Abstract
Acromelic frontonasal dysostosis (AFND; MIM #603671) is a rare autosomal dominant genetic disorder caused by a heterozygous mutation in the ZSWIM6 (KIAA1577) gene located at chromosome 5q12.1. It is phenotypically characterized by frontonasal malformation with hypertelorism, telecanthus, nasal clefting or bifid nasal tip, wide fontanels and sutures, brachycephaly, and cleft palate. The patients also present with central nervous system malformations such as encephalocele, agenesis of the corpus callosum, or interhemispheric lipoma. Limb malformations can also be found, including preaxial polydactyly of the feet and sometimes postaxial polydactyly of the hands, talipes equinovarus, or tibia malformations. Here, we present a case of early prenatal diagnosis of AFND with ultrasound and necropsy images which show the phenotypic findings of this syndrome.
Keywords: Acromelic frontonasal dysostosis, ZSWIM6, Craniofacial malformation, Limb malformation, Central nervous system malformation
Established Facts
Acromelic frontonasal dysostosis (AFND; MIM #603671) is a rare autosomal dominant genetic disorder caused by a heterozygous mutation in the ZSWIM6 (KIAA1577) gene located at chromosome 5q12.1
Novel Insights
Ultrasound at the 15th week of pregnancy showed the 3 main signs of AFND: facial malformation, hypertelorism, lower limb malformation, and central nervous system alterations.
Early prenatal diagnosis is possible.
Introduction
Frontonasal dysplasia is a craniofacial alteration which includes hypertelorism and varying degrees of median cleft face [Sedano et al., 1970]. When this malformation appears along with various alterations of the central nervous system and limb defects, it forms an entity called acromelic frontonasal dysostosis (AFND) [Verloes et al., 1992]. It is a rare autosomal dominant genetic disorder with a known molecular basis (MIM #603671), and it is caused by a heterozygous mutation in the ZSWIM6 (KIAA1577) gene located at chromosome 5q12.1. Its prevalence is less than 1/1,000,000 among newborns, being an extremely rare disease. To the best of our knowledge, this is the first case of this rare disease diagnosed in the early second trimester of pregnancy.
Case Report
We present a 39-year-old pregnant woman, Caucasian, born in Argentina, and living in Madrid (Spain). She had a history of preterm birth at 26 weeks of gestation 17 years before due to preeclampsia. Subsequently, she had a first trimester abortion. The pregnancy reported here was the first one with her current partner, also Caucasian and nonconsanguineous. She did not suffer from any disease of interest, did not take any drugs, and had not been exposed to any known toxic substance. A basic analytical study of the first trimester was performed, not finding any alterations. Screening for gestational diabetes turned out normal, her thyroid function was normal, and the serology for toxoplasmosis, syphilis, hepatitis B, hepatitis C, and HIV turned out negative, with a permanent immunity against rubella. Her blood type was O positive.
During the 13th week of pregnancy, she presented to the hospital for an ultrasound and a screening for chromosomopathies. The crown-rump length of the fetus corresponded to the amenorrhea, and the nuchal translucency measurement was normal. The combined screening results showed low-risk chromosomopathies. However, the morphological evaluation of the fetus showed abnormal lower limbs in which femurs were normal, but a mesomelic shortening and malposition of both feet were noticable (Fig. 1). The upper limbs seemed normal on ultrasound. Furthermore, the fetal profile showed an absent nasal bone and, evaluating the facies in a coronal cut in order to display the upper lip, a hypertelorism could be discerned (Fig. 1b). In suspicion of malformation of the lower limbs and of the craniofacial massif, a choroidal biopsy was taken. During the 15th week of pregnancy, ultrasound was repeated, revealing a fetal facies with an abnormal fetal profile due to an absent nose (Fig. 2A–C). In the coronal cut, marked hypertelorism was noticable (Fig. 3a, b. In the occipital area, there was a small encephalocele, as shown in Figure 4A–C, with a slightly flattened cerebellum. The lower limbs presented with the same findings seen on the prior ultrasound, with mesomelic shortening and malposition of both feet.
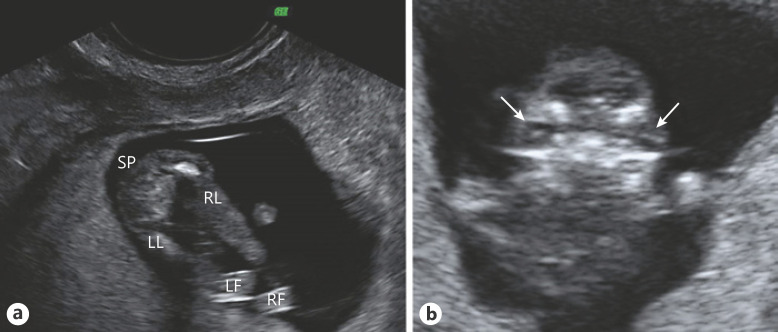
Fig. 1.
Ultrasound images at the 13th week of pregnancy. a Both lower limbs of the fetus. Note mesomelic shortening and malposition of both feet. b Coronal view of the fetal facies. The arrows point to both crystalline lens, showing pronounced hypertelorism. SP, spine; LL, left leg; RL, right leg; LF, left foot; RF, right foot.
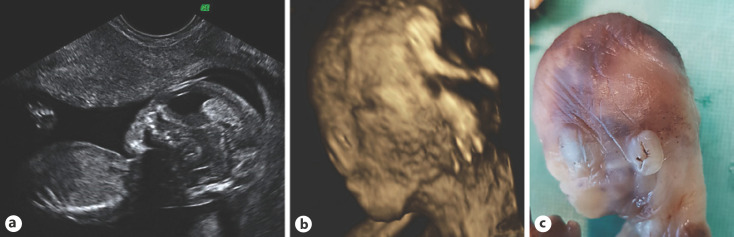
Fig. 2.
a Abnormal ultrasound image of the fetal profile at the 15th week of pregnancy with a flat profile and an absent nose. b 3D ultrasound reconstruction of the fetal head. The flat profile and the absent nose can be seen. Note nasal clefting. c Necropsy image additionally shows low-set ears.
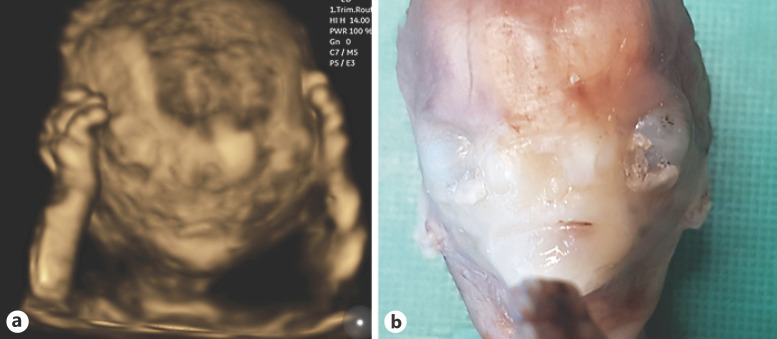
Fig. 3.
a 3D reconstruction of the fetal facies at the 15th week of pregnancy showing upper limbs on the side the head. Pronounced hypertelorism, as well as an abnormal development of the frontonasal area, with an undefined bifid nose are shown. b Fetal necropsy image demonstrating the same findings.
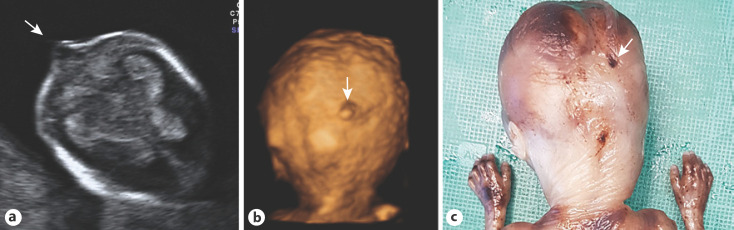
Fig. 4.
a Ultrasound image of the fetal head at the 15th week of pregnancy, in a transversal cut in transthalamic plane, which shows a small encephalocele (arrow). The 3D ultrasound reconstruction (b) and the photographic image of the fetal necropsy (c) show the fetal occipital area, and the arrows point to the small encephalocele. Note normal fetal hands.
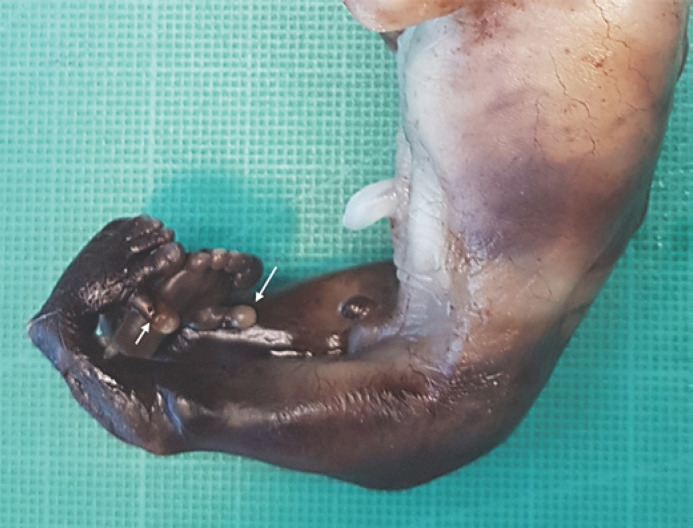
In view of the above findings, the couple decided to legally terminate the pregnancy. The anatomopathological study showed a male fetus consistent with 15 weeks of gestational age, with facial malformation and absent nasal bones. Other findings consisted of low-set ears, mesomelic shortening of lower limbs, and malposition of the feet. Preaxial polydactyly is shown in Figure 5. The encephalocele could not be confirmed, possibly due to the autolysis produced during delivery and the fixation of the fetus for necropsy, which can make a small injury go unnoticed. The fetus did not have internal malformations. The placenta and the umbilical cord did not show any pathological findings.
Fig. 5.
Fetal necropsy showing the malformation of the lower limbs affecting the mesomelic segment and both feet as well as bilateral preaxial polydactyly.
Methods
Karyotyping and array-CGH were performed in chorionic sample to rule out chromosomal alterations and copy number variants. Because AFND was suspected due to association of severe frontonasal dysplasia and limb abnormalities, a targeted next-generation sequencing skeletal dysplasia panel (Skeletal Seq.V7, 385 genes, Roche Nimblegen SeqCap EZ) was performed, which pointed to ZSWIM6 as the gene involved in the pathology. A heterozygous variant was identified in the ZSWIM6 gene, NM_020928.1:c.3487C>T [p.Arg1163Trp]: a missense mutation that affects a highly conserved amino acid. This mutation is not present in control population databases (gnomAD) and has been classified as pathogenic by bioinformatics pathogenicity prediction tools (CADD, Sift, PolyPhen-2, MutationTaster). In addition, this variant appears recurrently in individuals with AFND [Smith et al., 2014]. Therefore, the variant has been classified as likely pathogenic following acMG variant classification recommendations [Richards et al., 2015]. The mutation was confirmed as de novo by Sanger sequencing in the parents whose phenotype was normal. Gonadal mosaicism cases are also described, and the recurrence risk is set around 1%. Hence, prenatal diagnosis is recommended for future pregnancies.
Approximately 18 months later, the patient became pregnant again. During the 13th week of pregnancy, a gene screening study of a placenta sample taken by choroidal biopsy was conducted, which showed no alterations, and a normal full-term male infant was born.
Discussion and Conclusion
Prenatal ultrasound reveals fetal defects which entail a diagnostic challenge for the clinical geneticist. The observed polymalformative symptoms cannot always be associated with known syndromes; sometimes there are external features that cannot be seen due to fetal position and size. In this case, the polydactyly of both feet was not visible due to the position, whereas its absence in the hands could be spotted. In the first half of pregnancy, limitations are even greater because some defects may not be seen on unltrasound until later in pregnancy. The same is the case in some of the cardiopathies and many of the central nervous system alterations which affect the corpus callosum or produce an alteration in the cerebral cortex development, or those which appear along with a fetal growth defect.
AFND is an extremely rare entity, with less than 20 described cases in literature [Twigg et al., 2016], all of which were diagnosed postnatally, except one, diagnosed at the 20th week.
In the present case, the ultrasound at the 15th week of pregnancy showed the 3 main signs of AFND: facial malformation (hypertelorism and nasal malformation), lower limb malformation, and central nervous system alterations due to the presence of encephalocele.
Many of these manifestations can be detected on ultrasound during the first trimester, but others cannot. Other manifestations of the syndrome could never be suspected prenatally, such as absent olfactory bulbs, patella alterations, or hypopituitarism.
The responsible gene for this syndrome is ZSWIM6, located at chromosome 5q12.1. It has a critical role in neuronal development and function as well as in limb and craniofacial development. Therefore, apart from the alterations mentioned above, it also entails an important neurocognitive and motor delay.
The missense variant described within the C-terminal Sin3-like domain of ZSWIM6 was previously reported to cause AFND in 3 unrelated probands [Smith et al., 2014]. The p.Arg1163Trp variant was postulated to perturb the function of a highly conserved protein domain of ZSWIM6, resulting in a gain of function, which suggests a critical role for this domain in embryonic development.
The variable phenotypic expression observed in individuals with AFND suggests that mosaicism is involved in the phenotype. Consequently, it is very important to perform a clinical examination of the parents in order to rule out milder phenotypes, even though the usual techniques (Sanger sequencing) had excluded the mutation in both.
A recurrent de novo mutation in the penultimate exon 13 of the ZSWIM6 gene, c.2737C>T [p.Arg913Ter], has recently been reported in individuals with a severe neurocognitive disorder but without craniofacial and limb malformations. These different phenotypes can be explained by different molecular mechanisms [Palmer et al., 2017].
We can conclude that in some cases, even in early stages of the development, an exhaustive ultrasound screening of the fetus could detect a prenatal phenotype that leads the clinical geneticist to search for a possible genetic syndrome, which, if confirmed, would contribute to appropriate genetic counseling helping parents.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The paper is exempt from ethical committee approval because the manner in which it proceeds is part of the usual clinical practice. Parents have signed express consent for the case to be published.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no funding relevant to this study.
Author Contributions
Cristina Martínez-Payo: conception, design of the work, the acquisition and analysis, and interpretation of data. Fe Amalia García-Santiago: acquisition, analysis, interpretation of data, and drafting the work. Karen Heath: revised the manuscript critically for important intellectual content. Eduardo Gavin: acquisition, analysis, and interpretation of data. Elena Mansilla Aparicio: acquisition and analysis, interpretation of data and revising it critically for important intellectual content.
Acknowledgment
We would like to thank the Master Universitario en Comunicación Intercultural, Traducción e Interpretación en Servicios Públicos from the Universidad de Alcalá and the student Usue Menoyo García de Garayo for translating this work into English.
References
- Palmer EE, Kumar R, Gordon CT, Shaw M, Hubert L, Carroll R, et al. A Recurrent De Novo Nonsense Variant in ZSWIM6 Results in Severe Intellectual Disability without Frontonasal or Limb Malformations. Am J Hum Genet. 2017;101((6)):995–1005. doi: 10.1016/j.ajhg.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17((5)):405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedano HO, Cohen MM, Jr, Jirasek J, Gorlin RJ. Frontonasal dysplasia. J Pediatr. 1970;76((6)):906–13. doi: 10.1016/s0022-3476(70)80374-2. [DOI] [PubMed] [Google Scholar]
- Smith JD, Hing AV, Clarke CM, Johnson NM, Perez F, Park SS, et al. Exome sequencing identifies a recurrent de novo ZSWIM6 mutation associated with acromelic frontonasal dysostosis. Am J Hum Genet. 2014;95:235–40. doi: 10.1016/j.ajhg.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg SR, Ousager LB, Miller KA, Zhou Y, Elalaoui SC, Sefiani A, et al. Acromelic frontonasal dysostosis and ZSWIM6 mutation: phenotypic spectrum and mosaicism. Clin Genet. 2016;90((3)):270–5. doi: 10.1111/cge.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verloes A, Gillerot Y, Walczak E, Van Maldergem L, Koulischer L. Acromelic frontonasal dysplasia: further delineation of a subtype with brain malformation and polydactyly (Toriello syndrome) Am J Med Genet. 1992;42:180–3. doi: 10.1002/ajmg.1320420209. [DOI] [PubMed] [Google Scholar]