Abstract
Purpose
To calculate the negative predictive value (NPV) and false-negative rate (FNR) of molecular breast imaging (MBI) performed in patients who had low-suspicion index findings on mammograms and US images.
Materials and Methods
This retrospective study included patients who had undergone MBI between January 2015 and July 2017, who had index findings on screening mammograms and/or US images, and for whom either histopathologic results or a minimum of 1-year imaging follow-up results were available. A drawn dose of 8 mCi (296 MBq) of technetium 99m sestamibi was administered to all patients for MBI. The NPV and FNR of MBI was calculated for the cohort of 381 findings among 338 women (median age, 56 years; age range, 28–89 years) included in this study.
Results
Overall, 292 of the 381 (76.6%) MBI results were interpreted as negative. Of the 292, 27 patients underwent subsequent biopsies, results of which were negative for cancer; one patient underwent biopsy, and the result was positive for cancer; and 264 patients had true-negative findings based on follow-up imaging for a minimum of 1 year. Of the 89 MBI acquisitions interpreted as positive, there were 36 cancers. The NPV was calculated to be 99.7% (291 of 292, 95% confidence interval [CI]: 99.1%, 100%), and the FNR was 2.7% (one of 37, 95% CI: 0%, 7.9%). Interposing MBI reduced the number of biopsies by 67.5%.
Conclusion
The concept of the clinical utility of a negative MBI result may be valid but requires further testing.
Keywords: Breast, Molecular Imaging-Cancer
© RSNA, 2020
Summary
Molecular breast imaging scans obtained with a dual-head cadmium zinc telluride detector camera with a drawn dose of 8 mCi (296 MBq) of technetium 99m sestamibi have a very high negative predictive value and low false-negative rate, which may obviate biopsy of mammographic and US findings.
Key Points
■ This study showed a negative predictive value of 99.7% (291 of 292) and false-negative rate of 2.7% (one of 37) for molecular breast imaging in patients with index findings at mammography and US.
■ The negative predictive value of molecular breast imaging may become clinically important in light of its increased use for supplemental screening.
Introduction
Molecular breast imaging (MBI) is a functional imaging technique for the detection of breast cancer. Extensive recent literature reported on the success of MBI in supplemental screening of dense breasts. For example, it has been reported that MBI together with digital mammography detects 8.8 additional cancers per 1000 women compared with mammography alone (1). Another study found that MBI detects an additional 7.7 cancers per 1000 women with dense breasts who were screened (2). In an ongoing study (3), the preliminary data based on 537 women with dense breasts showed a cancer detection rate of 11.2 for MBI, compared with 1.9 for digital breast tomosynthesis, supporting the conclusion that MBI finds cancers not detected with digital breast tomosynthesis.
Although the benefits of supplemental screening with MBI in patients with dense breasts can be considered well established, the clinical utility of a negative MBI result has not been adequately addressed in the literature. Specifically, there remains the question of whether a negative MBI result can obviate a recall for additional evaluation and ultimately biopsy of low-suspicion mammographic and US findings. The utility of a negative MBI result may arise in a variety of situations, such as when screening mammography and supplemental screening MBI are performed together, and the mammogram is interpreted as Breast Imaging Reporting and Data System (BI-RADS) 0 and the MBI acquisition is interpreted as BI-RADS 1. In this scenario, it is unclear whether the mammogram finding can be safely followed for a short interval (6 months). Additional situations in which a negative MBI result may be of clinical help include evaluation of mammographic and/or US findings at a lumpectomy site to exclude recurrence, multiple suspicious findings at mammography and/or breast US and uncertainty about which ones to biopsy first, and other clinical scenarios.
The primary objective of this study was to evaluate the negative predictive value (NPV) and false-negative rate (FNR) of MBI among patients who had findings on screening mammograms and/or US images and who were referred for MBI with a dual-head cadmium zinc telluride (CZT) solid-state detector camera and a drawn dose of 8 mCi (296 MBq) of technetium 99m (99mTc) sestamibi for further evaluation.
Materials and Methods
Study Design
We retrospectively reviewed all patients who underwent MBI at our institution between January 2015 and July 2017. The study was approved by the hospital’s institutional review board, and the requirement to obtain informed consent was waived. This study was conducted in compliance with current Health Insurance Portability and Accountability Act regulations. All cases of MBI were potentially eligible for inclusion in this study. Exclusion criteria included (a) lack of an index finding at a recent screening mammography or US and (b) lack of correlating histopathologic results from either biopsy or surgery or lack of minimum 1-year imaging follow-up at our institution. Because this study was descriptive in nature, a power calculation was not conducted. There were 575 consecutive findings; 30 were excluded because of a lack of index finding at screening mammography or US and 164 were excluded because of inadequate follow-up. Thus, 381 findings among 338 unique women with a median age of 56 years (age range, 28–89 years) were eligible for inclusion in this retrospective study. This patient population was not previously reported on.
Breast Imaging Protocol and Workflow
In 2015, our institution’s protocol was revised such that combination two-dimensional and tomosynthesis screening and diagnostic mammography became routine. Therefore, 15 patients included in this study had only two-dimensional digital mammograms; 323 had combination two-dimensional and tomosynthesis mammograms. As a matter of routine, we informed patients of their breast density and, if requested, performed supplemental screening with breast US in extremely dense and heterogeneously dense breasts. Screening breast US was performed by sonographers with a handheld transducer, usually at the same appointment as the screening mammography. Six sonographers, each with at least 10 years of experience with diagnostic breast US, performed breast US included in this study. The screening mammography and screening handheld US were batch interpreted the next day by one of nine radiologists (including author R.J., 20 years of experience), ranging in experience from 3 to 20 years.
Prior Screening Evaluation and MBI Protocol
Index findings on screening mammograms were further evaluated with diagnostic mammograms. Screening US findings were further evaluated with diagnostic US findings unless the screening study already included images in two orthogonal planes, a video capture, and color Doppler US. US findings ranged in size from 2 mm to 27.3 mm (median, 8 mm). Lesion size affects MBI visibility, but the smallest size detected at MBI has progressively become smaller (4). If the interpreting radiologist concluded after the diagnostic evaluation that the index mammographic or US finding was possibly a candidate for BI-RADS 4a or 4b, the mechanism for obtaining an MBI was available. Whether the patient ultimately underwent MBI depended on an interplay of several factors, including the patient’s willingness to travel to another town for the MBI examination (breast imaging is performed in three facilities in our service area but MBI examinations are performed at just one facility), the timely availability of MBI appointments, the desire to avoid delay of the biopsy, the interpreting radiologist’s familiarity with availability of MBI, and the patient’s subjective choice. If no MBI was performed, the subsequent management was guided by the appropriate BI-RADS for the index findings. In instances in which MBI acquisitions were obtained and interpreted to be negative, the choice of biopsy or short-interval follow-up and assignment of a final BI-RADS category depended on the interpreting radiologist. Follow-up was performed at 6 months, 1 year, and 2 years. If the MBI result was positive, a biopsy was performed.
A dual-head MBI Discovery NM750b camera with CZT detectors, manufactured by GE Healthcare (Chicago, Ill), was used to obtain all MBI acquisitions. Approximately 8 mCi (296 MBq) (7.4–8.5 mCi [273.8–314.5 MBq]) of 99mTc sestamibi (Cardiolite; Lantheus Medical Imaging, North Billerica, Mass) was drawn in a syringe. On average, 6.9 mCi (6.5–7.2 mCi [240.5–266.4 MBq]) was injected in the patient’s antecubital fossa; the remainder was retained in the dead space of the syringe. Imaging began within 5 minutes after injection. Standard mediolateral oblique and craniocaudal views of the breasts were acquired for 8 minutes each. Additional views were acquired as deemed necessary by the interpreting radiologist. To increase blood flow to the breasts, the patient was asked not to engage in vigorous activity or exercise and to fast for 2 to 4 hours prior to the examination.
MBI Interpretation
Three radiologists (including the author R.J., 20 years of experience) interpreted the MBI acquisitions using the published lexicon (5). Their breast imaging experience ranged from 6 to 20 years. The images and reports of other breast imaging examinations were available to the interpreting radiologist. Invasive cancers, ductal carcinoma in situ (DCIS), and some benign and high-risk lesions appeared as “hot spots” on the images; these were evaluated in grayscale as well as color. Separate images for each detector were available, as were combined geometric-mean images of the two detectors. Finally, clinically actionable BI-RADS scores were assigned by the interpreting radiologist, combining the MBI results with all the other available imaging and clinical information. Index findings were biopsied or were followed with imaging for a minimum of 1 year. The images and reports of other breast imaging examinations, including the MBI, were available to the interpreting radiologist for subsequent imaging.
Statistical Analysis
Cases were analyzed on the basis of findings (ie, lesions on index examination). Therefore, a single patient could have more than one index finding and, in fact, some women had both mammographic and US findings that were deemed to be different lesions. Different index findings in the same location (eg, focal asymmetry and calcifications) were treated as one finding. In such cases, the finding was classified under the modality in which it was of higher suspicion. Mammographic findings included asymmetry (this common term was used for all asymmetry), mass, distortion, and calcifications; US findings included masses. The MBI results were compared with biopsy or surgery histopathologic results, when available, or with a minimum of 1 year of follow-up imaging. Interval breast cancers in an annual mammography screening program are defined as cancers detected within 1 year after a normal mammographic screening; the 1-year follow-up likewise has been used in evaluating diagnostic breast MRI performance (6). False-negative MBI results were defined as those which were interpreted as negative, but a cancer was found in the index finding within 1 year of the MBI study. False-positive MBI results were those which were interpreted as positive but subsequent biopsy or surgery of the index finding showed a noncancerous histopathologic finding. The NPV of MBI was the primary outcome measure. Secondary measures included positive predictive value (PPV), FNR, and additional descriptive statistics. All analyses were performed using JMP version 13.1 (SAS Institute, Cary, NC). All authors were responsible for data analysis.
Results
Patient Overview, PPV, NPV, and FNR
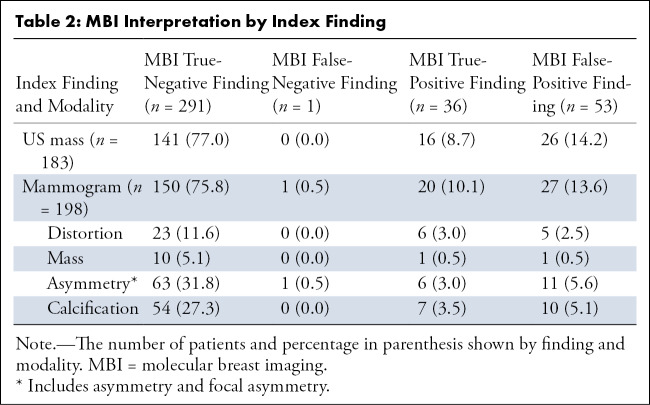
During the study period, there were 575 findings, of which 381 were included after applying exclusion criteria, as previously outlined. Table 1 details demographic data. Table 2 characterizes MBI interpretations by the kind of index finding for each of the two modalities: mammography and US.
Table 1:
Demographic Data
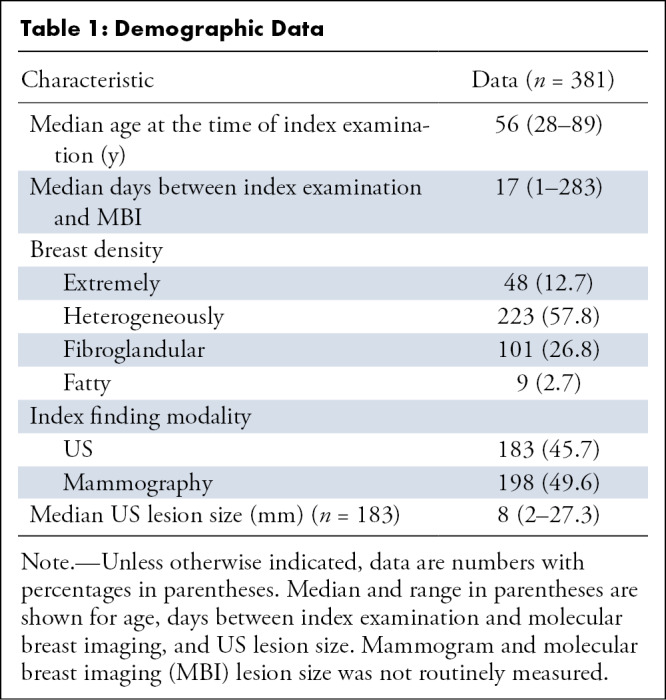
Table 2:
MBI Interpretation by Index Finding
PPV and False-Positive Findings at MBI
Overall, 89 of the 381 MBI examinations (23.4%) were interpreted as positive. All positive MBI cases had histopathologic results. Thirty-six cancers in the index findings were identified with MBI, including six that were DCIS only and 30 that were either invasive cancers only or invasive cancers and DCIS. Thus, the overall PPV was 40.4% (36 of 89; 95% confidence interval [CI]: 30.2%, 50.6%). The PPV for US findings was 38.1% (16 of 42) and for mammographic findings was 42.6% (20 of 47) (Table 2). The highest PPV was for the finding of distortion on mammograms (six of 11 [54.5%]), and the lowest was for mammogram asymmetry (six of 17 [35.3%]). However, these numbers should be interpreted with caution because of the small sample size.
There is interest in the size and grade of cancers depicted by the dual-head CZT camera using the low dose because it has been demonstrated that the size and grade of cancers affects the PPV of MBI studies (7). The cancers in our study ranged in size from 2 mm to 11 mm, with a median of 6 mm. Two cancer lesions were grade 3; six were grade 2; and the remaining 28 were grade 1. False-positive findings included seven atypical hyperplasias; some of the other 46 findings included fibroadenoma (n = 8), papilloma (n = 7), fat necrosis (n = 2), and phyllodes tumor (n = 2), and the rest (n = 27) were other benign entities such as fibrocystic changes, infectious and/or inflammatory process, hamartoma, benign lymph node, radial scar, pseudoangiomatous stromal hyperplasia, and other benign conditions.
Of note, there were seven patients in whom MBI results were interpreted as positive in unexpected locations (ie, not corresponding to the index mammogram or US findings). All underwent biopsy after targets were found at traditional imaging or MRI. One patient had atypical ductal hyperplasia, one had atypical lobular hyperplasia, and five had benign results, including fibroadenoma. Although we did not find an unexpected cancer, in three patients the extent of disease as indicated with MBI was more than that at mammography or US and was later confirmed with MRI or surgery.
NPV, FNR, and Reduction in the Biopsy Collection from MBI
Overall, MBI examinations of 292 of the 381 findings (76.6%) were interpreted as negative. Of the 292, 27 patients underwent subsequent biopsies, results of which were negative for cancer. One underwent biopsy of the index lesion, the result of which was positive for cancer, and 264 were true-negative findings based on follow-up imaging for 1 to 1.5 years (146 of 264 [55.3%]), or at least 2 years (118 of 264 [44.7%]). Thus, the NPV was 99.7% (291 of 292; 95% CI: 99.1%, 100%), the FNR was 2.7% (one of 37; 95% CI: 0%, 7.9%), sensitivity was 97.3% (36 of 37; 95% CI: 92.1%, 100%), and specificity was 84.6% (291 of 344; 95% CI: 80.8%, 88.4%).
Of the 381 findings included in this study, 117 underwent biopsy. In addition, seven biopsies were performed in unexpected positive MBI locations, resulting in 124 total biopsies. Thus, interposing MBI reduced the number of biopsies by 67.5% ([381 − 124] ÷ 381). We found 37 cancers in 381 index findings (ie, a rate of cancer of 9.7% in our cohort). Some typical cases are shown in Figures 1–4. All cases in the figures had true-negative MBI results: case one shows a new mammogram mass; case two shows a mass detected with US; case three shows distortion on the mammogram; and case four shows calcifications.
Figure 1a:
![Images in a 64-year-old woman. (a) The mammogram shows a new mass in the lateral right breast (arrow, coned magnification craniocaudal [CC] view). There is no US correlate. (b) The MBI result is negative. The mammogram remained stable for 2 years. MBI = molecular breast imaging, MLO = mediolateral oblique.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3909/7983715/38d14e91dee5/rycan.2020190096.fig1a.jpg)
Images in a 64-year-old woman. (a) The mammogram shows a new mass in the lateral right breast (arrow, coned magnification craniocaudal [CC] view). There is no US correlate. (b) The MBI result is negative. The mammogram remained stable for 2 years. MBI = molecular breast imaging, MLO = mediolateral oblique.
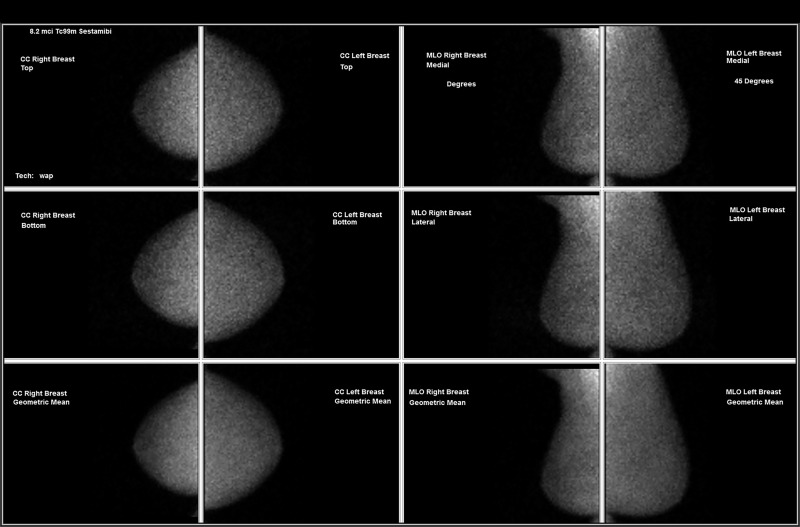
Figure 4a:
Images in a 64-year-old woman with (a) new grouping of calcifications in left breast (circled, coned magnification craniocaudal [CC]) but (b) with negative result at molecular breast imaging. No change on first short-interval follow-up (not shown); increased calcifications in the grouping 1 year later (not shown) led to stereotactic biopsy, results of which were negative for cancer.
Figure 1b:
Images in a 64-year-old woman. (a) The mammogram shows a new mass in the lateral right breast (arrow, coned magnification craniocaudal [CC] view). There is no US correlate. (b) The MBI result is negative. The mammogram remained stable for 2 years. MBI = molecular breast imaging, MLO = mediolateral oblique.
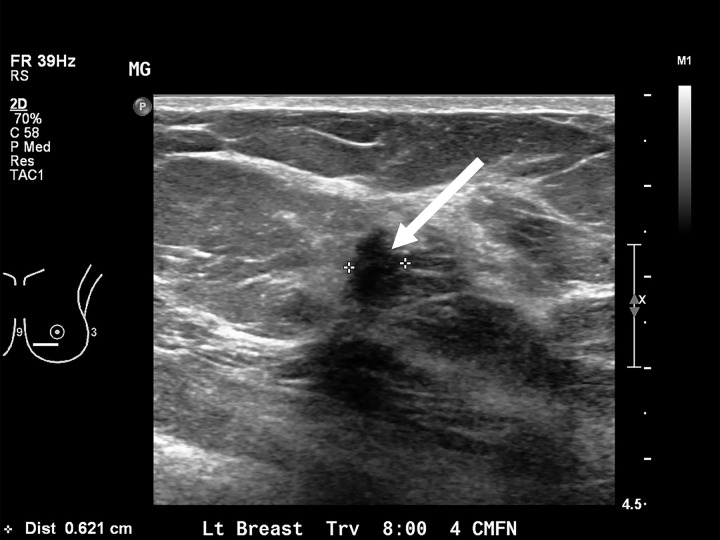
Figure 2a:
Images in a 71-year-old woman, after lumpectomy in the right breast. The mammogram in 2016 was negative. (a) US image obtained several days later shows a mass with posterior shadowing at eight o’clock position (arrow) in the left breast. (b) The molecular breast imaging result was negative. The subsequent MRI, mammographic, and US results were negative for 2 years. CC = craniocaudal, MLO = mediolateral oblique.
Figure 2b:
Images in a 71-year-old woman, after lumpectomy in the right breast. The mammogram in 2016 was negative. (a) US image obtained several days later shows a mass with posterior shadowing at eight o’clock position (arrow) in the left breast. (b) The molecular breast imaging result was negative. The subsequent MRI, mammographic, and US results were negative for 2 years. CC = craniocaudal, MLO = mediolateral oblique.
Figure 3a:
Images in a 55-year-old woman with (a) distortion in left breast (circle, coned magnification mediolateral oblique [MLO]) but (b) with negative molecular breast imaging. The faint activity seen on left craniocaudal (CC) of the molecular breast image was tissue bunching. There was no US correlate. Subsequent mammograms remained negative for 3 years.
Figure 3b:
Images in a 55-year-old woman with (a) distortion in left breast (circle, coned magnification mediolateral oblique [MLO]) but (b) with negative molecular breast imaging. The faint activity seen on left craniocaudal (CC) of the molecular breast image was tissue bunching. There was no US correlate. Subsequent mammograms remained negative for 3 years.
Figure 4b:
Images in a 64-year-old woman with (a) new grouping of calcifications in left breast (circled, coned magnification craniocaudal [CC]) but (b) with negative result at molecular breast imaging. No change on first short-interval follow-up (not shown); increased calcifications in the grouping 1 year later (not shown) led to stereotactic biopsy, results of which were negative for cancer.
Case Examples
In only one case, the MBI result was interpreted as negative, but the biopsy results of the index finding showed cancer. This case related to a very posterior asymmetry on the mammogram. Routine positioning of the breast on the MBI camera did not include this finding in the field of view. This shortcoming did not come to light until after the patient had left the department. We considered this a limitation of the MBI modality and included this case as a false-negative finding. We did not encounter any other case in which the MBI result was outright falsely negative (ie, an index finding turned out to be cancer even though the MBI result was technically adequate and interpreted as negative).
In one other patient, MBI was performed to evaluate a mass depicted at US in the left breast and was interpreted as negative. Six months later, the patient underwent annual mammography which showed new right breast calcifications which were proven to be malignant at biopsy. The original mass depicted at US disappeared subsequently. Yet another patient had a new mass identified at a routine US 1 year from a negative result at MBI. The new mass was proven to be malignant at biopsy. We did not count these two cases as false negative for the purposes of this study which was done to evaluate the NPV and FNR of MBI in the index findings. However, these two cases may have been considered false negative in a screening MBI study.
Discussion
This article reports our initial results of the NPV and FNR of MBI in cases which had low-suspicion findings at screening mammography or US. We found that MBI had a very high NPV of 99.7% (291 of 292, 95% CI: 99.1%, 100%) in our cohort of patients.
Previous reports of NPV for MBI included 94.3% (50 of 53) (4) and 96% (611 of 634) (8) based on single-head sodium iodide crystal technology. The interval leap in technology to cameras with dual-head CZT solid-state detectors is expected to result in improved detection of cancers (9). A preliminary study (10) regarding the accuracy of MBI in suspicious calcifications on mammograms, using a dual-head CZT detector camera, reported the NPV of MBI was only 81% (48 of 59). In this study, 20 of 24 cancers found were DCIS (10). Substantial heterogeneity in the lesions associated with mammographic calcifications may contribute to discordant conclusions about the performance of MBI. We speculate that invasive cancer and DCIS lesions vary in metabolic needs and bulk-forming tendency, resulting in varying visibility at MBI. However, calcifications have been difficult to characterize even with breast MRI (11).
We found a 67.5% reduction in the number of breast biopsies, compared with if all index findings had undergone biopsy. On the basis of a FNR of 8%, Weigert et al (8) concluded that a negative breast gamma imaging examination could not replace biopsy. Our FNR was 2.7% (one of 37). The use of an adjunct diagnostic test to reduce the number of breast biopsies is controversial (12). An imaging-guided breast biopsy is considered relatively safe and simple. Conversely, in the United States, approximately 75% (12) of the 1.6 million annual (13) breast biopsies triggered by screening mammographic findings are negative for cancer (12). There is growing literature regarding false-positive mammographic results (14) and the emotional, physical, and monetary cost of biopsies triggered by false-positive screenings (15). Most of the negative breast biopsy findings are BI-RADS 4a and 4b, which have a chance of malignancy of 7.6% and 22%, respectively (16). There is interobserver variability in assignment between the BI-RADS categories, specifically with respect to BI-RADS 3 and 4a (17–20). Some patients with BI-RADS 3 findings choose to undergo biopsy rather than endure the anxiety of waiting 6 months for follow-up imaging. Furthermore, some patients may be repeatedly placed in BI-RADS 3 or 4 in consecutive years with ultimately a benign outcome, which can erode patient confidence in the process. Researchers have attempted to decrease unnecessary breast biopsies by radiomic image assessment (21) and contrast material–enhanced mammography (22). Efforts using other techniques and modalities to downgrade BI-RADS 4a and 4b findings have been reported (23), including through the use of breast MRI (24). Zuley et al (25) found an NPV of 98.3% for contrast-enhanced digital mammography adequate for reducing the number of benign biopsies.
If an imaging test like MBI is found to be reliable in excluding cancer in certain mammographic and US findings, it would be a positive step forward for patients. The use of MBI may become more widespread for several reasons: the passing of the federal breast density law may further heighten the awareness of the need for supplemental screening in patients with dense breasts; US screening is not ideal because while it helps in detection it also adds substantially to the false-positive pool (26); and because many small- to medium-size hospitals cannot afford to dedicate an MRI unit solely to breast imaging. Additionally, the radiation dose concerns with MBI may recede (27,28), and MBI may come to be perceived as safe. It is therefore necessary to look at the potential benefits of a negative MBI result.
Some have cautioned about the radiation risk posed by MBI. They used a theoretical benefit-to-radiation risk metric to compare MBI to mammography-based techniques (29,30), concluding that MBI fell short on the metric. However, there are substantial challenges to the validity of this metric and the use of a linear nonthreshold model for risk calculations (28). The whole-body effective dose of 2 mSv from an MBI examination is at or below the natural background radiation.
Our study had several limitations. It was conducted at a single institution with a relatively nondiverse patient population. We did not collect detailed data on patients who underwent biopsy but not MBI. The factors that contributed to whether a patient underwent MBI may have affected the proportions of BI-RADS category 4a and 4b lesions in the MBI group, compared with the entire 4a and 4b population at our institution. However, our work was not aimed at examining a particular mix of 4a and 4b lesions. Indeed, the proportion may likely vary between institutions. A multi-institutional trial is needed to assess whether the NPV is as high among thousands of 4a and 4b lesions as it was among our cohort. In addition, the sample size of our study was relatively small compared with screening MBI studies, although it was similar to those in published MRI studies (6,24).
Our results can be considered as the initial proof of concept. Future research will recruit patients headed for biopsy based on mammographic findings to participate in a prebiopsy MBI study to directly compare the MBI and biopsy results in a variety of BI-RADS 4 findings. If this work continues to show very high NPV, a multi-institutional trial would be needed. In summary, MBI with dual-head CZT detector camera and with a drawn dose of 8 mCi (296 MBq) of 99mTc–sestamibi had a very high NPV, which may obviate biopsy of mammographic and US findings.
Acknowledgments
Acknowledgments
We gratefully acknowledge the generous grant received in support of this work from the Connecticut Breast Health Initiative.
Supported by a grant from the Connecticut Breast Health Initiative.
Disclosures of Conflicts of Interest: R.J. Activities related to the present article: institution received grant from Connecticut Breast Health Initiative and institution paid author for working as principal investigator. Activities not related to the present article: author received honorarium for lectures by Dilon Technologies. Other relationships: disclosed no relevant relationships. D.R.K. disclosed no relevant relationships. A.D.K. Activities related to the present article: institution received grant from Connecticut Breast Health Initiative but CT BHI did not have any influence on study design, data analysis, and so forth. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships.
Abbreviations:
- BI-RADS
- Breast Imaging Reporting and Data System
- CI
- confidence interval
- CZT
- cadmium zinc telluride
- DCIS
- ductal carcinoma in situ
- FNR
- false-negative rate
- MBI
- molecular breast imaging
- NPV
- negative predictive value
- PPV
- positive predictive value
References
- 1.Rhodes DJ, Hruska CB, Conners AL, et al. Journal club: molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. AJR Am J Roentgenol 2015;204(2):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shermis RB, Wilson KD, Doyle MT, et al. Supplemental breast cancer screening with molecular breast imaging for women with dense breast tissue. AJR Am J Roentgenol 2016;207(2):450–457. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes D, Hunt K, Conners A, et al. Molecular breast imaging and tomosynthesis to eliminate the reservoir of undetected cancer in dense breasts: The Density MATTERS trial [abstr]. Cancer Res 2019;79(4 Suppl):PD405. [Google Scholar]
- 4.Brem RF, Floerke AC, Rapelyea JA, Teal C, Kelly T, Mathur V. Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. Radiology 2008;247(3):651–657. [DOI] [PubMed] [Google Scholar]
- 5.Conners AL, Hruska CB, Tortorelli CL, et al. Lexicon for standardized interpretation of gamma camera molecular breast imaging: observer agreement and diagnostic accuracy. Eur J Nucl Med Mol Imaging 2012;39(6):971–982. [DOI] [PubMed] [Google Scholar]
- 6.Niell BL, Bhatt K, Dang P, Humphrey K. Utility of Breast MRI for Further Evaluation of Equivocal Findings on Digital Breast Tomosynthesis. AJR Am J Roentgenol 2018;211(5):1171–1178. [DOI] [PubMed] [Google Scholar]
- 7.Tadwalkar RV, Rapelyea JA, Torrente J, et al. Breast-specific gamma imaging as an adjunct modality for the diagnosis of invasive breast cancer with correlation to tumour size and grade. Br J Radiol 2012;85(1014):e212–e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigert JM, Bertrand ML, Lanzkowsky L, Stern LH, Kieper DA. Results of a multicenter patient registry to determine the clinical impact of breast-specific gamma imaging, a molecular breast imaging technique. AJR Am J Roentgenol 2012;198(1):W69–W75. [DOI] [PubMed] [Google Scholar]
- 9.Hruska CB. Molecular Breast Imaging for Screening in Dense Breasts: State of the Art and Future Directions. AJR Am J Roentgenol 2017;208(2):275–283. [DOI] [PubMed] [Google Scholar]
- 10.Hruska C, Hunt K, Johnson M, et al. Accuracy of Molecular Breast Imaging in Patients with Suspicious Calcifications. Radiological Society of North America; 2018 Scientific Assembly and Annual Meeting, Chicago, IL, November 25–30, 2018. http://archive.rsna.org/2018/18011888.html. Accessed December 2019. [Google Scholar]
- 11.Bennani-Baiti B, Baltzer PA. MR Imaging for Diagnosis of Malignancy in Mammographic Microcalcifications: A Systematic Review and Meta-Analysis. Radiology 2017;283(3):692–701. [DOI] [PubMed] [Google Scholar]
- 12.Lee CI, Bensink ME, Berry K, et al. Performance goals for an adjunct diagnostic test to reduce unnecessary biopsies after screening mammography: analysis of costs, benefits, and consequences. J Am Coll Radiol 2013;10(12):924–930. [DOI] [PubMed] [Google Scholar]
- 13.Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 2015;313(11):1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med 2011;155(8):481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch HG, Passow HJ. Quantifying the benefits and harms of screening mammography. JAMA Intern Med 2014;174(3):448–454. [DOI] [PubMed] [Google Scholar]
- 16.Elezaby M, Li G, Bhargavan-Chatfield M, Burnside ES, DeMartini WB. ACR BI-RADS Assessment Category 4 Subdivisions in Diagnostic Mammography: Utilization and Outcomes in the National Mammography Database. Radiology 2018;287(2):416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KA, Talati N, Oudsema R, Steinberger S, Margolies LR. BI-RADS 3: Current and Future Use of Probably Benign. Curr Radiol Rep 2018;6(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology 2006;239(2):385–391. [DOI] [PubMed] [Google Scholar]
- 19.Pistolese CA, Tosti D, Citraro D, et al. Probably Benign Breast Nodular Lesions (BI-RADS 3): Correlation between Ultrasound Features and Histologic Findings. Ultrasound Med Biol 2019;45(1):78–84. [DOI] [PubMed] [Google Scholar]
- 20.Redondo A, Comas M, Macià F, et al. Inter- and intraradiologist variability in the BI-RADS assessment and breast density categories for screening mammograms. Br J Radiol 2012;85(1019):1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drukker K, Giger ML, Joe BN, et al. Combined Benefit of Quantitative Three-Compartment Breast Image Analysis and Mammography Radiomics in the Classification of Breast Masses in a Clinical Data Set. Radiology 2019;290(3):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jochelson M. Contrast-enhanced digital mammography. Radiol Clin North Am 2014;52(3):609–616. [DOI] [PubMed] [Google Scholar]
- 23.Menezes GLG, Pijnappel RM, Meeuwis C, et al. Downgrading of Breast Masses Suspicious for Cancer by Using Optoacoustic Breast Imaging. Radiology 2018;288(2):355–365. [DOI] [PubMed] [Google Scholar]
- 24.Strobel K, Schrading S, Hansen NL, Barabasch A, Kuhl CK. Assessment of BI-RADS category 4 lesions detected with screening mammography and screening US: utility of MR imaging. Radiology 2015;274(2):343–351. [DOI] [PubMed] [Google Scholar]
- 25.Zuley ML, Bandos AI, Abrams GS, et al. Contrast Enhanced Digital Mammography (CEDM) Helps to Safely Reduce Benign Breast Biopsies for Low to Moderately Suspicious Soft Tissue Lesions. Acad Radiol 2019. 10.1016/j.acra.2019.07.02. Published online September 5, 2019. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Arao RF, Sprague BL, et al. Performance of Screening Ultrasonography as an Adjunct to Screening Mammography in Women Across the Spectrum of Breast Cancer Risk. JAMA Intern Med 2019;179(5):658–667 [Published correction appears in JAMA Intern Med 2019;179(5):733.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao AT, Hruska CB, Conners AL, et al. Dose Reduction in Molecular Breast Imaging With a New Image-Processing Algorithm. AJR Am J Roentgenol 2020;214(1):185–193. [DOI] [PubMed] [Google Scholar]
- 28.Hruska CB. Let’s Get Real about Molecular Breast Imaging and Radiation Risk. Radiol Imaging Cancer 2019;1(1):e190070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrick RE, Tredennick T. Benefit to radiation risk of breast-specific gamma imaging compared with mammography in screening asymptomatic women with dense breasts. Radiology 2016;281(2):583–588. [DOI] [PubMed] [Google Scholar]
- 30.Brown M, Covington MF. Comparative Benefit-to–Radiation Risk Ratio of Molecular Breast Imaging, Two-Dimensional Full-Field Digital Mammography with and without Tomosynthesis, and Synthetic Mammography with Tomosynthesis. Radiol Imaging Cancer 2019;1(1):e190005. [DOI] [PMC free article] [PubMed] [Google Scholar]



![Images in a 64-year-old woman with (a) new grouping of calcifications in left breast (circled, coned magnification craniocaudal [CC]) but (b) with negative result at molecular breast imaging. No change on first short-interval follow-up (not shown); increased calcifications in the grouping 1 year later (not shown) led to stereotactic biopsy, results of which were negative for cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3909/7983715/9644b55cecb7/rycan.2020190096.fig4a.jpg)
![Images in a 64-year-old woman. (a) The mammogram shows a new mass in the lateral right breast (arrow, coned magnification craniocaudal [CC] view). There is no US correlate. (b) The MBI result is negative. The mammogram remained stable for 2 years. MBI = molecular breast imaging, MLO = mediolateral oblique.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3909/7983715/5f7932ceeae5/rycan.2020190096.fig1b.jpg)
![Images in a 55-year-old woman with (a) distortion in left breast (circle, coned magnification mediolateral oblique [MLO]) but (b) with negative molecular breast imaging. The faint activity seen on left craniocaudal (CC) of the molecular breast image was tissue bunching. There was no US correlate. Subsequent mammograms remained negative for 3 years.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3909/7983715/237437f0904d/rycan.2020190096.fig3a.jpg)
![Images in a 55-year-old woman with (a) distortion in left breast (circle, coned magnification mediolateral oblique [MLO]) but (b) with negative molecular breast imaging. The faint activity seen on left craniocaudal (CC) of the molecular breast image was tissue bunching. There was no US correlate. Subsequent mammograms remained negative for 3 years.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3909/7983715/c8df20034af4/rycan.2020190096.fig3b.jpg)
![Images in a 64-year-old woman with (a) new grouping of calcifications in left breast (circled, coned magnification craniocaudal [CC]) but (b) with negative result at molecular breast imaging. No change on first short-interval follow-up (not shown); increased calcifications in the grouping 1 year later (not shown) led to stereotactic biopsy, results of which were negative for cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3909/7983715/2233f858be6e/rycan.2020190096.fig4b.jpg)