Abstract
Background:
Persons with multiple sclerosis are often at higher risk for falling, but clinical disability scales and fall risk questionnaires are subjective and don’t provide specific feedback about why an individual is unstable. The purpose of this study was to determine how relationships between trunk and foot acceleration variability relate to physiological impairments, clinical disability scales, and mobility questionnaires in persons with multiple sclerosis.
Methods:
15 fallers and 25 non-fallers with multiple sclerosis walked on a treadmill at normal walking speed while trunk and foot accelerations were recorded with wireless accelerometers and variability measures were extracted and used to calculate the gait stability index metrics as a ratio of trunk acceleration variability divided foot acceleration variability. Subjects’ sensorimotor delays and lower extremity vibration sensitivity were tested. Subjects also completed clinical disability scales (Guy’s Neurological Disability Scale and Patient Reported Expanded Disability Status Scale) and mobility questionnaires (Falls Efficacy Scale, Activities Balance Confidence Scale, 12 Item Multiple Sclerosis Walk Scale).
Findings:
Multiple gait stability index metrics were significantly correlated with clinical measures of disability and mobility in multiple sclerosis subjects (r=0.354–0.528), but no correlations were found for sensorimotor delays or lower extremity sensation. Multiple gait stability indices performed at least as well as clinical questionnaires for separating fallers from non-fallers.
Interpretation:
The gait stability indices can potentially be used outside of a laboratory setting to measure walking characteristics related to fall history and disability level in people with multiple sclerosis.
Keywords: Gait, multiple sclerosis, accelerometers, falls
1. Introduction
Multiple sclerosis (MS) is an inflammatory demyelination disorder which disrupts neural signaling in the central nervous system resulting in a wide range of disabilities [1]. Approximately 80% of persons with MS (PwMS) report difficulty with walking and balance [2], and half of PwMS experience at least one fall per year [3]. Falling is a significant problem for PwMS as falls can lead to injury [4] and decreased activity which subsequently leads to deconditioning and worse quality of life [5]. Previous studies have shown that MS symptomology includes physiological deficits that can have an effect on the gait and balance function in PwMS [6–9]. PwMS have been shown to have delayed sensorimotor responses to standing surface translations compared to healthy controls [6, 8], with longer sensorimotor delays being shown to be related to larger standing sway area and larger sagittal plane trunk range of motion during walking [8]. PwMS also commonly experience altered or lost sensation in the extremities [10], with worse vibration sensation at the feet being shown to be related to worse dynamic stability during walking [9]. Because stability during walking involves a combination of physiological mechanisms, it is important to understand how these altered physiological mechanisms in PwMS are related to gait stability.
Current clinical outcomes for monitoring walking and balance deficits in PwMS include tests of walking speed such as the timed 25-foot walk and self-report questionnaires related to self-perceived functional status such as the Activities Balance Confidence scale [11]. Unfortunately, these assessments are often subjective and lack sensitivity, which limits their ability to accurately detect changes in function over time or predict risk of future falls [12, 13]. In addition, these questionnaires don’t specifically measure the source of an individual’s instability. Therefore, there is a need for objective assessments that can be used in the clinic or in real-world environments to supplement patient outcomes and quantify risk of falls in PwMS. Such an objective assessment could be useful for monitoring progression of disease, efficacy of treatment, and tracking changes in walking function over time. Wireless inertial sensors have been growing in popularity as a feasible alternative for collecting data during walking in clinical and real-world settings [14, 15]. Previous studies using accelerometers have found that variability of accelerations can be a sensitive indicator of fall risk [16–19]. Van Schooten et al found that acceleration range and Lyapunov exponents significantly contributed to a model that predicted future falls with good accuracy [19]. These previous studies examined movement of individual segments during walking, however it is likely that whole body stability requires a controlled interaction between upper and lower body segments [15]. Instead of evaluating only one body segment at a time to quantify stability, the gait stability index which examines acceleration variability at the trunk and at the foot segment simultaneously during walking to quantify whole body stability [20, 21].
The purpose of this study was to determine how the gait stability index metrics are related to physiological impairments, clinical disability scales, and mobility questionnaires in PwMS. Additionally, the current study determined if the gait stability index metrics are capable of separating MS fallers from MS non-fallers. Coordination of upper and lower body movement during walking may be controlled by underlying sensorimotor feedback within the central nervous system which has been shown to be altered in PwMS [6, 8, 22]. We hypothesize that 1) the GSI metrics will show moderate to strong correlations with measures of pathophysiology and self-report disability scales in PwMS, and 2) the GSI will more sensitively differentiate fallers from non-fallers compared to self-report mobility questionnaires.
2. Methods
2.1. Participants
The current study enrolled 25 PwMS with no fall history, and 15 PwMS with a history of 2 or more falls in the previous 12 months [23]. PwMS were excluded if they were currently prescribed symptom targeting medication (i.e. Fampridine) due to its proposed effect on gait, if they had experienced an MS symptom exacerbation in the previous 60 days that required treatment, or if they were unable to walk 100 meters without assistance or use of a walking aid corresponding to a Kurtzke Expanded Disability Status Scale (EDSS) score ≤ 5.5 [24]. Participants were excluded if they were not between 20 – 60 years of age, had vestibular impairments, diabetes, pre-existing conditions that could make exercise dangerous (i.e. myocardial infarction, chest pain, etc.). Female participants were excluded if they were pregnant, or within 3 months post-partum. Subjects were free of any additional diagnosed neurological or musculoskeletal impairment that could affect their balance or gait. All subjects gave informed written consent, and all study protocols were reviewed by the University of Kansas Medical Center Institutional Review Board. Demographic information for all subjects is listed in Table 1.
Table 1.
Summary of subject demographics.
| MS Non-fallers N = 25 | MS Fallers N = 15 | |
|---|---|---|
| Age | 44 (9.9) yrs | 48 (9.6) yrs |
| M / F | 5 / 20 | 7 / 8 |
| BMI | 46.9 (8.7) | 53.4 (6.5) |
| EDSS | 3.3 (1.9) | 4.4 (1.1) |
| Falls in previous 12 months | 0 | 3.75 (1.7) |
2.2. Treadmill Walking
Subjects wore two wireless inertial sensors (Opal sensors, APDM, Portland, OR, USA) secured by elastic straps during the entirety of testing. The lumbar sensor was mounted over the posterior surface of the lumbar spine at the L5 level, and the foot sensor was mounted on the lateral surface of the distal shank just superior to the ankle joint [25]. Subjects’ comfortable walking speed was measured three times over a 10-meter walkway, and this preferred walking speed was used as subjects’ preferred walking speed on the treadmill (Woodway Bari-Mill, Eugene, OR, USA) [26]. Subjects completed one walking trial at this preferred speed on the treadmill while the wireless inertial sensors recorded at 128 Hz for 90-seconds.
2.3. Data Analysis
The acceleration time series from each sensor was translated from local Cartesian coordinates to resultant frontal and sagittal plane time series local to each sensor. To account for differences in number of strides across subjects’ walking speeds, the middle 60 strides of each trial were selected for analysis [27, 28]. Acceleration data was left unfiltered for accurate analysis of variability within the time series [29]. Linear and nonlinear variability metrics were calculated using custom Matlab programs (MATLAB version R2013b, MathWorks, Natick, Massachusetts, USA) to quantify the amount and the temporal structure of variability respectively [30–32]. Linear metrics included range and root mean square (RMS), nonlinear metrics included sample entropy (SaEn) and the maximum Lyapunov exponent (LyE). RMS was calculated as the square root of the mean of squares over the entire time series and was used to quantify the average dispersion of the acceleration traces. Range was calculated as the difference between the maximum and minimum acceleration values with the time series. LyE and SaEn are nonlinear variability measures which quantify the predictability and regularity of the cyclical gait pattern. Delay-embedded state spaces were reconstructed for each anatomical plane. Embedding dimensions were found using the global false nearest neighbor algorithm and the median of 8 was used for the LyE analysis [33]. Time delays for each individual subject were found using the average mutual information algorithm and ranged from 8 to 27. LyE was calculated using Wolf’s algorithm which identifies the maximum LyE [33, 34]. SaEn was calculated using a vector length m = 3, and tolerance r = 0.2 (20% of the time series standard deviation) as these parameters were shown to have good relative consistency [35].
The gait stability index (GSI) metrics were calculated as the ratio of lumbar acceleration (ACC) variability divided foot acceleration variability for each of the 4 variability measures (RMS, range, SaEn, LyE) in the frontal and sagittal planes [36, 37]. Therefore, four GSI metrics were calculated in each plane using each of the four variability measures: GSIRMS, GSIRange, GSISaEn, GSILyE, resulting in 8 GSI metrics total used in the statistical analysis.
| Eq. (1) |
2.4. Sensorimotor Delays
Subjects stood on a servo-controlled motorized treadmill which was translated forward to cause a backward body sway [8]. The treadmill translated 4cm forward at a rate of approximately 15cm/s which elicited a step response from participants in order to regain balance. Surface electromyography (EMG) electrodes (Trigno Lab, Delsys Inc., Boston, MA) were placed bilaterally over the tibialis anterior. EMG signals were sampled at 1800Hz, amplified, band-pass filtered at 70–2000Hz. The participant’s sensorimotor delay was measured as the time between the beginning of treadmill surface translation and the onset of muscle activity. Muscle activity onset was defined as EMG activity greater than 2 standard deviations above the resting average sustained for at least 50ms [6, 8]. The average sensorimotor delay was found from three trials including both legs for each MS subject [8].
2.5. Lower Extremity Sensation
Lower extremity vibrotactile sensation was measured using a Vibratron II (Physitemp Instruments, Clifton, NJ). Subjects were seated and asked to touch their big toe to two pedestal pedestals and say which one was vibrating. The vibration amplitude of the pedestals was decreased until the subject could no longer feel the vibration which defined the vibration sensation threshold [38].
Lower extremity cutaneous sensation was measured using a monofilament test. Subjects were seated with eyes closed and a monofilament was pressed against the anterior surface of the foot, just proximal to the first metotarsophalangeal joint. Subjects were asked to raise their hand when they felt the touch on their foot. Testing began with the highest stiffness monofilament and proceed through 5 total filaments of decreasing stiffness until the subject could no longer feel the touch of the filament which defined a cutaneous sensation threshold of that monofilament’s weight [39].
2.6. Clinical Disability Scales and Questionnaires
All MS subjects self-reported number of falls from previous 12 months, with 2 or more falls categorizing them as faller [23]. Disability status was assessed using the patient reported EDSS [40], and the Guy’s Neurological Disability Scale [41]. Subjects also completed the Falls Efficacy Scale-International (FES-I), Activities-specific Balance Confidence scale (ABC), and the 12-Item Multiple Sclerosis Walking scale (MSW12) which have all been validated in MS [42–44].
2.7. Statistical Analysis
Pearson’s correlations were used to determine how the gait stability index metrics were related to fall history, sensorimotor delays, lower extremity sensation thresholds, and disability status in PwMS. All data was found to be normally distributed by Shapiro-Wilks tests. A receiver operating characteristic (ROC) curve was constructed for each gait stability index and clinical questionnaire (FES-I, ABC, MSW12), and the area under the curve (AUC) was calculated and compared to random chance (AUC=0.5) to determine each measure’s strength of separation between MS fallers and MS non-fallers. Target significance was set as p<0.05, and the Holm-Bonferroni correction was applied to test for significance within the multiple correlations [45].
3. Results
Fall history was significantly correlated with GSISaEn (r=0.528, p=0.002) and GSILyE (r=−0.354, p=0.047) in the frontal plane (Figure 1A and 1B). The Guy’s Neurological Disability Scale was significantly correlated with GSISaEn in the frontal (r=0.435, p=0.016) and sagittal (r=0.428, p=0.021) planes (Figure 1C and 1D). Patient reported EDSS was significantly correlated with GSILyE (r=−0.484, p=0.005) in the sagittal plane (Figure 1E). There were no correlations between any GSI metrics and sensorimotor delays or lower extremity sensation thresholds. All correlations are reported in Table 2.
Figure 1.
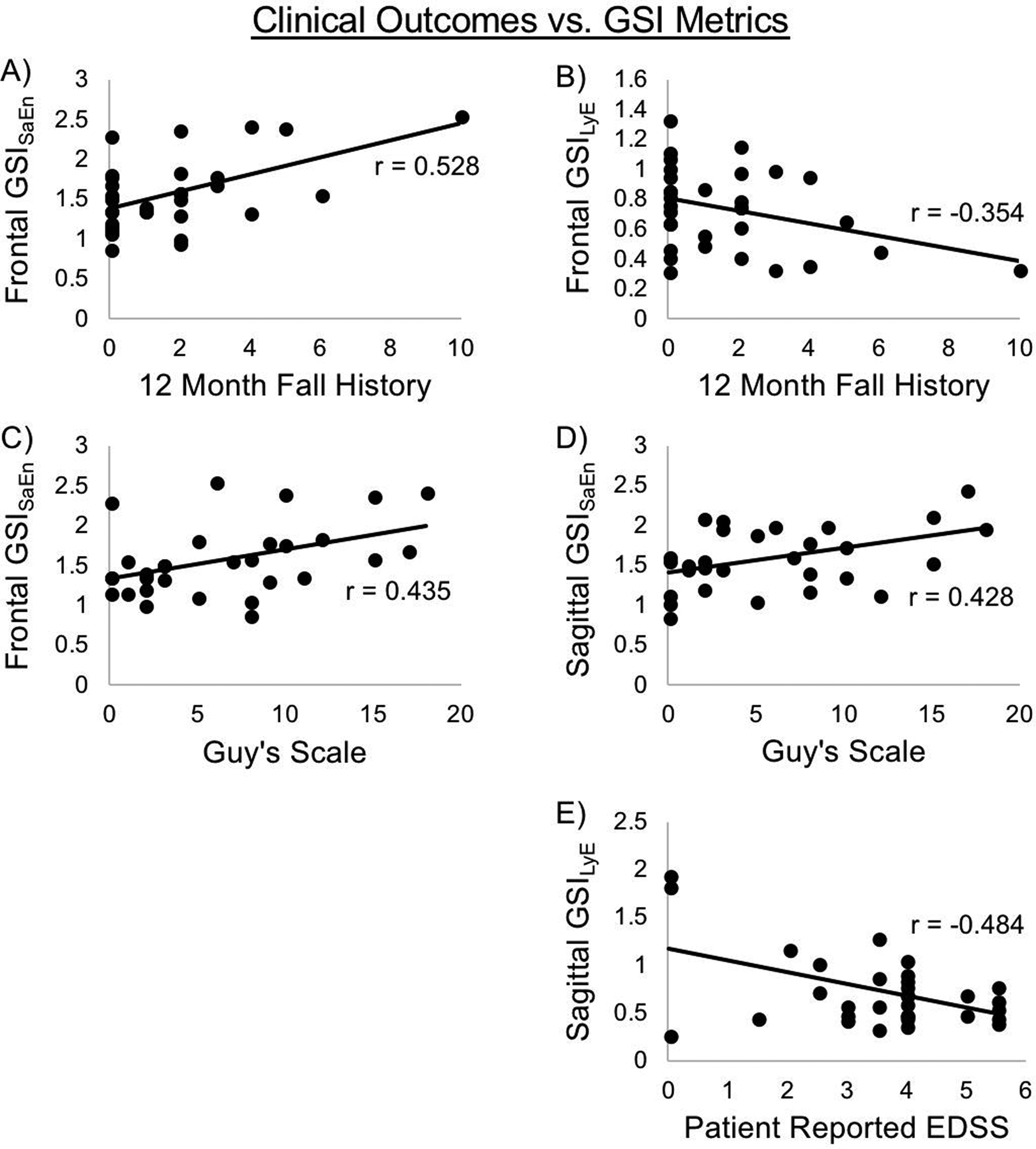
Scatter plots of significant correlations between clinical outcomes and GSI metrics. A) Fall history vs. frontal GSISaEn; B) Fall history vs. frontal GSILyE; C) Guy’s scale vs. frontal GSISaEn; D) Guy’s Scale vs. sagittal GSISaEn; E) Patient reported EDSS vs. sagittal GSILyE. All correlations significant.
Table 2.
Pearson’s correlation coefficients (and p-value) between clinical outcomes and GSI metrics. Significant correlations in bold following Holm-Bonferroni correction.
| Fall History | Vibration Threshold | Monofilament Test | Guy’s Scale | Patient EDSS | Sensorimotor Delay | ||
|---|---|---|---|---|---|---|---|
| Frontal Plane | GSIRMS | −0.033 (0.860) | 0.068 (0.715) | −0.045 (0.819) | −0.278 (0.145) | −0.061 (0.742) | 0.169 (0.380) |
| GSIRange | −0.210 (0.241) | 0.008 (0.964) | 0.181 (0.339) | −0.014 (0.942) | −0.131 (0.466) | −0.102 (0.586) | |
| GSISaEn | 0.528 (0.002) | −0.062 (0.735) | −0.156 (0.420) | 0.435 (0.016) | 0.203 (0.266) | 0.142 (0.456) | |
| GSILyE | −0.354 (0.047) | 0.058 (0.753) | 0.247 (0.196) | −0.220 (0.242) | −0.090 (0.623) | −0.277 (0.139) | |
| GSIRMS | −0.318 (0.106) | −0.200 (0.317) | −0.039 (0.854) | −0.349 (0.081) | −0.041 (0.839) | −0.168 (0.422) | |
| GSIRange | −0.114 (0.549) | 0.076 (0.691) | −0.026 (0.898) | 0.057 (0.772) | 0.037 (0.845) | −0.057 (0.773) | |
| GSISaEn | 0.354 (0.055) | 0.179 (0.343) | −0.031 (0.879) | 0.428 (0.021) | 0.240 (0.202) | 0.057 (0.771) | |
| GSILyE | −0.151 (0.410) | −0.093 (0.612) | −0.117 (0.544) | −0.221 (0.241) | −0.484 (0.005) | −0.222 (0.230) |
For the GSI metrics, the strongest separator of MS fallers from MS non-fallers was the GSISaEn in the frontal plane, with an AUC = 0.920 (p=0.001). In the frontal plane, the AUC for GSIRMS was 0.630 (p=0.326), GSIRange was 0.660 (p=0.226), and GSILyE was 0.640 (p=0.290). In the sagittal plane, the AUC for GSIRMS was 0.800 (p=0.023), GSIRange was 0.730 (p=0.082), GSISaEn was 0.760 (p=0.049), and GSILyE was 0.690 (p=0.151). For the clinical questionnaires, the strongest separator of MS fallers from MS non-fallers was MSWS12 with an AUC of 0.900 (p=0.002). The AUC for the ABC scale was 0.760 (p=0.049), and for FES-I was 0.770 (p=0.041). ROC curves for separating MS fallers from non-fallers are shown for the best performing GSI metric frontal plane GSISaEn and best performing clinical questionnaire MSWS12 in Figure 2.
Figure 2.
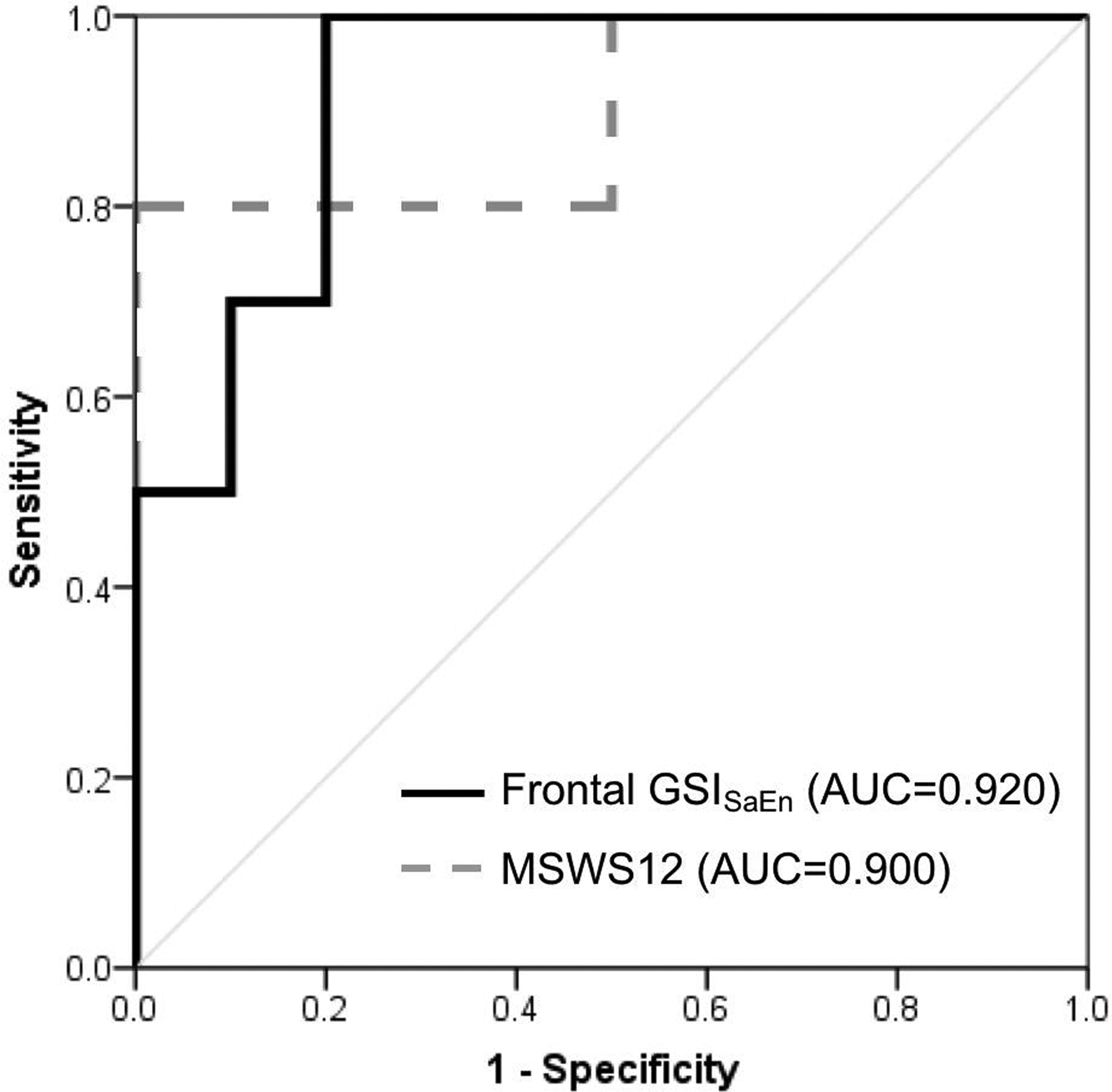
Receiver operating characteristic curves for the best performing GSI metric and clinical questionnaire for separating MS fallers from MS non-fallers. Frontal plane GSISaEn – Black; MSWS12 – Gray.
4. Discussion
The goal of the current study was to determine how the gait stability index metrics are related to physiological impairments, clinical disability scales, and mobility questionnaires in PwMS. Additionally, the current study determined if the gait stability index metrics are capable of separating MS fallers from MS non-fallers. Acceleration data was collected from wireless sensors and the GSI metrics were calculated to examine how trunk movement was controlled relative to foot movement. We hypothesized that 1) the GSI metrics would show moderate to strong correlations with measures of pathophysiology and disability in PwMS, and 2) the GSI would more sensitively differentiate fallers from non-fallers compared to standard clinical questionnaires. Our results partially supported our first hypothesis, as GSI metrics did show relationships to disability level in MS, but there were no significant correlations found between GSI metrics and sensorimotor delays or lower extremity sensation thresholds. Our results did support our second hypothesis, as multiple GSI metrics separated MS fallers from MS non-fallers as well or better than the clinical mobility questionnaires.
Our results show that fall history was significantly correlated with the GSISaEn and GSILyE in the frontal plane. During walking, movement in the frontal plane is considered to be governed by active control systems which use closed-loop feedback to make minor adjustments and maintain stability from step to step [46, 47]. This active control uses sensory feedback to drive motor output in both upper [48, 49] and lower [8, 50] body musculature in order to maintain stability throughout the gait cycle. The current results demonstrate that the frontal plane GSI metrics which use nonlinear variability were related to fall history. Specifically, individuals who had more falls demonstrated more irregular and less divergent frontal plane trunk accelerations relative foot accelerations during walking. These results may indicate that persons who have a greater history of falls have altered frontal plane control over their trunk segment during walking, which may be an underlying characteristic driving their increased number of falls. An inability to maintain appropriate trunk motion during walking likely affects an individual’s ability to respond to and attenuate any perturbation during walking [51, 52], making individuals more at risk of falls.
The frontal and sagittal GSISaEn and the sagittal GSILyE were significantly related to self-report disability level in PwMS. Our results showed that individuals with worse disability as measured by the Guy’s Neurological Disability Scale tended to have more irregular accelerations at the trunk relative to the feet, and individuals with worse disability as rated by the patient reported EDSS tended to have less divergence in sagittal trunk accelerations relative to the feet. The self-report disability scales used in the current study include a wide range of symptom types including sensory and motor symptoms, cognition, fatigue, bowel and bladder, vision, and speech. Many of these symptoms are not directly involved in walking and balance maintenance, which may be why more relationships with the GSI metrics were not found. Altered and inappropriate control of trunk and foot movement during walking in individuals with more falls follows the loss of complexity hypothesis which indicates a decreased amount of adaptability in the sensorimotor control system, which could ultimately give rise to increased fall risk [53]. This loss of complexity in sensorimotor control may be related to other, more widespread functional deficits measured by other physiological categories assessed in the self-report disability scales. These results demonstrate that the GSI metrics may provide different information that can supplement clinical assessments of disability in PwMS.
Surprisingly the results of the current study did not show any correlations between any GSI metrics and sensorimotor delays or sensation thresholds. Previous studies have shown that PwMS demonstrate delayed sensorimotor responses to postural perturbations compared to healthy adults [6, 8]. These delayed sensorimotor responses have also been showed to be related to measures of dynamic stability during walking [9]. We expected to find that individuals with worse sensation and longer sensorimotor delays would also demonstrate more altered relationships between their trunk and foot acceleration variability during walking, but this was not the case even though the GSI metrics were related to fall history (Table 2) and were able to separate fallers from non-fallers (Figure 1). One potential reason for this finding is that the important gait adjustments that are critical to maintaining stability may happen over a longer time scale than what is examined in the sensorimotor delay testing and may not be solely driven by the same pathways which are examined in sensorimotor delay testing. Sensorimotor delays range from 100–200 ms in PwMS [8, 9], while a single stride occurs over approximately one second [54]. Therefore, while sensorimotor delays may be one factor related to maintaining stability during gait, there may be other mechanisms on longer time-scales that are driving the results of the GSI metrics. For example, PwMS experience strength asymmetries [55], overall muscle weakness [56], and altered timing of trunk muscle activation [49]. This altered control of muscle activation could result in an inability of the trunk to appropriately respond to and attenuate perturbations over one or more steps during walking. Future studies could examine how the GSI metrics relate to altered strength or control of muscle activation during gait.
Our second hypothesis that GSI metrics would separate fallers from non-fallers at least as well as clinical mobility questionnaires was partially supported, as multiple GSI metrics were as good or better at separating MS fallers from non-fallers compared to the clinical disability and mobility questionnaires. The frontal plane GSISaEn was stronger than all clinical questionnaires, while sagittal plane GSIRMS, GSIRange, and GSISaEn also demonstrated strong separation between MS fallers and non-fallers. These results provide support for the ability to use the GSI metrics as a mobility assessment. Current clinical questionnaires don’t directly assess gait stability, and worse outcomes on these questionnaire scores are indicative of a fear of falling but are not directly associated with risk of future falls [57]. Using a measure of fall risk based on segmental control relationships can provide clinicians with an objective measure of stability, as the relationship between the center of mass and base of support is directly tied to stability [58]. Prevention and rehabilitation care in aging and neuropathological populations would benefit from access to an objective measure of gait stability to track how a person’s mobility may change over time. Future studies will determine whether or not it is feasible to measure the GSI metrics in clinical and real-world settings, and which GSI metrics should be used for such fall risk assessments.
Because of the wide range of disability subcategories examined in the various clinical questionnaires and disability scales, examining relationships between the GSI metrics and disability subscales may be warranted in a larger cohort of MS subjects with a larger range of mobility disability. One should also keep in mind that the GSI metrics in the current study were calculated during treadmill walking, while practical application of these measures for clinical or real-world fall risk assessment will likely use overground walking. While it is possible that there may be differences between treadmill and overground walking, it is likely that the GSI metrics during overground walking would demonstrate similar trends to those seen from treadmill walking in the current study. However, future work will need to investigate the GSI metrics during overground walking to validate their use for separating MS fallers from MS non-fallers.
5. Conclusions
Symptoms of MS can widely vary, but walking disability is reported to be one of the most detrimental symptoms by persons with MS. It is important to identify objective measures that are clinically valid and sensitive to different mobility levels in PwMS. The current study demonstrates that the GSI metrics may be valid for use as a clinical assessment, and that they relate to standard clinical questionnaires and disability scales. However, the GSI metrics were not related to lower extremity sensation thresholds or postural response latencies indicating that coordination of the trunk and feet during walking may be driven by other factors that were not assessed in the current study. Additionally, the GSI metrics showed strong separation of MS fallers from MS non-fallers. Future studies will need to determine how the GSI metrics perform during overground walking as will be necessary for application in clinical and real-world environments.
Acknowledgements:
This work was supported by the National Multiple Sclerosis Society RG 4914A1/2; the NIH National Center for Advancing Translational Science 1KL2TR00011; and the NIH Ruth L. Kirschstein National Research Service Award T32 HD057850 from the National Institute of Child Health and Human Development.
References
- 1.Compston A and Coles A, Multiple sclerosis. Lancet, 2008. 372(9648): p. 1502–17. [DOI] [PubMed] [Google Scholar]
- 2.Souza A, et al. , Multiple sclerosis and mobility-related assistive technology: systematic review of literature. J Rehabil Res Dev, 2010. 47(3): p. 213–23. [DOI] [PubMed] [Google Scholar]
- 3.Nilsagard Y, et al. , Clinical relevance using timed walk tests and ‘timed up and go’ testing in persons with Multiple Sclerosis. Physiotherapy Research International, 2007. 12(2): p. 105–114. [DOI] [PubMed] [Google Scholar]
- 4.Peterson EW, et al. , Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil, 2008. 89(6): p. 1031–7. [DOI] [PubMed] [Google Scholar]
- 5.Peterson EW, Cho CC, and Finlayson ML, Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler, 2007. 13(9): p. 1168–75. [DOI] [PubMed] [Google Scholar]
- 6.Cameron MH, et al. , Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens Mot Res, 2008. 25(2): p. 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaneo D and Jonsdottir J, Sensory impairments in quiet standing in subjects with multiple sclerosis. Mult Scler, 2009. 15(1): p. 59–67. [DOI] [PubMed] [Google Scholar]
- 8.Huisinga JM, et al. , Postural response latencies are related to balance control during standing and walking in patients with multiple sclerosis. Arch Phys Med Rehabil, 2014. 95(7): p. 1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peebles AT, et al. , Dynamic balance is related to physiological impairments in persons with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frohman EM, Racke MK, and Raine CS, Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med, 2006. 354(9): p. 942–55. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo P, et al. , Fall Risk Assessment Tools for Elderly Living in the Community: Can We Do Better? PLoS ONE, 2015. 10(12): p. e0146247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry E, et al. , Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatrics, 2014. 14: p. 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattaneo D, Regola A, and Meotti M, Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil, 2006. 28(12): p. 789–95. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh JJ and Menz HB, Accelerometry: a technique for quantifying movement patterns during walking. Gait Posture, 2008. 28(1): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 15.Craig JJ, et al. , Instrumented balance and walking assessments in persons with multiple sclerosis show strong test-retest reliability. J Neuroeng Rehabil, 2017. 14(1): p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Zhang X, and Lockhart TE, Fall risk assessments based on postural and dynamic stability using inertial measurement unit. Saf Health Work, 2012. 3(3): p. 192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menz HB, Lord SR, and Fitzpatrick RC, Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. J Gerontol A Biol Sci Med Sci, 2003. 58(5): p. M446–52. [DOI] [PubMed] [Google Scholar]
- 18.Rispens SM, et al. , Identification of Fall Risk Predictors in Daily Life Measurements: Gait Characteristics’ Reliability and Association With Self-reported Fall History. Neurorehabil Neural Repair, 2014. [DOI] [PubMed] [Google Scholar]
- 19.van Schooten KS, et al. , Ambulatory fall-risk assessment: amount and quality of daily-life gait predict falls in older adults. J Gerontol A Biol Sci Med Sci, 2015. 70(5): p. 608–15. [DOI] [PubMed] [Google Scholar]
- 20.Craig J, Bruetsch A, Horak F, Lynch S, Huisinga J, The relationship between trunk and foot movement variability during walking is altered in persons with multiple sclerosis with history of falls, in 5th International Symposium on Gait and Balance in Multiple Sclerosis. 2017: Portland, OR. [Google Scholar]
- 21.Craig JB, A; Lynch S; Huisinga J, The relationship between trunk and foot movement variability during walking is sensitive to separate fallers from non-fallers, in World Congress of Biomechanics. 2018: Dublin, Ireland. [Google Scholar]
- 22.Gagliardo A, et al. , Motor evoked potentials in multiple sclerosis patients without walking limitation: amplitude vs. conduction time abnormalities. J Neurol, 2007. 254(2): p. 220–7. [DOI] [PubMed] [Google Scholar]
- 23.Garcia PA, et al. , Prospective monitoring and self-report of previous falls among older women at high risk of falls and fractures: a study of comparison and agreement. Brazilian Journal of Physical Therapy, 2015. 19(3): p. 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtzke JF, Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology, 1983. 33(11): p. 1444–52. [DOI] [PubMed] [Google Scholar]
- 25.Craig JJ, et al. , The relationship between trunk and foot acceleration variability during walking shows minor changes in persons with multiple sclerosis. Clin Biomech, 2017. 49: p. 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franz JR, et al. , Advanced age brings a greater reliance on visual feedback to maintain balance during walking. Hum Mov Sci, 2015. 40C: p. 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehdizadeh S and Sanjari MA, Effect of noise and filtering on largest Lyapunov exponent of time series associated with human walking. J Biomech, 2017. [DOI] [PubMed] [Google Scholar]
- 28.Terrier P and Reynard F, To what extent does not wearing shoes affect the local dynamic stability of walking?: effect size and intrasession repeatability. J Appl Biomech, 2014. 30(2): p. 305–9. [DOI] [PubMed] [Google Scholar]
- 29.Mees AI and Judd K, Dangers of geometric filtering. Physica D: Nonlinear Phenomena, 1993. 68(3–4): p. 427–436. [Google Scholar]
- 30.Huisinga JM, et al. , Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Ann Biomed Eng, 2013. 41(8): p. 1670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang HG and Dingwell JB, Dynamic stability of superior vs. inferior segments during walking in young and older adults. Gait Posture, 2009. 30(2): p. 260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tochigi Y, et al. , Entropy analysis of tri-axial leg acceleration signal waveforms for measurement of decrease of physiological variability in human gait. J Orthop Res, 2012. 30(6): p. 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stergiou N, Nonlinear Analysis for Human Movement Variability. 2016: CRC Press. [Google Scholar]
- 34.Wolf A, et al. , Determining Lyapunov exponents from a time series. Physica D: Nonlinear Phenomena, 1985. 16(3): p. 285–317. [Google Scholar]
- 35.Richman JS and Moorman JR, Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol, 2000. 278(6): p. H2039–49. [DOI] [PubMed] [Google Scholar]
- 36.Craig J, Bruetsch A, Huisinga J , Segment relationships adapt to walking speed differently in healthy young and elderly adults, in American Society of Biomechanics. 2017: Boulder, CO. [Google Scholar]
- 37.Craig J, Bruetsch A, Lynch S, Huisinga J , Variability of the center of mass and base of support during gait altered in persons with multiple sclerosis, in International Society of Posture and Gait Research World Congress. 2016: Fort Lauderdale, FL. [Google Scholar]
- 38.Martin CL, et al. , Vibration Perception Threshold as a Measure of Distal Symmetrical Peripheral Neuropathy in Type 1 Diabetes: Results from the DCCT/EDIC study. Diabetes Care, 2010. 33(12): p. 2635–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson MP, et al. , Threshold for detection of diabetic peripheral sensory neuropathy using a range of research grade monofilaments in persons with Type 2 diabetes mellitus. Journal of Foot and Ankle Research, 2008. 1(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowen J, et al. , Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Mult Scler, 2001. 7(3): p. 201–6. [DOI] [PubMed] [Google Scholar]
- 41.Sharrack B and Hughes RA, The Guy’s Neurological Disability Scale (GNDS): a new disability measure for multiple sclerosis. Mult Scler, 1999. 5(4): p. 223–33. [DOI] [PubMed] [Google Scholar]
- 42.van Vliet R, et al. , Falls efficacy scale-international: a cross-sectional validation in people with multiple sclerosis. Arch Phys Med Rehabil, 2013. 94(5): p. 883–9. [DOI] [PubMed] [Google Scholar]
- 43.Nilsagard Y, Carling A, and Forsberg A, Activities-specific balance confidence in people with multiple sclerosis. Mult Scler Int, 2012. 2012: p. 613925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hobart JC, et al. , Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology, 2003. 60(1): p. 31–6. [DOI] [PubMed] [Google Scholar]
- 45.Holm S, A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics, 1979: p. 65–70. [Google Scholar]
- 46.O’Connor SM and Kuo AD, Direction-dependent control of balance during walking and standing. J Neurophysiol, 2009. 102(3): p. 1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauby CE and Kuo AD, Active control of lateral balance in human walking. Journal of Biomechanics, 2000. 33(11): p. 1433–1440. [DOI] [PubMed] [Google Scholar]
- 48.Arvin M, et al. , Effects of narrow base gait on mediolateral balance control in young and older adults. J Biomech, 2016. 49(7): p. 1264–7. [DOI] [PubMed] [Google Scholar]
- 49.Ketelhut NB, et al. , Core muscle characteristics during walking of patients with multiple sclerosis. J Rehabil Res Dev, 2015. 52(6): p. 713–24. [DOI] [PubMed] [Google Scholar]
- 50.Peterka RJ and Loughlin PJ, Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol, 2004. 91(1): p. 410–23. [DOI] [PubMed] [Google Scholar]
- 51.Arvin M, van Dieen JH, and Bruijn SM, Effects of constrained trunk movement on frontal plane gait kinematics. J Biomech, 2016. 49(13): p. 3085–3089. [DOI] [PubMed] [Google Scholar]
- 52.Kavanagh J, Barrett R, and Morrison S, The role of the neck and trunk in facilitating head stability during walking. Exp Brain Res, 2006. 172(4): p. 454–63. [DOI] [PubMed] [Google Scholar]
- 53.Stergiou N, Harbourne R, and Cavanaugh J, Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther, 2006. 30(3): p. 120–9. [DOI] [PubMed] [Google Scholar]
- 54.Givon U, Zeilig G, and Achiron A, Gait analysis in multiple sclerosis: characterization of temporal-spatial parameters using GAITRite functional ambulation system. Gait Posture, 2009. 29(1): p. 138–42. [DOI] [PubMed] [Google Scholar]
- 55.Rudroff T, et al. , Asymmetric glucose uptake in leg muscles of patients with Multiple Sclerosis during walking detected by [18F]-FDG PET/CT. NeuroRehabilitation, 2014. 35(4): p. 813–23. [DOI] [PubMed] [Google Scholar]
- 56.Hoang PD, Gandevia SC, and Herbert RD, Prevalence of joint contractures and muscle weakness in people with multiple sclerosis. Disabil Rehabil, 2014. 36(19): p. 1588–93. [DOI] [PubMed] [Google Scholar]
- 57.Maki BE, Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc, 1997. 45(3): p. 313–20. [DOI] [PubMed] [Google Scholar]
- 58.Winter DA, Human balance and posture control during standing and walking. Gait & Posture, 1995. 3(4): p. 193–214. [Google Scholar]


