: a modelling study. Lancet HIV. 2016; 3(12):e592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem Av, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic [Internet]. Geneva: UNAIDS; 2014. October [cited 2019 Apr 30]. Available for download from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf [Google Scholar]
- 13.World Health Organization. Global action plan for the prevention and control of noncommunicable diseases: 2013–2020 [Internet]. Geneva: WHO; 2013. [cited 2019 May 3]. Available from: https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf [Google Scholar]
- 14.Zaidi J, Grapsa E, Tanser F, Newell ML, Bärnighausen T. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS. 2013;27(14):2301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wachira J, Ndege S, Koech J, Vreeman RC, Ayuo P, Braitstein P. HIV testing uptake and prevalence among adolescents and adults in a large home-based HIV testing program in Western Kenya. J Acquir Immune Defic Syndr. 2014;65(2): e58–66. [DOI] [PubMed] [Google Scholar]
- 16.Moucheraud C Service readiness for noncommunicable diseases was low in five countries in 2013–15. Health Aff (Millwood). 2018;37(8):1321–30. [DOI] [PubMed] [Google Scholar]
- 17.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013; 339(6122):966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hontelez JA, Lurie MN, Bärnighausen T, Bakker R, Baltussen R, Tanser F, et al. Elimination of HIV in South Africa through expanded access to antiretroviral therapy: a model comparison study. PLoS Med. 2013;10(10): e1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.To access the appendix, click on the Details tab of the article online.
- 20.Wander GS, Ram CVS. Global impact of 2017 American Heart Association/American College of Cardiology hypertension guidelines: a perspective from India. Circulation. 2018;137(6): 549–50. [DOI] [PubMed] [Google Scholar]
- 21.Whitworth JA, Chalmers J. World Health Organisation–International Society of Hypertension (WHO/ISH) hypertension guidelines. Clin Exp Hypertens. 2004;26(7–8):747–52. [DOI] [PubMed] [Google Scholar]
- 22.Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep. 2012; 14(3):254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim MM, Damasceno A. Hypertension in developing countries. Lancet. 2012;380(9841):611–9. [DOI] [PubMed] [Google Scholar]
- 24.Tanser F, Hosegood V, Bärnighausen T, Herbst K, Nyirenda M, Muhwava W, et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol. 2008;37(5):956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry KM, Parker WA, Mchiza ZJ, Sewpaul R, Labadarios D, Rosen S, et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012. BMJ Glob Health. 2017;2(3): e000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenya Ministry of Health. Kenya STEPwise Survey for Non Communicable Diseases Risk Factors 2015 report [Internet]. Nairobi: Ministry of Health, Division of Non-Communicable Diseases; [cited 2019 May 3]. Available from: http://aphrc.org/wp-content/uploads/2016/04/Steps-Report-NCD-2015.pdf [Google Scholar]
- 27.Kenya Ministry of Health, National AIDS and STI Control Programme. Kenya HIV County Profiles: 2016 [Internet]. Nairobi: National AIDS Control Council; 2016. [cited 2019 May 3]. Available from: https://nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-County-Profiles-2016.pdf [Google Scholar]
- 28.National AIDS and STI Control Programme. Kenya AIDS Indicator Survey 2007: final report [Internet]. Nairobi: NASCOP; 2009. June [cited 2019 May 7]. Available from: http://guidelines.health.go.ke:8000/media/KAIS_2007_Final.pdf [Google Scholar]
- 29.Kenya Ministry of Health, National AIDS and STI Control Programme. Kenya AIDS Indicator Survey 2012: final report [Internet]. Nairobi: NASCOP; 2014. June [cited 2019 May 3]. Available from: https://nacc.or.ke/wp-content/uploads/2015/10/KAIS-2012.pdf [Google Scholar]
- 30.Smit M, Olney J, Ford NP, Vitoria M, Gregson S, Vassall A, et al. The growing burden of noncommunicable disease among persons living with HIV in Zimbabwe. AIDS. 2018;32(6):773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekker LG, Alleyne G, Baral S, Cepeda J, Daskalakis D, Dowdy D, et al. Advancing global health and strengthening the HIV response in the era of the Sustainable Development Goals: the International AIDS Society–Lancet Commission. Lancet. 2018;392(10144):312–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips-Howard PA, Laserson KF, Amek N, Beynon CM, Angell SY, Khagayi S, et al. Deaths ascribed to non-communicable diseases among rural Kenyan adults are proportionately increasing: evidence from a health and demographic surveillance system, 2003–2010. PLoS One. 2014;9(11):e114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathi A, Jerrell JM, Skelton TN, Nickels MA, Duffus WA. Incidence of primary hypertension in a population-based cohort of HIV-infected compared with non-HIV-infected persons and the effect of combined antiretroviral therapy. J Am Soc Hypertens. 2015;9(5):351–7. [DOI] [PubMed] [Google Scholar]
- 34.Malaza A, Mossong J, Bärnighausen T, Newell ML. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PLoS One. 2012;7(10): e47761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manne-Goehler J, Montana L, Gómez-Olivé FX, Rohr J, Harling G, Wagner RG, et al. The ART advantage: health care utilization for diabetes and hypertension in rural South Africa. J Acquir Immune Defic Syndr. 2017;75(5):561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayosi BM, Flisher AJ, Lalloo UG, Sitas F, Tollman SM, Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374(9693):934–47. [DOI] [PubMed] [Google Scholar]
- 37.Shisana O, Labadarios D, Rehle T, Simbayi L, Zuma K, Dhansay A, et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: the health and nutritional status of the nation [Internet]. 2nd ed. Cape Town: HSRC Press; 2014. [cited 2019 May 3]. Available for download from: http://www.hsrc.ac.za/en/research-outputs/view/6493 [Google Scholar]
- 38.Juma PA, Mapa-Tassou C, Mohamed SF, Matanje Mwagomba BL, Ndinda C, Oluwasanu M, et al. Multi-sectoral action in non-communicable disease prevention policy development in five African countries. BMC Public Health. 2018;18(Suppl 1):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.